Abstract
Background
Epidermal growth factor receptor (EGFR) amplification refers to the copy number increase of EGFR gene, and is often identified as a “bypass” way of Epidermal growth factor receptor Tyrosine kinase inhibitors (EGFR-TKI) resistance. We aimed to explore the effect of EGFR amplification on EGFR mutation treatment-naive advanced non-squamous non-small cell lung cancer (NSCLC) patients.
Methods
We conducted a prospective observational study in single center, enrolling advanced non-squamous NSCLC patients receiving Tyrosine kinase inhibitors (TKIs) between March 3, 2019, and February 1, 2022. Next-generation sequencing (NGS) was used to detect genetic alterations in tumor tissue samples. Progression-free survival (PFS) curves were performed using the Kaplan-Meier method. Univariate and multivariate analyses were used to evaluate factors affecting the efficacy of TKIs.
Results
A total of 117 treatment-naive advanced NSCLC patients were identified in this study. EGFR amplification was found in 22 of 117 (18.8%) patients with EGFR mutations. Of 22 patients with EGFR amplification, 10 patients harbored EGFR 19 del, 11 patients with 21-L858R. The median follow-up time was 22.47 months. The median PFS of the patients with or without EGFR amplification was 8.25 months and 10.67 months, respectively (log-rank test, P = 0.63). In multivariate analysis, EGFR amplification was not an independent prognosis factor for the patients receiving first-line TKIs [HR = 1.38, 95%CI (0.73–2.58), P = 0.321]. Subgroup analysis revealed that EGFR amplification is a risk factor for progression in the brain metastasis population. [HR = 2.28, 95%CI (1.01, 5.14), P = 0.047].
Conclusion
EGFR amplification is not an independent prognosis factor for PFS in advanced non-squamous NSCLC patients receiving first-line TKIs. However, it is an independent risk factor for PFS in the brain metastasis population.
Similar content being viewed by others
Introduction
Epidermal growth factor receptor (EGFR) mutation is the most common gene alteration in NSCLC, which accounts for approximately 15% of the Caucasians and 50% of Eastern Asians [1]. EGFR-TKI is the standard first-line treatment for EGFR-mutated lung cancer patients [2,3,4,5]. At present, the third generation EFGR-TKIs, such as Osimertinib or Aumolertinib, significantly improve response rates, progression-free survival (PFS), and overall survival (OS) in lung cancer patients with EGFR mutations [6, 7], but the outcome of partial patients remains extremely poor.
Both patient-related and tumor-related factors could affect the efficacy and survival of EGFR-TKI treatment in lung cancer patients. Different types of EGFR mutations may have different responses to the EGFR-TKIs, such as patients with EGFR exon 19 deletion (19del) having a longer PFS compared to patients with EGFR exon 21 Leu858Arg (21-L858R) mutation [8,9,10,11,12]. Furthermore, other studies have found that patients with different Eastern Cooperative Oncology Group (ECOG) scores, gender, and smoking status show different responses to TKIs [8, 13, 14]. In addition, co-mutations of EGFR mutation lung adenocarcinoma patients may also influence the response to TKI treatment. It has been reported that co-occurring abnormalities including TP53, RB1, PTEN, ERBB2, and MET are unfavorable clinical prognosis factors for lung adenocarcinoma patients receiving first-line EGFR-TKIs [15,16,17].
EGFR amplification means an increase in the copy number of the EGFR gene, which is generally recognized as a “bypass” way of EGFR-TKI resistance [18, 19]. Previous studies reported that EGFR amplification occurred in about 9–64% NSCLC patients [20,21,22,23], and EGFR amplification also occurs in treatment-naive EGFR-mutated patients [15, 24,25,26]. A post hoc analysis of the Iressa Pan-Asia Study (IPASS) reported that EGFR amplification alone is not a predictive factor for patients receiving first-line EGFR-TKIs treatment [27]. A retrospective study suggested that EGFR amplification was a predictive factor for a better survival benefit from first-line TKIs treatment [26]. Ruiz-Patino A et al. discovered a significant difference in PFS and OS between EGFR amplification patients with 19del and 21-L858R [25]. However, Gao X et al. found that EGFR amplification is an independent poor factor in NSCLC patients with EGFR exon 20 insert receiving TKIs [24]. Meanwhile, a retrospective study found that EGFR amplification is an unfavorable factor when patients are treated with third-generation TKIs [15]. Therefore, it is not clear whether EGFR amplification is an independent prognosis factor in EGFR mutation patients receiving TKIs.
In this study, we conducted a prospective observational study to evaluate the relationship between EGFR amplification and PFS in EGFR-mutated NSCLC patients.
Methods
Study design
Advanced non-squamous NSCLC patients with EGFR mutations were consecutively collected between March 3, 2019 and February 1, 2022 at the Second Affiliated Hospital of Nanchang University. Inclusion criteria are as follows: (1) older than 18 years; (2) TNM IV stage; (3) patients with pathologically confirmed lung adenocarcinoma, adenosquamous carcinoma, or other non-specific pathologic types; and (4) EGFR mutation confirmed by NGS of tissue specimens. Those who had a history of additional malignancies, previous treatment, or had incomplete clinical data were excluded. Patients’ characteristics of demographic, genomic, and clinical, including age, sex, smoking, ECOG score, type of pathology, TNM stage, distant metastases status, EGFR mutation, T790M mutation, TP53 mutation, radiotherapy, and type of EGFR-TKIs, were collected from the hospital information system. This study conformed to the Declaration of Helsinki and was approved by the Institutional Ethics Committee of the Second Affiliated Hospital of Nanchang University.
Next-generation sequencing (NGS) detection
Lung tissues were obtained from percutaneous lung biopsies or bronchoscopy, and fixed in the Department of Pathology using paraffin wax. Formalin-fixed paraffin-embedded (FFPE) tumor tissues were collected for genetic variation detection. Gene mutation analysis of FFPE tumor tissue was determined by capture single molecule amplification and resequencing technology(capSMART) for 31or 457 cancer-related genes (Berry Oncology Beijing, China). In a nutshell, genomic DNA from tumor tissue samples was extracted using the DNeasy Tissue kit (Qiagen). The concentration of the purified DNA was determined by the QubitR dsDNA HS Assay Kit (Life Technologies, Grand Island, NY, United States). DNA libraries were constructed as previously described and the target-enriched library was then paired end (PE) sequenced (2 × 150 bp) on the NovaSeq platform (Illumina) according to the manufacturer’s instructions with high, uniform median coverage (> 1000×) and assessed for base substitutions, short insertions and deletions, copy number alterations, and gene fusions/rearrangements [28]. We determined the log ratio of DoC for each target (tumor versus control) and then used the circular binary segmentation (CBS) algorithm to segment the log ratio profile into segments of equal copy number for copy number analysis of the normalized collection of somatic variations [29]. We extracted CNV genes from the CBS segments. At first, genes with less than five target (target number ≤ 4) were filtered out. Then, for each gene target, we calculated the segment value presenting the mean log ratio of all target of this segment. When the segment value was ≥ 0.35, we consider this target as a gain target gain. If the number of gain targets / all targets for this gene was ≥ 0.7, and then this gene was considered as a gene amplification. Finally, we used the all of the targets of this gene to calculate the average log ratio, and calculated the average copy number. We used the formula to calculate the mutation of copy number nmut =VAF1ppCNt + CNn1-p [30]. While CNt means the tumor locus specific copy number, CNn represents the normal locus specific copy number(assumed to be 2), p is the tumor purity calculated by Facet [31] and VAF represents the variant allele frequency. EGFR amplification was defined as the copy number over 4.
Treatment and follow-up procedures
All the patients were treated with TKIs-based regimens, including TKIs alone or TKIs combined Anlotinib. PFS was calculated from the date of the TKI treatment to disease progression or death due to any cause. Evaluation of treatment effects was conducted according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The last follow-up time was June 22, 2022. The median follow-up time was 22.47 months.
Statistical analysis
All the continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as percentages. Independent sample t-tests and Fisher’s exact test were used for comparisons between the groups. Survive curves were drawn using the Kaplan-Meier method and compared by log-rank tests. Univariate and multivariate Cox proportional hazards models were used to evaluate independent predictive factors of each demographic, genomic, and clinical character associated with survival. We took relevant factors including age, ECOG score, TNM stage, bone metastasis, liver metastasis, lung metastases, pleural metastasis, EGFR mutation, T790M, TP53 mutation, radiotherapy, and type of TKIs into multivariate analysis. A multivariable stratified analysis adjusted with age, was performed as the sensitivity analysis to assess the effect of EGFR amplification in the subgroup patients, expressed as a hazard ratio with a 95% CI. Multivariable HRs for progression based on EGFR amplification status stratified by gender, brain metastasis, EGFR mutation, TP53 mutation, and TKI type with adjusted age. P values less than 0.05 were recognized as statistically significant. The statistical software packages R (version 3.6.3, http://www.R-project.org, The R Foundation) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) were used to analyze all data.
Results
Patient characteristics
A total of 117 non-squamous NSCLC patients with EGFR mutations were enrolled in our study, and eventually EGFR amplification was found in 22 of 117(18.8%) patients. The selection procedure was presented in Fig. 1. Table 1 showed that the enrolled patients included 70 females (59.83%), 88 patients (75.21%) aged under 70, and 16 patients (13.68%) with a smoking history. Seven patients (5.98%) received treatment combining first-generation EGFR-TKIs and Anlotinib, 65 patients (55.56%) were treated with the first-generation TKI, and 31patients (29.06%) were exposed to third-generation TKI.
Molecular characteristics
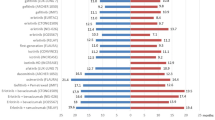
There were 49 (41.9%) patients harboring 19del, 60 (51.3%) with 21-L858R mutations, and 8 with uncommon EGFR mutations (2 patients with L861Q, 2 patients with I740_K745dupIPVAIK, 1 patient with 21p.L858_A859delinsRS, 1patent with G719X/V834L and 1 patient with G719X/A767V). Twenty-two EGFR amplification patients were detected with co-occurring alterations, including 10 (45.5%) EGFR19 del, 11(50%) 21-L858R. The detailed genomic alterations of patients with EGFR amplification were described in Fig. 2A. Table 1 showed that the proportion of females was higher in patients with EGFR amplification compared to those without (77.27% vs. 55.79%). Moreover, patients with EGFR amplification more often had co-mutation of TP53 compared with those without EGFR amplification (86.36% vs. 58.95%, P = 0.016). There were only 16 patients received NGS test again when the disease progresses. Of 16 patients, 1 patient carry MET amplification, 3 patients harboring T790M.
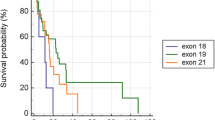
Gene distribution and the survival swimming plots of lung adenocarcinoma patients with EGFR mutation and EGFR amplification. A Gene distribution of lung adenocarcinoma patients with EGFR mutation and EGFR amplification; B survival swimming plots of lung adenocarcinoma patients with EGFR mutation and EGFR amplification
Survival analysis
The median follow-up time was 22.47 months. The median PFS was 11.43 months, and 11.43 months for 19del, 11.23 months for 21-L858R, and 14.07 months for rare EGFR mutations in the entire group. Figure 2B displayed survival time of these 22 patients with EGFR amplification. The median PFS of patients with EGFR amplification and without EGFR amplification were 9.93months and 11.47 months respectively (P = 0.63, Fig. 3). Then, we conducted univariate analysis to evaluate factors affecting the efficacy of TKIs (Table 2). Univariate analysis showed that bone metastasis is associated with shorter PFS [HR = 1.58, 95%CI (1.01–2.50), p = 0.047]. In contrast, 3rd generation TKI is associated with favorable PFS [HR = 0.41, 95%CI (0.23, 0.73) P = 0,003]. However, EGFR amplification was not a favorable prognostic factor for PFS [HR = 1.15, 95%CI (0.66–2.01), P = 0.63] in univariate analysis. Multivariate analysis indicated that EGFR amplification was still not a favorable prognostic factor of PFS [HR = 1.38, 95% (0.73, 2.58), P = 0.321]. Stratified analysis was further conducted to assess the effect of EGFR amplification in the patient subgroups (Table 3), which revealed that EGFR amplification is a risk factor for PFS [HR = 2.28, 95%CI (1.01, 5.14), P = 0.469] in the brain metastasis population.
Discussion
To the best of our knowledge, this is the first prospective observational cohort study evaluating the EGFR amplification prognostic value in EGFR-mutated NSCLC patients treated with first-line TKIs. We identified a total of 22 (18.8%) patients with EGFR amplification. Patients with EGFR amplification often have TP53 mutations. Our results revealed that concomitant concurrence of EGFR amplification is not associated with longer PFS in patients receiving first-line EGFR-TKIs. But subgroup analysis revealed that EGFR amplification is a risk factor for progression in patients with brain metastasis.
The proportion of EGFR amplification in our study was lower than previous studies, which reported EGFR amplification appeared in approximately 30-47% EGFR-mutated NSCLC patients [24,25,26]. The discrepancy might derive from sample size and methods of detecting EGFR amplification. In the study conducted by Ling Shana et al. and Ruiz-Patino A et al., they used the Dual-color Silver in situ Hybridization (DISH) or the fluorescence in situ hybridization (FISH) to detect EGFR amplification [25, 26]. Consistent with us, a study about EGFR 20 insert also used NGS to examine EGFR amplification, the definition of EGFR amplification was gene copy number > 2.75 in the 520 panel and > 2.25 in the other panels [24]. But in our study, EGFR amplification was defined as a copy number greater than 4 regardless of the small or large panels. Among the assay methods of NGS, there is not a consensus definition of EGFR amplification [32, 33]. Shan L et al. firstly demonstrated that EGFR amplification can influence treatment effects for NSCLC EGFR-mutated patients receiving TKIs [26]. The study among Hispanic patients also identified EGFR amplification as a prognosis factor for containing EGFR mutation patients treated with erlotinib, and further suggested that there is a significant difference between EGFR amplification patients with 19del and 21-L858R no matter in PFS or OS [25]. But our results suggest that co-occurring EGFR amplification is not a prognosis factor for patients treated with first-line TKIs, and we did not find any significant difference between patients with 19del and 21-L858R in PFS. Several reasons might account for the discrepancy. Firstly, both studies by Ling Shana and Ruiz-Patino A et al. were retrospective, while our study was a prospective observational study. In addition, these two did not include the other potentially coexisting factors, such as TP53 mutation and radiotherapy, which might influence the therapeutic efficacy of TKIs. Moreover, our study included different types of TKIs while enrolled patients were treated with 1st generation TKIs in their studies. Another study used a novel method to assess the heterogeneity of EGFR copy number gain [22]. Their results indicated that the EGFR copy number is significantly heterogeneous in different NSCLC patients. This discovery may help to explain the conflicting clinical data on EGFR amplification.
In addition to EGFR amplification, lots of studies have identified co-alterations including TP53 as a negative prognostic factor for EGFR-mutated NSCLC and a consistent predictor of poor survival outcome of EGFR-mutated patients receiving TKIs treatment [15,16,17, 34]. Paolo Bironzo et al. find that co-mutations including TP53 are not a predictive factor for patients treated with first-line TKIs [35]. However, we failed to identify TP53 as a prognosis factor. The main reasons might come from the size of the sample and heterogeneity of the included study population and usage of different EGFR-TKIs. Consistent with the study conducted by Bironzo P et al., the third-generation TKI was an independent predictor for patients with EGFR mutations [35]. Their study was also a single institution study based on real world data, including patients treated with first, second, and third generation TKIs. Both study of Bironzo P et al. and us are small sample studies, while our studies was prospective.
It has been established that EGFR copy gain is related to activating mutations only at the mutated oncogene locus but not in other oncogene loci [36, 37]. It is one of the typical examples that mutant allele specific imbalance caused by copy number gain or uniparental disomy. It frequently occurs in an important subset of cells containing mutant oncogenes due to complete loss of wild type allele [36, 38]. Besides, a growing body of research indicated that EGFR mutations are closely related to tumor onset, while increased copy number is associated with tumor progression [39, 40]. Therefore, the sensitivity to EGFR-TKIs between patients with coexisting EGFR mutation and amplification and patients with EGFR mutation may be different. However, our study proved that EGFR amplification is not associated with PFS. The discrepancy might be driven by real clinical practice, which contains lots of confounding factors such as heterogeneity of individuals and different types of TKIs. And these confounding factors might offset the effect of EGFR amplification. The advantage of this study is that it is a prospective observational study from the real world. However, the findings of this study should to be explained interpreted cautiously. Because our findings are based on single-center study, a multi-center and larger sample size study should be warranted to further confirm our conclusion.
Conclusion
Taken together, we found that EGFR amplification is not an independent prognostic factor for EGFR-mutated non-squamous NSCLC patients receiving first-line EGFR-TKI. However, EGFR amplification was a risk factor for progression in patients with brain metastasis. These findings indicated that EGFR amplification may be a prognosis factor in specific populations, and prospective identification of patients with EGFR amplification should be enrolled in clinical trials to further verify it.
Availability of data and materials
The datasets used and/or analyzed during the current study be deposited in Dryad Data. Link: https://datadryad.org/stash/share/ew_zc6wxeYIayI36cus1F_WkMweJpU43A-eKfW9G39w.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- EGFR-TKIs:
-
Epidermal growth factor receptor Tyrosine kinase inhibitors
- TKIs:
-
Tyrosine kinase inhibitors
- PFS:
-
Progression-free survival
- NGS:
-
Next-generation sequencing
- OS:
-
Overall survival
- 19del:
-
EGFR exon 19 deletion
- 21-L858R:
-
Exon 21 Leu858Arg
- FFPE:
-
Formalin-fixed paraffin-embedded
- TNM:
-
Tumor Node Metastasis
References
Suda K, Tomizawa K, Mitsudomi T. Biological and clinical significance of KRAS mutations in lung cancer: an oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev. 2010;29(1):49–60.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8.
Wu Y-L, Zhou C, Hu C-P, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–22.
Takeda M, Nakagawa K. First- and second-generation EGFR-TKIs are all replaced to Osimertinib in chemo-naive EGFR mutation-positive non-small cell lung cancer? Int J Mol Sci. 2019;20(1):146.
Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(9):841–9.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25.
Lu S, Wang Q, Zhang G, Dong X, Yang CT, Song Y, Chang GC, Lu Y, Pan H, Chiu CH, et al. Efficacy of aumolertinib (HS-10296) in patients with advanced EGFR T790M + NSCLC: updated post-national medical products administration approval results from the APOLLO Registrational Trial. J Thorac Oncol. 2022;17(3):411–22.
Lee CK, Wu YL, Ding PN, Lord SJ, Inoue A, Zhou C, Mitsudomi T, Rosell R, Pavlakis N, Links M, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol. 2015;33(17):1958–65.
Zhou J, Ben S. Comparison of therapeutic effects of EGFR-tyrosine kinase inhibitors on 19Del and L858R mutations in advanced lung adenocarcinoma and effect on cellular immune function. Thorac Cancer. 2018;9(2):228–33.
Zhang Y, Sheng J, Kang S, Fang W, Yan Y, Hu Z, Hong S, Wu X, Qin T, Liang W, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS One. 2014;9(9):e107161.
Castellanos E, Feld E, Horn L. Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12(4):612–23.
Chiu CH, Yang CT, Shih JY, Huang MS, Su WC, Lai RS, Wang CC, Hsiao SH, Lin YC, Ho CL, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol. 2015;10(5):793–9.
Hasegawa Y, Ando M, Maemondo M, Yamamoto S, Isa S, Saka H, Kubo A, Kawaguchi T, Takada M, Rosell R, et al. The role of smoking status on the progression-free survival of non-small cell lung cancer patients harboring activating epidermal growth factor receptor (EGFR) mutations receiving first-line EGFR tyrosine kinase inhibitor versus platinum doublet chemotherapy: a meta-analysis of prospective randomized trials. Oncologist. 2015;20(3):307–15.
Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, Vynnychenko I, Park K, Eberhardt WE, de Marinis F, et al. Prognostic factors in patients with advanced non-small cell lung cancer: data from the phase III FLEX study. Lung Cancer. 2012;77(2):376–82.
Cheng Y, Ma L, Liu Y, Zhu J, Xin Y, Liu X, Wang Y, Zhang T, Yang C, Wang S, et al. Comprehensive characterization and clinical impact of concomitant genomic alterations in EGFR-mutant NSCLCs treated with EGFR kinase inhibitors. Lung Cancer. 2020;145:63–70.
Yu HA, Suzawa K, Jordan E, Zehir A, Ni A, Kim R, Kris MG, Hellmann MD, Li BT, Somwar R, et al. Concurrent alterations in EGFR-mutant lung cancers associated with resistance to EGFR kinase inhibitors and characterization of MTOR as a mediator of resistance. Clin Cancer Res. 2018;24(13):3108–18.
Kim Y, Lee B, Shim JH, Lee SH, Park WY, Choi YL, Sun JM, Ahn JS, Ahn MJ, Park K. Concurrent genetic alterations predict the progression to target therapy in EGFR-mutated advanced NSCLC. J Thorac Oncol. 2019;14(2):193–202.
Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29(suppl_1):i10–9.
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725–37.
Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, Yamamoto S, Nokihara H, Yamamoto N, Sekine I, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23(28):6829–37.
Douillard JY, Shepherd FA, Hirsh V, Mok T, Socinski MA, Gervais R, Liao ML, Bischoff H, Reck M, Sellers MV, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28(5):744–52.
Grob TJ, Hoenig T, Clauditz TS, Atanackovic D, Koenig AM, Vashist YK, Klose H, Simon R, Pantel K, Izbicki JR, et al. Frequent intratumoral heterogeneity of EGFR gene copy gain in non-small cell lung cancer. Lung Cancer. 2013;79(3):221–7.
Bell DW, Lynch TJ, Haserlat SM, Harris PL, Okimoto RA, Brannigan BW, Sgroi DC, Muir B, Riemenschneider MJ, Iacona RB, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005;23(31):8081–92.
Gao X, Wei XW, Zheng MY, Chen ZH, Zhang XC, Zhong WZ, Yang JJ, Wu YL, Zhou Q. Impact of EGFR amplification on survival of patients with EGFR exon 20 insertion-positive non-small cell lung cancer. J Thorac Dis. 2020;12(10):5822–32.
Ruiz-Patino A, Castro CD, Ricaurte LM, Cardona AF, Rojas L, Zatarain-Barron ZL, Wills B, Reguart N, Carranza H, Vargas C, et al. EGFR amplification and sensitizing mutations correlate with survival in lung adenocarcinoma patients treated with Erlotinib (MutP-CLICaP). Target Oncol. 2018;13(5):621–9.
Shan L, Wang Z, Guo L, Sun H, Qiu T, Ling Y, Li W, Li L, Liu X, Zheng B, et al. Concurrence of EGFR amplification and sensitizing mutations indicate a better survival benefit from EGFR-TKI therapy in lung adenocarcinoma patients. Lung Cancer. 2015;89(3):337–42.
Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu DT, Saijo N, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–74.
Huang L, Jiang XL, Liang HB, Li JC, Chin LH, Wei JP, Wang RR, Cai J, Xiong Q, Wang LT, et al. Genetic profiling of primary and secondary tumors from patients with lung adenocarcinoma and bone metastases reveals targeted therapy options. Mol Med. 2020;26(1):88.
Olshen AB, Venkatraman ES, Lucito R, Wigler M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics. 2004;5(4):557–72.
Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376(22):2109–21.
Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 2016;44(16):e131.
Zhang YC, Chen ZH, Zhang XC, Xu CR, Yan HH, Xie Z, Chuai SK, Ye JY, Han-Zhang H, Zhang Z, et al. Analysis of resistance mechanisms to abivertinib, a third-generation EGFR tyrosine kinase inhibitor, in patients with EGFR T790M-positive non-small cell lung cancer from a phase I trial. EBioMedicine. 2019;43:180–7.
Riess JW, Gandara DR, Frampton GM, Madison R, Peled N, Bufill JA, Dy GK, Ou SI, Stephens PJ, McPherson JD, et al. Diverse EGFR exon 20 insertions and co-occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J Thorac Oncol. 2018;13(10):1560–8.
Labbe C, Cabanero M, Korpanty GJ, Tomasini P, Doherty MK, Mascaux C, Jao K, Pitcher B, Wang R, Pintilie M, et al. Prognostic and predictive effects of TP53 co-mutation in patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung Cancer. 2017;111:23–9.
Bironzo P, Reale ML, Sperone T, Tabbo F, Caglio A, Listi A, Passiglia F, Di Maio M, Righi L, Bussolino F, et al. Clinical and molecular features of epidermal growth factor receptor (EGFR) mutation positive non-small-cell lung cancer (NSCLC) patients treated with tyrosine kinase inhibitors (TKIs): predictive and prognostic role of co-mutations. Cancers (Basel). 2021;13(10):2425.
Soh J, Okumura N, Lockwood WW, Yamamoto H, Shigematsu H, Zhang W, Chari R, Shames DS, Tang X, MacAulay C, et al. Oncogene mutations, copy number gains and mutant allele specific imbalance (MASI) frequently occur together in tumor cells. PLoS ONE. 2009;4(10):e7464.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94.
Gandhi J, Zhang J, Xie Y, Soh J, Shigematsu H, Zhang W, Yamamoto H, Peyton M, Girard L, Lockwood WW, et al. Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS ONE. 2009;4(2):e4576.
Gazdar AF, Minna JD. Deregulated EGFR signaling during lung cancer progression: mutations, amplicons, and autocrine loops. Cancer Prev Res. 2008;1(3):156–60.
Zhao ZR, Wang JF, Lin YB, Wang F, Fu S, Zhang SL, Su XD, Jiang L, Zhang YG, Shao JY, et al. Mutation abundance affects the efficacy of EGFR tyrosine kinase inhibitor readministration in non-small-cell lung cancer with acquired resistance. Med Oncol. 2014;31(1):810.
Acknowledgements
We sincerely thank the patients treated in our hospital.
Funding
None.
Author information
Authors and Affiliations
Contributions
Zhimin Zeng: Conceptualization and project administration; Duanyang Peng: Data acquisition, methodology, and writing original draft. Pingan Liang: Data acquisition, methodology, and writing original draft. Congying Zhong: Methodology and writing original draft. Yanqing He, Yuxi Luo, Anwen Liu: Data acquisition and revising the manuscript. Xia Wang: Writing assistance. Peng Xu: Writing assistance and revising the manuscript. All authors have read and approved the manuscript. Duanyang Peng, Pingan Liang and Congying Zhong contributed equally.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by institutional ethics committee of Second Affiliated Hospital of Nanchang University, Nanchang, China. As it was an observational study, informed consent for this study was deemed not required by ethics committee of Second Affiliated Hospital of Nanchang University, Nanchang, China. Approval Number was Review [2018] No. (108).
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Peng, D., Liang, P., Zhong, C. et al. Effect of EGFR amplification on the prognosis of EGFR-mutated advanced non–small-cell lung cancer patients: a prospective observational study. BMC Cancer 22, 1323 (2022). https://doi.org/10.1186/s12885-022-10390-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10390-0