Abstract
Background
Epidermal growth factor receptor (EGFR)-targeting therapies dramatically modified the prognosis of stage 4 non-small-cell lung cancer. Sensitizing EGFR mutations are the best efficacy factor of these treatments. In 2006, the French National Cancer Institute launched a network of 28 centers for EGFR molecular analysis in routine practice. The aim of this retrospective study was to describe the results of routine EGFR analysis in one of these centers (Lyon University Hospital) and to assess outcomes in patients with the mutation.
Methods
EGFR mutations were analyzed for exons 18–21 by direct sequencing. The characteristics of each sample were retrospectively collected from the lab archives. Subsequent outcomes for patients harboring at least one mutation were retrospectively collected from each referring physician.
Results
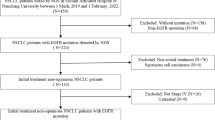
During 1 year, 792 samples were analyzed, corresponding to 753 patients. A total of 133 mutations were diagnosed in 124 samples (15.7 %), corresponding to 121 patients. Most of them (77.4 %) were sensitizing mutations and were located in exons 19 and 21. Others were resistance mutations (8.3 %) or rare mutations (14.3 %) for which effects on tyrosine kinase inhibitor (TKI) sensitivity are unknown. The rate of indeterminate results (i.e., no sequencing of the entire exon 19 or 21) was 6.3 % (n = 50 samples). The only factor statistically associated with a risk of failure was sample from bone tissue: 13.7 % gave incomplete results (i.e., no whole sequencing of exons 18–21).
Conclusions
Eighty-five of the 121 patients with EGFR mutations were treated with TKI. There were no differences in progression free survival (PFS) according to the type of molecule (erlotinib or gefitinib) or to the line of prescription of TKI. By contrast, exon 18 sensitizing mutations showed a worse PFS than exon 19 or 21 mutations. Finally, dose reduction was significantly more frequent in the erlotinib group than in the gefitinib group.


Similar content being viewed by others
References
Pao W, Miller VA (2005) Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol 23(11):2556–2568
Sharma SV et al (2007) Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 7(3):169–181
He M et al (2012) EGFR exon 19 insertions: a new family of sensitizing EGFR mutations in lung adenocarcinoma. Clin Cancer Res 18(6):1790–1797
Somatic Mutations in Epidermal Growth Factor Receptor Database (2008). www.somaticmutations-egfr.org. Accessed 30 May 2013
Dahabreh IJ et al (2010) Somatic EGFR mutation and gene copy gain as predictive biomarkers for response to tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 16(1):291–303
Girard N et al (2012) Nomogram to predict the presence of EGFR activating mutation in lung adenocarcinoma. Eur Respir J 39(2):366–372
Rosell R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246
Zhou C et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12(8):735–742
Mitsudomi T et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11(2):121–128
Maemondo M et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388
Institut National du Cancer (INCA) (2010) Cancer du poumon non à petites cellules. Formes localisées non opérables, localement avancées et métastatiques. Recommandations & référentiels. www.e-cancer.fr. Accessed 30 May 2013
Keedy VL et al (2011) American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 29(15):2121–2127
Andre F et al (2012) Biomarker discovery, development, and implementation in France: a report from the French National Cancer Institute and cooperative groups. Clin Cancer Res 18(6):1555–1560
Beau-Faller M et al (2011) Cross-validation study for epidermal growth factor receptor and KRAS mutation detection in 74 blinded non-small cell lung carcinoma samples: a total of 5550 exons sequenced by 15 molecular French laboratories (evaluation of the EGFR mutation status for the administration of EGFR-TKIs in non-small cell lung carcinoma [ERMETIC] project—part 1). J Thorac Oncol 6(6):1006–1015
Ruppert AM et al (2011) A simple view on lung cancer biology: the EGFR pathway. Rev Mal Respir 28(4):565–577
Han JY et al (2012) First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 30(10):1122–1128
Fukuoka M et al (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29(21):2866–2874
Talaulikar D et al (2008) A comparative study of the quality of DNA obtained from fresh frozen and formalin-fixed decalcified paraffin-embedded bone marrow trephine biopsy specimens using two different methods. J Clin Pathol 61(1):119–123
Wickham CL et al (2000) Formic acid decalcification of bone marrow trephines degrades DNA: alternative use of EDTA allows the amplification and sequencing of relatively long PCR products. Mol Pathol 53(6):336
Pirker R et al (2010) Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J Thorac Oncol 5(10):1706–1713
Institut National du Cancer (INCA) (2012) Molecular genetic testing for equal access to targeted therapies in France in 2011, Reports & Summaries. http://www.e-cancer.fr. Accessed 30 May 2013
Rosell R et al (2009) Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361(10):958–967
de Mello RA et al (2012) EGFR exon mutation distribution and outcome in non-small-cell lung cancer: a Portuguese retrospective study. Tumour Bio 33(6):2061–2068
Sahoo R et al (2011) Screening for EGFR mutations in lung cancer, a report from India. Lung Cancer 73(3):316–319
Greulich H et al (2005) Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med 2(11):e313
Jiang J et al (2005) Epidermal growth factor-independent transformation of Ba/F3 cells with cancer-derived epidermal growth factor receptor mutants induces gefitinib-sensitive cell cycle progression. Cancer Res 65(19):8968–8974
Petrelli F et al (2012) Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small-cell lung cancer: a meta-analysis of 13 randomized trials. Clin Lung Cancer 13(2):107–114
Besse B, Zalcman G (2011) What is the best sequence of treatment for patients with EGFR mutations? Rev Pneumol Clin 67(Suppl 1):S24–S29
Nowak F et al (2012) Tumour molecular profiling for deciding therapy—the French initiative. Nat Rev Clin Oncol 9(8):479–486
Conflict of interest
SC declares that he has received fees for consulting, attending a meeting, and conducting a lecture from Roche, Astra Zeneca, Lilly, Glaxo-Smith Kline, Chugai, Vitalaire, Air-Product; and also research grants were paid at his institution from Pfizer, Roche, Astra Zeneca, Lilly, Pierre-Fabre, Boeringher Ingelheim, Laidet, Chugai. Other authors have none to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
408_2013_9482_MOESM1_ESM.docx
Electronic supplementary material The online version of this article (doi: 10.1007/s00408-013-9482-2) contains supplementary material which is available to authorized users. (DOCX 14 kb)
Collaborators of This Study
Collaborators of This Study
Philippe Ardisson, Dominique Arpin, Aline Bajard, Dominique Beal-Ardisson, Denis Beaute, Jacques Berger, Elisabeth Biron, Christian Bonnamour, Marie-Laure Braud, Philippe Brun, Serge Cabuzel, Gérard Chatte, Vincent Cottin, Jérôme Dagognet, Sylvie Demolombe, Arlette Desira, Gilles Devouassoux, Nouredine Douissa, Bernard Duvert, Bénédicte Etienne-Mastroianni, Lionel Falchero, Eric Fauchon, Patrick Fournel, Nathalie Freymond, Cécile Garnier, Béatrice gentil, Etienne Giroux Le Prieur, Valérie Grangeon, Patrick Hyvernat, Stéphane Hominal, Zouhair Jaouali, Géraldine Raichon-Patru, Sébastien Larive, Geneviève Letanche, Aurore Mallet, Philippe Mallinger, Catherine Marichy, Yann Martinat, Bernard Montagnon, Alain Penet, Maurice Perol, Marielle Perrichon, Bernard Raphanel, Anne-Claire Ravel, Paul Rebattu, Marielle Roux, Léa Saban Roche, Bernard Sanjuan, Jean François Vidal, Michel Vincent, Alain Voloch.
Rights and permissions
About this article
Cite this article
Locatelli-Sanchez, M., Couraud, S., Arpin, D. et al. Routine EGFR Molecular Analysis in Non-Small-Cell Lung Cancer Patients is Feasible: Exons 18–21 Sequencing Results of 753 Patients and Subsequent Clinical Outcomes. Lung 191, 491–499 (2013). https://doi.org/10.1007/s00408-013-9482-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-013-9482-4