Abstract
Childhood maltreatment (CM) is a non-specific risk factor for eating disorders (ED) and is associated with a greater severity in their clinical presentation and poorer treatment outcome. These data suggest that maltreated people with ED may be biologically other than clinically different from non-maltreated people. The aim of the present study was to investigate cortical thickness (CT), a possible biomarker of neurodevelopment, in people with ED with or without history of CM and in healthy women. Twenty-four healthy women, 26 with anorexia nervosa and 24 with bulimia nervosa underwent a 3T MRI scan. All participants filled in the childhood trauma questionnaire. All neuroimaging data were processed by FreeSurfer. Twenty-four participants with ED were identified as maltreated and 26 participants with ED as non-maltreated. All healthy women were non-maltreated. Compared to healthy women, maltreated people with ED showed lower CT in the left rostral anterior cingulate gyrus, while compared to people with ED without history of CM showed lower CT values in the left superior frontal and in right caudal middle frontal and superior parietal gyri. No significant differences emerged in CT measures between healthy women and people with ED without history of CM. The present findings show for the first time that in adult people with ED childhood maltreatment is associated with cortical thinning in areas implicated in the modulation of brain processes that are acknowledged to play a role in the psychopathology of ED.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early traumatic experiences are recognized as non-specific risk factor for the onset of various psychiatric disorders [1,2,3]. These experiences include both childhood abuse and neglect [4] and produce physiological and neurohumoral reactions that may affect brain development with possible psychopathological consequences in genetically vulnerable individuals [5,6,7]. Other than increasing the risk of onset of various psychiatric disorders, childhood maltreatment (CM) may allow to clinically and biologically distinguish individuals with the same psychiatric disorder. Indeed, for example, maltreated individuals with different psychiatric disorders have been proved to show earlier age at onset, greater symptom severity, more comorbidity, greater risk for suicide, and poorer treatment outcome than non-maltreated people [1]. Moreover, maltreated individuals with schizophrenia, other psychotic disorders, major depression or antisocial personality disorder presented neuroanatomical differences with respect to healthy controls in terms of reduced volume of anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (PFC), whereas non-maltreated individuals with the same psychiatric diagnosis did not [8,9,10]. All these findings support the existence of a “maltreated ecophenotype” biologically and clinically different from the non-maltreated one [1, 2].
People with eating disorders (ED) exhibit a prevalence of CM higher than general population [11] and, as for other psychiatric conditions, a history of CM in the context of ED has been found associated with an earlier age at onset, a greater clinical severity, a more frequent comorbidity with other psychiatric conditions [12] and a poorer treatment response [13]. Neuroendocrine modifications [14] as well as a heightened biological and emotional vulnerability to acute social stressor exposure [15] have been reported in people with ED and history of CM. This evidence suggests the possibility to identify a “maltreated ecophenotype” also in people affected by ED.
To our knowledge, only one study investigated the association between CM and brain morphology in people with EDs and found a reduced gray matter volume in the right paracentral lobule and in the left inferior temporal gyrus of maltreated patients [16]. However, cortical thickness (CT), which reflects the number of neurons within cortical columns [17] and is believed to represent a measure of the neurodevelopment process more specific and biologically more significant than brain volume [18], although explored in people with ED, has been never assessed in relation to the individual history of CM. Indeed, several studies lead to the conclusion that cortical thinning is frequently observed in the acute phase of ED and is related to malnutrition, especially in anorexia nervosa (AN) [19,20,21]. However, other studies have reported the persistence of cortical thinning in weight-recovered people with AN [22] or a higher CT in underweight people with AN compared to healthy controls [23]. In people with bulimia nervosa (BN), there were findings of both higher and lower CT values compared to healthy control in frontal and temporo-parietal areas [20, 21]. Other than the heterogeneity of the studies samples, a further variable that can influence CT in people with ED and explain part of the literature inconsistency could be a history of CM. Therefore, the aim of the present study was to identify and estimate specific variations in CT associated with a history of CM in people with ED. Based on previous findings in maltreated individuals [8,9,10], we hypothesized that people with ED and history of CM should show a cortical thinning with respect to both people with ED without history of CM and to a group of age- and sex-matched healthy controls.
Materials and methods
Subjects and clinical assessment
Study participation was proposed to patients consecutively admitted to the adult ED outpatient centers of the Departments of Psychiatry of the University of Campania “Luigi Vanvitelli” and the University of Salerno. Participants with ED had to meet the following inclusion/exclusion criteria: (1) diagnosis of current AN or bulimia nervosa (BN), according to DSM-5, confirmed by the Structured Clinical Interview for DSM-5 Disorders—Research Version [24]; (2) age ≥ 18 years; (3) absence of severe physical disorders or current comorbid psychiatric disorders; (4) no history of psychoactive substance use or head trauma; (5) no use of medications or oral contraceptives in the past 2 months; (6) right handed; (7) willingness to cooperate in the experimental procedures and to sign a written informed consent. Diagnostic assessment and collection of sociodemographic and clinical data were performed by trained psychiatrists.
Healthy women were recruited through advertisements on the campus of the University of Salerno. They had to be drug-free and physically and mentally healthy, as assessed by a routine physical examination and the Mini International Neuropsychiatric Interview [25]. All subjects gave their written consent after being fully informed of the nature and procedures of the study. The study was approved by the Ethics Committee of the University of Campania “Luigi Vanvitelli” and performed in accordance with 1964 Declaration of Helsinki and its later amendments.
All the participants completed the short form of the Childhood Trauma Questionnaire (CTQ) [26] to evaluate childhood trauma exposure. The CTQ is a 28-item questionnaire investigating childhood experience across five types of CM: emotional neglect, emotional abuse, sexual abuse, physical neglect and physical abuse. For each subscale, validated cutoff scores indicating the occurrence of maltreatment have been provided [27]: emotional neglect ≥ 15, emotional abuse ≥ 10, sexual abuse ≥ 8, physical abuse ≥ 8, physical neglect ≥ 8. We classified as “maltreated” (Mal) participants who scored higher than the threshold in at least one subscale and as “non-maltreatment” (noMal) those who scored below the threshold for all five subscales.
Image acquisitions
All subjects underwent a 3T scanner (MAGNETOM Skyra, Siemens, Erlangen, Germany). The image protocol consisted of the acquisition of T1-weighted 3D Magnetization Prepared RApid Gradient Echo (MPRAGE), sagittal orientation, matrix size 256 × 240, FOV 240 × 256 mm, 136 slices, slice thickness 1.2 mm, in-plane voxel size 1 × 1 mm, flip angle 9°, TR/TE 2300/2.98 ms, one average.
All participants with ED were outpatients and underwent the MRI scanning within two weeks from the first assessment, before starting any type of treatment. Participants from the control group and menstruating patients were tested in the follicular phase of their menstrual cycle.
Processing and measurements of cortical thickness
All data were processed using FreeSurfer (FS) version 6.0 (https://surfer.nmr.mgh.harvard.edu/), on Hewlett-Packard workstation equipped with an Intel® CoreTM i5-4590S CPU @3.00 GHz and 8 GB of RAM and running Linux Ubuntu 20.04 LTS.
Raw T1w images of all subjects were imported in FS and submitted to the standard structural image preprocessing and reconstruction pipeline of FS via the “recon-all" command (for a detailed description of this procedure please see https://surfer.nmr.mgh.harvard.edu/fswiki/ReconAllTableStableV5.3) [28,29,30,31,32,33,34,35].
Preprocessed data were visually inspected and corrected to remove non-gray matter tissues (e.g. dura mater and blood vessels) incorrectly included into the gray matter volume.
After this editing step the “autorecon-pial” command of the FS pipeline was launched.
Each subject folder was used to extract the CT measurements. Maps of CT were computed in order to perform a vertex-by-vertex analysis.
Statistical analysis
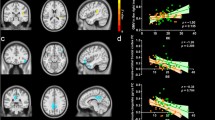
CT maps underwent a general linear model (GLM) analysis to evaluate differences in the following contrasts: HC vs maltreated people with ED, maltreated people with ED vs non-maltreatment people with ED and HC vs non-maltreatment people with ED. Age and body mass index (BMI) were included as nuisance covariates. Statistical maps were then corrected for multiple comparisons using Monte Carlo simulation with 10,000 iterations and a statistical threshold of p = 0.05. We extracted from each cluster showing significant differences the mean CT values to plot the trend of these measures across groups. Then, because it has been shown that CT may have a nonlinear relationship with age [36], a generalized linear model was performed including linear and quadratic age effects.
Differences in demographic and clinical variables among the groups were tested by means of one-way analysis of variance (ANOVA) followed by the post hoc Tukey’s test.
Results
Twenty-four healthy women, 26 with AN (19 restrictive subtype and 7 binge-purging subtype) and 24 with BN participated in the study. Based on CTQ cutoff scores, 12 participants with AN and 12 with BN were identified as maltreated and 14 participants with AN and 12 with BN as non-maltreatment. All healthy women were “non-maltreated.” Therefore, participants were split in 3 groups: 26 maltreated participants with ED, 24 non-maltreatment participants with ED and healthy control (HC). The groups did not differ in age, age at onset and illness duration, while healthy women showed a BMI significantly higher than participants with ED both with and without history of childhood maltreatment (Table 1). Twenty-four patients with a diagnosis of AN (12 maltreated and 12 not-maltreatment) were amenorrheic. The mean duration of amenorrhea was 5.9 ± 4.2 years. Fifteen participants with ED (30%) disclosed emotional neglect, 16 (32%) emotional abuse, 5 (10%) sexual abuse, 8 (16%) physical neglect, 7 (14%) physical abuse. Among maltreated people with ED, 10 (38.5%) reported only one type of CM, while 16 participants (61.5%) reported 2 or more different types of CM.
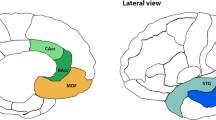
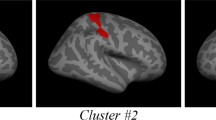
Compared to HC, maltreated people with ED showed lower CT values in the left rostral anterior cingulate gyrus (Fig. 1), while compared to non-maltreatment people with ED showed lower CT values in the left superior frontal, in right caudal middle frontal and in right superior parietal gyri (Fig. 2). No significant differences emerged in CT measures between HC and non-maltreatment people with ED. Table 2 shows the coordinates (in the Talairach space) of the peaks and the cluster sizes of significant clusters.
Results from generalized linear model including both linear and quadratic effects of age are reported in supplementary materials.
Discussion
According to our study hypothesis, maltreated people with ED showed a significant cortical thinning compared to non-maltreatment people with ED and HC, whereas measures of CT in non-maltreatment people with ED did not differ significantly from those of HC. These results are in line with a large body of the literature, showing that CM is associated with cortical thinning in both clinical and non-clinical populations [37,38,39,40]. In our previous study we found a reduced cortical volume in the right paracentral lobule and in the left inferior temporal gyrus in maltreated people with ED compared to non-maltreatment ones [16], while in the present study, we have shown that CT is not affected by CM in those areas. Since CT contributes with cortical surface area in the determination of the cortical gray matter volume [41], the present findings support the idea that CM may be associated with a reduced cortical surface rather than cortical thinning in inferior temporal gyrus and paracentral lobule. However, this hypothesis needs to be confirmed.
Interestingly, the localization of cortical thinning in ACC and PFC is consistent with reports of previous studies in non-clinical populations with history of CM [37,38,39]. The ACC is the cortical region most frequently affected in maltreated individuals with findings of reduced volume [42], connectivity [43] and thickness [38]. This area plays a role in emotional regulation processes [44], self-awareness [45] and reward [46]. Emotion dysregulation [47] has been identified as a variable making emotionally or sexually abused individuals more vulnerable to EDs. Moreover, reflection on one’s own physical characteristics is a key feature of ED psychopathology [48], and it has been reported to mediate early life adverse experience and ED core psychopathology [49]. Finally, hypoactivation of brain areas of reward system has been found in people with an history of CM and in people with ED, even after recovery [50, 51]. These findings, taken together, not only support the involvement of reward mechanisms in the pathophysiology of ED, but they also suggest that dysregulation in reward mechanisms may be, at least in part, linked to CM [52, 53].
The detection of a significant reduction of ACC CT in maltreated people with ED compared to HC, but not with respect to non-maltreatment people with ED, suggests that structural alterations in ACC associated with history of CM may contribute to the greater severity of ED psychopathology of maltreated patients. Indeed, structural and functional differences in the cingulate cortex have been found related to disease severity in AN [54].
The finding of a reduced CT in frontal gyri in maltreated people with ED is consistent with a previous report of cortical thinning in lateral PFC in non-clinical adolescents with a history of CM [55]. The PFC is implicated in specific types of emotion regulation, particularly in response to social exclusion [56], and CM severity was associated with PFC responsivity to social exclusion in young adults [57]. Alterations in social functioning have been found in people with ED either before or after illness onset [58], and people with EDs showed blunted cortisol and heightened emotional responses to a psychosocial stressor [59]. People with an EDs showed vigilance to rejection and avoidance of social reward [60]. Moreover, attentional bias to rejecting faces was correlated with adverse childhood experiences [60].
Several limitations of this study need to be acknowledged. First, the relatively small size of our sample, although consistent with most of the neuroimaging studies in people with EDs [61], did not allow to identify differences between ED diagnostic groups and could be a possible confounding factor for the study findings. To overcome this issue, BMI and age, which can affect CT [62], were included as nuisance covariates in the GLM analysis.
Second, we did not take into account the possible association between different types of trauma and CT alterations. Thus, alterations reported in maltreated people with EDs likely reflect additive effects of several forms of maltreatment, which often co-occur [63]. Third, the lack of a non-clinical population with a history of childhood maltreatment did not allow us to disentangle the effect of psychiatric disorder from that of childhood maltreatment. However, the study design is consistent with those already conducted in clinical population with CM [9]. Finally, another limitation is the retrospective self-reports of childhood experiences which does not exclude recall bias. However, it has been showed that false negative reports are more frequent than false positive, leading to downward biases in estimated associations between CM and outcome variables [64].
In conclusion, the present findings show that in people with ED childhood maltreatment is associated with cortical thinning in several areas that could play a role in the onset and maintenance of EDs. Moreover, this study corroborated the hypothesis of the existence of a “maltreated ecophenotype,” which recommends grouping individuals affected by the same psychiatric condition into subgroups characterized by different clinical and biological correlates [65]. Further studies are needed to ascertain whether changes in CT in adult people with ED represent a biological marker of CM exposure and whether these alterations could mediate the risk of CM to develop an ED in the adulthood.
References
Teicher MH, Samson JA (2013) Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry 170:1114–1133. https://doi.org/10.1176/appi.ajp.2013.12070957
Arango C, Dragioti E, Solmi M, Cortese S, Domschke K, Murray RM, Jones PB, Uher R, Carvalho AF, Reichenberg A, Shin JI, Andreassen OA, Correll CU, Fusar-Poli P (2021) Risk and protective factors for mental disorders beyond genetics: an evidence-based atlas. World Psychiatry 20:417–436. https://doi.org/10.1002/wps.20894
Karatzias T, Shevlin M, Hyland P, Ben-Ezra M, Cloitre M, Owkzarek M, McElroy E (2020) The network structure of ICD-11 complex post-traumatic stress disorder across different traumatic life events. World Psychiatry 19:400–401. https://doi.org/10.1002/wps.20795
McLaughlin KA, Sheridan MA, Lambert HK (2014) Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 47:578–591. https://doi.org/10.1016/j.neubiorev.2014.10.012
Teicher MH (2000) Wounds that time won’t heal: the neurobiology of child abuse. Cerebrum 4:50–67
Bloomfield MAP, Chang T, Woodl MJ, Lyons LM, Cheng Z, Bauer-Staeb C, Hobbs C, Bracke S, Kennerley H, Isham L, Brewin C, Billings J, Greene T, Lewis G (2021) Psychological processes mediating the association between developmental trauma and specific psychotic symptoms in adults: a systematic review and meta-analysis. World Psychiatry 20:107–123. https://doi.org/10.1002/wps.20841
Feldman R (2020) What is resilience: an affiliative neuroscience approach. World Psychiatry 19:132–150. https://doi.org/10.1002/wps.20729
Malykhin NV, Carter R, Hegadoren KM, Seres P, Coupland NJ (2012) Fronto-limbic volumetric changes in major depressive disorder. J Affect Disord 136:1104–1113. https://doi.org/10.1016/j.jad.2011.10.038
Kumari V, Uddin S, Premkumar P, Young S, Gudjonsson GH, Raghuvanshi S, Barkataki I, Sumich A, Taylor P, Das M (2014) Lower anterior cingulate volume in seriously violent men with antisocial personality disorder or schizophrenia and a history of childhood abuse. Aust N Z J Psychiatry 48:153–161. https://doi.org/10.1177/0004867413512690
Sheffield JM, Williams LE, Woodward ND, Heckers S (2013) Reduced gray matter volume in psychotic disorder patients with a history of childhood sexual abuse. Schizophr Res 143:185–191. https://doi.org/10.1016/j.schres.2012.10.032
Caslini M, Bartoli F, Crocamo C, Dakanalis A, Clerici M, Carrà G (2016) Disentangling the association between child abuse and eating disorders: a systematic review and meta-analysis. Psychosom Med 78:79–90. https://doi.org/10.1097/PSY.0000000000000233
Molendijk ML, Hoek HW, Brewerton TD, Elzinga BM (2017) Childhood maltreatment and eating disorder pathology: a systematic review and dose-response meta-analysis. Psychol Med 471:402–1416. https://doi.org/10.1017/S0033291716003561
Castellini G, Lelli L, Cassioli E, Ciampi E, Zamponi F, Campone B, Monteleone AM, Ricca V (2018) Different outcomes, psychopathological features, and comorbidities in patients with eating disorders reporting childhood abuse: a 3-year follow-up study. Eur Eat Disord Rev 26:217–229. https://doi.org/10.1002/erv.2586
Monteleone AM, Marciello F, Cascino G, Cimino M, Ruzzi V, Pellegrino F, Del Giorno C, Monteleone P (2020) Early traumatic experiences impair the functioning of both components of the endogenous stress response system in adult people with eating disorders. Psychoneuroendocrinology 115:104644. https://doi.org/10.1016/j.psyneuen.2020.104644
Monteleone AM, Cascino G, Ruzzi V, Pellegrino F, Patriciello G, Barone E, Carfagno M, Monteleone P, Maj M (2021) Emotional traumatic experiences significantly contribute to identify a maltreated ecophenotype sub-group in eating disorders: experimental evidence. Eur Eat Disord Rev 29:269–280. https://doi.org/10.1002/erv.2818
Monteleone AM, Monteleone P, Esposito F, Prinster A, Ruzzi V, Canna A, Aiello M, Di Salle F, Maj M (2019) The effects of childhood maltreatment on brain structure in adults with eating disorders. World J Biol Psychiatry 20:301–309. https://doi.org/10.1080/15622975.2017.1395071
Rakic P (1988) Specification of cerebral cortical areas. Science 241:170–176. https://doi.org/10.1126/science.3291116
Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS (2009) Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 19:2728–2735. https://doi.org/10.1093/cercor/bhp026
King JA, Geisler D, Ritschel F, Boehm I, Seidel M, Roschinski B, Soltwedel L, Zwipp J, Pfuhl G, Marxen M, Roessner V, Ehrlich S (2015) Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biol Psychiatry 77:624–632. https://doi.org/10.1016/j.biopsych.2014.09.005
Cyr M, Kopala-Sibley DC, Lee S, Chen C, Stefan M, Fontaine M, Terranova K, Berner LA, Marsh R (2017) Reduced inferior and orbital frontal thickness in adolescent bulimia nervosa persists over two-year follow-up. J Am Acad Child Adolesc Psychiatry 56:866–874. https://doi.org/10.1016/j.jaac.2017.08.008
Cascino G, Canna A, Monteleone AM, Russo AG, Prinster A, Aiello M, Esposito F, Salle FD, Monteleone P (2020) Cortical thickness, local gyrification index and fractal dimensionality in people with acute and recovered Anorexia Nervosa and in people with Bulimia Nervosa. Psychiatry Res Neuroimaging 299:111069. https://doi.org/10.1016/j.pscychresns.2020.111069
Brodrick BB, Adler-Neal AL, Palka JM, Mishra V, Aslan S, McAdams CJ (2021) Structural brain differences in recovering and weight-recovered adult outpatient women with anorexia nervosa. J Eat Disord 9:108. https://doi.org/10.1186/s40337-021-00466-w
Lavagnino L, Amianto F, Mwangi B, D’Agata F, Spalatro A, Zunta Soares GB, Daga GA, Mortara P, Fassino S, Soares JC (2016) The relationship between cortical thickness and body mass index differs between women with anorexia nervosa and healthy controls. Psychiatry Res Neuroimaging 248:105–109. https://doi.org/10.1016/j.pscychresns.2016.01.002
First MB, Williams JBW, Karg RS, Spitzer RL (2015). Structured clinical interview for DSM-5 research version. American Psychiatric Association Washington D.C.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:22–33
Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Zule W (2003) Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl 27:169–190. https://doi.org/10.1016/S0145-2134(02)00541-0
Walker EA, Gelfand A, Katon WJ, Koss MP, Von Korff M, Bernstein D, Russo J (1999) Adult health status of women with histories of childhood abuse and neglect. Am J Med 107:332–339. https://doi.org/10.1016/S0002-9343(99)00235-1
Dale AM, Sereno MI (1993) Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 5:162–170. https://doi.org/10.1162/jocn.1993.5.2.162
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9:179–194. https://doi.org/10.1006/nimg.1998.0395
Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage 9:195–207. https://doi.org/10.1006/nimg.1998.0396
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. https://doi.org/10.1073/pnas.200033797
Fischl B, Liu A, Dale AM (2001) Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20:70–80. https://doi.org/10.1109/42.906426
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: neurotechnique automated labeling of neuroanatomical structures in the human brain. Neuron 33:241–355. https://doi.org/10.1016/S0896-6273(02)00569-X
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B (2004) Thinning of the cerebral cortex in aging. Cereb Cortex 14:721–730. https://doi.org/10.1093/cercor/bhh032
Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004) A hybrid approach to the skull stripping problem in MRI. Neuroimage 22:1060–1075. https://doi.org/10.1016/j.neuroimage.2004.03.032
Steffener J (2021) Education and age-related differences in cortical thickness and volume across the lifespan. Neurobiol Aging 102:102–110. https://doi.org/10.1016/j.neurobiolaging.2020.10.034
Tomoda A, Polcari A, Anderson CM, Teicher MH (2012) Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS ONE 7:e52528. https://doi.org/10.1371/journal.pone.0052528
Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC (2013) Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am J Psychiatry 170:616–623. https://doi.org/10.1176/appi.ajp.2013.12070950
Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, McCrory EJ (2013) Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol Psychiatry 74:845–852. https://doi.org/10.1016/j.biopsych.2013.06.020
Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H et al (2020) Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med 50:1020–1031. https://doi.org/10.1017/S003329171900093X
Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC (2010) Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 53:1135–1146. https://doi.org/10.1016/j.neuroimage.2009.12.028
Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM (2006) Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry 59:975–982. https://doi.org/10.1016/j.biopsych.2005.12.016
Teicher MH, Anderson CM, Ohashi K, Polcari A (2014) Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatry 76:297–305. https://doi.org/10.1016/j.biopsych.2013.09.016
Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, Webb AG, Warren SL, Miller GA (2007) Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 44:343–351. https://doi.org/10.1111/j.1469-8986.2007.00515.x
Wagner G, Koch K, Schachtzabel C, Peikert G, Schultz CC, Reichenbach JR, Sauer H, Schlösser RG (2012) Self-referential processing influences functional activation during cognitive control: an fMRI study. Soc Cogn Affect Neurosci 8:828–837. https://doi.org/10.1093/scan/nss074
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. https://doi.org/10.1038/npp.2009.129
Mills P, Newman EF, Cossar J, Murray G (2015) Emotional maltreatment and disordered eating in adolescents: testing the mediating role of emotion regulation. Child Abuse Negl 39:156–166. https://doi.org/10.1016/j.chiabu.2014.05.011
Cascino G, Castellini G, Stanghellini G, Ricca V, Cassioli E, Ruzzi V, Monteleone P, Monteleone AM (2019) The role of the embodiment disturbance in the anorexia nervosa psychopathology: a network analysis study. Brain Sci 9:276. https://doi.org/10.3390/brainsci9100276
Monteleone AM, Cascino G, Martini M, Patriciello G, Ruzzi V, Delsedime N, Abbate-Daga G, Marzola E (2021) Confidence in one-self and confidence in one’s own body: the revival of an old paradigm for anorexia nervosa. Clin Psychol Psychother 28:818–827. https://doi.org/10.1002/cpp.2535
Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, Frank GK, Bailer UF, Fischer L, Nguyen V, Carter C, Putnam K, Kaye WH (2007) Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry 164:1842–1849. https://doi.org/10.1176/appi.ajp.2007.07040575
Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, Baumeister S, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M (2014) Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS ONE 9:e104185. https://doi.org/10.1371/journal.pone.0104185
Monteleone AM, Castellini G, Volpe U, Ricca V, Lelli L, Monteleone P, Maj M (2018) Neuroendocrinology and brain imaging of reward in eating disorders: a possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog Neuropsychopharmacol Biol Psychiatry 80:132–142. https://doi.org/10.1016/j.pnpbp.2017.02.020
Kotov R, Jonas KG, Carpenter WT, Dretsch MN, Eaton NR, Forbes MK, Forbush KT, Hobbs K, Reininghaus U, Slade T, South SC, Sunderland M, Waszczuk MA, Widiger TA, Wright AGC, Zald DH, Krueger RF, Watson D, Workgroup HU (2020) Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): I. Psychosis superspectrum. World Psychiatry 19:151–172. https://doi.org/10.1002/wps.20730
Bär KJ, de la Cruz F, Berger S, Schultz CC, Wagner G (2015) Structural and functional differences in the cingulate cortex relate to disease severity in anorexia nervosa. J Psychiatry Neurosci 40:269–279. https://doi.org/10.1503/jpn.140193
Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert HK, Alves S, Pine DS, McLaughlin KA (2016) Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. J Child Psychol Psychiatry 57:1154–1164. https://doi.org/10.1111/jcpp.12630
Eisenberger NI (2012) The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci 13:421–434. https://doi.org/10.1038/nrn3231
van Harmelen AL, Hauber K, Gunther Moor B, Spinhoven P, Boon AE, Crone EA, Elzinga BM (2014) Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults. PLoS ONE 9:e85107. https://doi.org/10.1371/journal.pone.0085107
Treasure J, Willmott D, Ambwani S, Cardi V, Clark Bryan D, Rowlands K, Schmidt U (2020) Cognitive interpersonal model for anorexia nervosa revisited: the perpetuating factors that contribute to the development of the severe and enduring illness. J Clin Med 9:630. https://doi.org/10.3390/jcm9030630
Monteleone AM, Cascino G, Ruzzi V, Pellegrino F, Carfagno M, Raia M, Del Giorno C, Monteleone P, Maj M (2020) Multiple levels assessment of the RDoC “system for social process” in eating disorders: biological, emotional and cognitive responses to the Trier Social Stress Test. J Psychiatr Res 130:160–166. https://doi.org/10.1016/j.jpsychires.2020.07.039
Cardi V, Di Matteo R, Corfield F, Treasure J (2013) Social reward and rejection sensitivity in eating disorders: an investigation of attentional bias and early experiences. World J Biol Psychiatry 4:622–633. https://doi.org/10.3109/15622975.2012.665479
Frank GKW (2019) Neuroimaging and eating disorders. Curr Opin Psychiatry 32:478–483. https://doi.org/10.1097/YCO.0000000000000544
Bang L, Tamnes CK, Norbom LB, Thomassen RA, Holm JS, Skotte LH, Juliusson PB, Mejlaender-Evjensvold M, Rø Ø (2021) Associations of age, body mass index and biochemical parameters with brain morphology in patients with anorexia nervosa. Eur Eat Disord Rev 29:74–85. https://doi.org/10.1002/erv.2803
Kim K, Mennen FE, Trickett PK (2017) Patterns and correlates of co-occurrence among multiple types of child maltreatment. Child Fam Soc Work 22:492–502. https://doi.org/10.1111/cfs.12268
Hardt J, Rutter M (2004) Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry 45:260–273. https://doi.org/10.1111/j.1469-7610.2004.00218.x
Teicher MH, Samson JA, Anderson CM, Ohashi K (2016) The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 17:652–666. https://doi.org/10.1038/nrn.2016.111
Funding
Open access funding provided by Università degli Studi di Salerno within the CRUI-CARE Agreement. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cascino, G., Canna, A., Russo, A.G. et al. Childhood maltreatment is associated with cortical thinning in people with eating disorders. Eur Arch Psychiatry Clin Neurosci 273, 459–466 (2023). https://doi.org/10.1007/s00406-022-01456-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01456-y