Abstract
Purpose
Fetal growth restriction (FGR) management and delivery planning is based on a multimodal approach. This meta-analysis aimed to evaluate the prognostic accuracies of the aortic isthmus Doppler to predict adverse perinatal outcomes in singleton pregnancies with FGR.
Methods
PubMed, EMBASE, the Cochrane Library, ClinicalTrials.gov and Google scholar were searched from inception to May 2021, for studies on the prognostic accuracy of anterograde aortic isthmus flow compared with retrograde aortic isthmus flow in singleton pregnancy with FGR. The meta-analysis was registered on PROSPERO and was assessed according to PRISMA and Newcastle–Ottawa Scale. DerSimonian and Laird’s random-effect model was used for relative risks, Freeman-Tukey Double Arcsine for pooled estimates and exact method to stabilize variances and CIs. Heterogeneity was quantified using I2 statistics.
Results
A total of 2933 articles were identified through the electronic search, of which 6 studies (involving 240 women) were included. The quality evaluation of studies revealed an overall acceptable score for study group selection and comparability and substantial heterogeneity. The risk of perinatal death was significantly greater in fetuses with retrograde Aortic Isthmus blood flow, with a RR of 5.17 (p value 0.00001). Similarly, the stillbirth rate was found to have a RR of 5.39 (p value 0.00001). Respiratory distress syndrome had a RR of 2.64 (p value = 0.03) in the group of fetuses with retrograde Aortic Isthmus blood flow.
Conclusion
Aortic Isthmus Doppler study may add information for FGR management. However, additional clinical trial are required to assess its applicability in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aortic isthmus Doppler represents an addionatl doppler marker useful for FGR management. However, its role is not entirely known, and our meta-analysis demonstrated that the aortic isthmus Doppler study could predict neonatal adverse outcomes. |
Introduction
Fetal growth restriction (FGR) occurs in around 10% of gestations and represents a significant cause of perinatal morbidity and mortality [1, 2]. The stillbirth incidence is twice (1.5%) in these fetuses than in fetuses with normal growth [3, 4]. FGR is a complicated obstetrical dilemma with different queries, such as low detection rates or few prophylactic interventions. FGR can be classified as early-onset or late-onset based on gestational age at prenatal ultrasound diagnosis. FGR classification had several implications for diagnosis, treatment and prognosis. Early-onset FGR is diagnosed before 32 weeks of gestation [5]. This type of FGR is usually severe, follows a well-established Doppler deterioration pattern, and is frequently correlated with hypertensive disorders of pregnancy [2]. Late-onset FGR is diagnosed after 32 weeks of gestation and is correlated with placental insufficiency and chronic intrauterine hypoxia more frequently than gestational hypertensive disorders. Once early-onset or late-onset FGR has been diagnosed, further evaluation and monitoring are required to determine the optimal delivery timing [5, 6]. Management also provides several criticisms. Different management algorithms have been proposed, involving the use of ultrasound (US) and cardiotocography evaluations [5, 7,8,9,10,11,12,13,14]. Umbilical artery (UA) Doppler has been proven to be an essential surveillance tool, mainly in the presence of an absent or reversed end-diastolic flow (EDF) [7, 15,16,17]. Additional Doppler parameters as the fetal middle cerebral artery (MCA), the cerebroplacental ratio (CPR) and the ductus venosus (DV) are progressively integrated into the FGR management [10, 18,19,20,21,22]. Altered MCA and CPR are considered expression of the “brain sparing effect”, a signal of fetal circulation redistribution [23,24,25,26]. The correlation between MCA Doppler and the adverse fetal outcome has been described [27, 28] and the same was reported for an abnormal CPR [29, 30]. Uterine artery Doppler (UtA) abnormalities have also been associated with the occurrence of stillbirth and adverse perinatal outcomes [31,32,33]. However, as the risks associated with iatrogenic prematurity are very high before 32 weeks’ gestation, additional Doppler parameters are needed to assess fetal compromise and to indicate delivery. According to this, before 32 weeks’ gestation, the main sign of fetal distress to be considered is the presence of abnormal venous flow findings, including reversed flow in the DV during atrial contraction. However, the recognition of such venous alterations is frequently associated with advanced fetal compromise and therefore signs of acidemia and cardiac decompensation. The aortic isthmus (AoI) Doppler has also been studied in fetuses with FGR, as a potential indicator of worsening of the fetal hemodynamic state [34,35,36,37,38,39,40]. AoI provides information on fetal haemodynamic circulation, mainly on the ventricular performance [41]. A FGR fetus with normal anterograde flow in AoI, provides a preferential supply of oxygenated blood to the coronary and cerebral circulation [42]. Otherwise, a predominant reverse diastolic blood flow through the AoI, led to a significant decrease of oxygen supply to the brain [43, 44]. Interestingly, the AoI Doppler abnormalities were reported to occur prior to ductus venosus, suggesting that reversed aortic isthmus flow may represent an intermediate step between placental insufficiency-hypoxemia and cardiac failure, a further step in the sequence of Doppler deterioration beginning with the UA and MCA Dopplers [45]. On these considerations, we provided a systematic review and meta-analysis of studies comparing anterograde to retrograde AoI flow as a predictor of adverse perinatal outcomes in fetuses with FGR.
Materials and methods
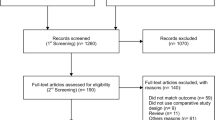
We carried the meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (www.prisma-statement.org) and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statement guidelines [46, 47]. The study protocol was developed before and registered in the International Prospective Register of Systematic Reviews database (PROSPERO ID: CRD42020160983). From the beginning until May 1st 2021, extensive research was conducted using PubMed, EMBASE, the Cochrane Library, ClinicalTrials.gov, and Google Scholar. Constrained words (MeSH in PubMed, Emtree in EMBASE) were combined with free-text keywords (Cochrane Library and Google scholar). The following terms were used as index terms or free-text words: (“fetus” or “fetal”), (“aorta/thoracic” or “isthmus” or “Aortic isthmus”), (“fetal growth retardation” or “Intrauterine growth retardation” or “IUGR” or “Growth retardation”), and (“ultrasonography” or “doppler” or “ultrasound”). Duplicated articles were omitted, and prior reviews were searched for additional articles that met the inclusion criteria [48,49,50]. Our search strategy is presented in Fig. 1 using a PRISMA diagram. Language restriction was not applied; one of the retrieved articles was in French [35]. We screened for studies comparing anterograde to retrograde aortic isthmus flow in women with FGR in the absence of any documented chromosomal or anatomical abnormalities. The meta-analysis includes studies reporting on the association between AoI Doppler and one of the following adverse perinatal outcomes: perinatal death, stillbirth, neonatal death, severe neonatal morbidity, necrotizing enterocolitis (NEC), sepsis, respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH) (Grade III-IV), bronchopulmonary dysplasia (BPD), adverse neurological outcomes, and admission to a neonatal intensive care unit (NICU). When the same author published multiple papers, only data reporting on the larger study group were included. Review articles and studies reporting on AoI Doppler with no information on the neonatal outcome were excluded.
Data extraction and quality assessment
Two reviewers (M.L.V and G.R.) reviewed all abstracts and articles independently. We collected complete reports on research that were evaluated potentially acceptable by at least one of the reviewers. The two reviewers (M.L.V. and G.R.) determined whether or not to include the complete reports and extracted data. In case of uncertainty, a third reviewer assessed the manuscript (M.M.). For each included paper, information about the first author, nation, journal, year of publication, setting of recruitment, sample size, and patient characteristics were collected. The AoI flow Doppler (anterograde or retrograde flow) and neonatal outcomes were analyzed. Whenever AoI Doppler results were shown, 2 × 2 tables were extracted. Two reviewers (M.L.V. and G.R.) used the Newcastle–Ottawa scale to evaluate the risk of bias [51].
Statistical analysis
Meta-analyses were conducted to determine the following adverse perinatal outcomes in singleton pregnancies with growth-restricted fetuses: perinatal death, stillbirth, neonatal death, necrotizing enterocolitis, sepsis, respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH, Grade III-IV), bronchopulmonary dysplasia (BPD), and admission to NICU. These outcomes were chosen by consensus, because they are the most uniformly defined and less influenced by ascertainment bias. According to the Doppler direction of diastolic blood flow, the AoI was categorized as reversed or anterograde. The FGR was defined as an estimated fetal weight (EFW) below the 10th percentile using local reference curves [52, 53]. For each outcome analyzed, the predictive accuracy of anterograde versus retrograde AoI flow was compared. Additionally, this method was utilized to explore heterogeneity. In a conservative approach, the random-effect estimates of event proportion (ES), allowing for variation of true proportion across studies, were considered the ‘main results’, which was calculated using the method of DerSimonian and Laird. To stabilize variances, the pooled estimate was computed using the Freeman-Tukey Double Arcsine Transformation. The exact procedure was used to estimate the confidence interval (CI). Using the I2-statistic, we measured heterogeneity as the proportion of total variance across trials that can be attributed to heterogeneity rather than chance. I2-values of 25%, 50%, and 75% corresponded to cut-off criteria for low, moderate, and high degrees of heterogeneity, respectively, in our meta-analysis. All other proportions having a relative confidence interval (CI) were examined in the same manner. RevMan 5.3 (The Nordic Cochrane Centre, 2014, Copenhagen, Denmark) was utilized to extract data and generate forest plots, while Stata 14.1 (Stata corp., College Station, TX, USA) was utilized to analyze bivariate models.
Results
A total of 2933 articles were identified and assessed with respect to their eligibility for inclusion (Fig. 1). After deleting duplicates, we examined the remaining 2813 titles and abstracts, yielding 89 possibly suitable papers. 82 of these were excluded for the reasons described in Fig. 1. As a consequence, 6 studies were included [35,36,37,38,39,40], with a total of 240 pregnant women (Table 1 and Fig. 1). In five of the six studies, neonatal outcomes were evaluated [36,37,38,39,40]. The missing data were requested to the authors via email, to minimize the risk of bias and to increase the accuracy of the statistical analysis. Table 1 summarizes the features of the studies included. In all the selected studies data were collected prospectively. The trials included 31 to 51 pregnant women. The mean or median age of the mother varied between 25.19 and 32.8 years. All the six studies included FGR fetuses with an EFW below the 10th percentile for gestational age (Table 1). Three studies included FGR with an abnormal umbilical artery Doppler [36, 39, 40], and one study included fetuses with FRG and a cerebroplacental ratio below the 5th centile [38]. Concerning the relationship between neonatal outcomes and anterograde and retrograde AoI Doppler flow, all studies assessed perinatal mortality, intrauterine death, and neonatal death rates. The neonatal outcomes assessed were: RDS, NEC, neonatal sepsis, NICU admission > 14 days. The prevalence of RDS and NEC was reported in six studies, while IVH [35,36,37, 40] and BPD were reported in four studies [35, 36, 38, 40]. Except for one study [37], all the studies considered late decelerations on the cardiotocography an indication for immediate delivery, whereas US Doppler findings were interpreted in different ways. Del Rio et al. [38] and Hidar et al. [35] evaluated delivery in the context of Doppler parameter decline. Abdelrazzq et al. [36] performed the delivery when reversed “A wave” in the ductus venosus Doppler was observed, while Bhagat et al. [40] considered umbilical artery's reversed flow as indication to delivery. Women with anterograde AoI Doppler flow had a mean or median gestational age of 32.2–37.6 weeks, whereas those with retrograde AoI Doppler flow had a mean or median gestational age of 27.2–35.33 weeks. Tantuway et al. [39] did not report the gestational age at the moment of delivery. Five of the six studies assessed the fetal AoI Doppler using either the longitudinal aortic arch or the three-vessel and trachea views with a 30° insonation angle. While Hidar et al. [35] evaluated the fetal AoI only in the longitudinal aortic arch view [54]. The Newcastle–Ottawa Scale was used to evaluate the quality of the research, and the results revealed an overall high score for the selection and comparability of study groups (five studies out of six), as well as for the determination of the outcomes of interest (Table 2)[51]. RDS, IVH, BPD, NEC, sepsis, and NICU hospitalization > 14 days were studied comparing anterograde and retrograde AoI flow. Table 3 describes all the outcomes analyzed. The overall rate of perinatal mortality and stillbirth were higher in FGR fetuses with AoI retrograde flow, with a risk ratio of 5.17 (2.52–10.62, 95% CI; I2 = 35%; P < 0.00001) (Fig. 2) and 5.58 (2.95–10.52, 95% CI; I2 0%; P < 0.00001) (Fig. 3), respectively. In the AoI retrograde flow group the risk ratio of neonatal mortality and RDS were 4.81 (1.68–13.73, 95% CI; I2 9%; P = 0.003) (Fig. 4). and 3.25 (1.59–6.63, 95%CI; I2 0%; P = 0.001), respectively (Fig. 5). For NICU admission and sepsis, the risk ratio in the AoI retrograde flow group were 1.58 and 1.67, respectively (Figs. 6,7,8, 9 and 10).
Discussion
In FGR fetuses, it is critical to establish the optimal time of delivery based on US Doppler examination and other fetal well-being indicators [55]. In the early stage of FGR, increased pulsatility index (PI) in the UA preceded a sequence of Doppler changes, before the occurrence of fetal acidosis [18, 56]. Subsequently, the absence and the reversed EDF in the UA, are expression of a late decline in early FGR fetuses. These findings are finally followed by abnormalities in the ductus venosus, absence or reversed a-wave, indicative of cardiovascular and metabolic failure [57, 58]. Steroids administration for lung maturation, magnesium sulfate prophylaxis for neuroprotection, delivery in a tertiary care center, and close fetal monitoring to define the optimal delivery time, are the only effective interventions to reduce the occurrence of acidosis-related complications in early FGR fetuses [59, 60]. Neonatal complications due to iatrogenic prematurity are even higher [61]. The Trial of Randomized Umbilical and Fetal Flow in Europe (TRUFFLE) revealed that following a strict protocol including DV Doppler assessment and computerized cardiotocography (cCTG) for fetal monitoring and decision making for time of delivery, significantly improves fetal and neonatal outcomes for early FGR fetuses [62]. The management protocol from the TRUFFLE study has been subsequently included in the majority of guidelines and recommendations on the management of FRG fetuses by the main international societies. AoI Doppler assessment was not included in the management protocol used in the TRUFFLE. In the case of late-onset FGR, several guidelines have proposed different management, including fetal surveillance combining a weekly UA Doppler and cCTG, with increased fetal controls if the UA is abnormal [7, 16]. IUSOG guidelines recommend monitoring according to FGR severity and UA Doppler abnormalities [10]. Other studies demonstrated a strong association between MCA Doppler alterations and fetal morbidity and mortality in late-onset FGR after 35 gestational weeks [63]. Low CRP was also related to a greater risk of fetal distress in labor, a lower fetal pH, and an increased risk of caesarean section and neonatal intensive care unit admission in fetuses with late-onset FGR [11, 63,64,65]. Other guidelines recommend delivery in cases of late-onset FGR with brain sparing [66]. In addition, several studies suggested that changes in the AoI might represent an intermediate step between placental insufficiency-hypoxemia and fetal cardiac failure, occurring prior to DV abnormalities which are associated with fetal cardiovascular deterioration. Using the AoI Doppler, it is possible to estimate the fetal ventricular and cardiovascular hemodynamic status. Anterograde AoI blood flow redirects oxygenated blood to the heart and brain circulations [43], in animal models, it has been proven that when the fetal AoI flow is anterograde, brain oxygenation is preserved [67, 68]. Placental insufficiency is usually associated with an increase in placental vascular resistance. This condition, together with the fetal cerebral vasodilation, can be responsible of the reduction in the anterograde blood flow within the aortic isthmus, and in more severe cases of the retrograde flow. When the blood flow within the aortic isthmus is reversed, the more oxygenated blood coming from the pulmonary artery and the aorta is directed to the placenta, and therefore the brain will be perfused by poorly oxygenated blood, inappropriate for normal neurodevelopment, [43, 67, 69]. Some authors have suggested to include reversed AoI in the management of FGR fetuses, as a sign of severe placental insufficiency that could be useful for decision making on timing of delivery beyond 34 weeks of gestation [12, 13]. However, more data are needed to support this intervention. Our meta-analysis showed that AoI retrograde flow is a reliable predictor of neonatal outcomes. We discovered that an AoI retrograde Doppler flow significantly increased the risk of perinatal death, stillbirth, and RDS. Our meta-analysis represents the most extensive analysis on the AoI Doppler flow in case of FGR, with good heterogeneity in all the outcomes reported. However, there are some limitations to be considered. In terms of comparability and reported outcomes, half of the studies had a risk of bias (Table 2). Secondly, in all the included studies, the delivery timing was established according to local protocols, which may have influenced some of the fetal and neonatal outcomes. Furthermore, because the study populations ranged from early to late severe FGR, these differences may have affected the prevalence of unfavorable perinatal outcomes. Finally, the sub-analysis of preterm birth complications was limited given the small sample of publications that clearly recorded all the neonatal outcomes.
Conclusion
AoI Doppler assessment may increase the accuracy of the prediction of adverse perinatal outcomes in singleton pregnancies affected by FGR. The findings from this meta-analysis might be taken into account to determine the appropriate time of delivery for fetuses with FGR, despite the limited evidence provided from the available literature. The usefulness of the AoI Doppler for directing clinical management in FGR fetuses needs to be assessed via clinical trials, which are now underway.
Data availability
The data are available.
References
IM Bernstein JD Horbar GJ Badger 2000 Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction Am J Obstet Gynecol 182 1 198 206
J Unterscheider K O'Donoghue S Daly 2014 Fetal growth restriction and the risk of perinatal mortality-case studies from the multicentre PORTO study BMC Pregnancy Childbirth 11 14 63
A Ego D Subtil G Grange 2006 Customized versus population-based birth weight standards for identifying growth restricted infants: a French multicenter study Am J Obstet Gynecol 194 4 1042 1049
D Getahun CV Ananth WL Kinzler 2007 Risk factors for antepartum and intrapartum stillbirth: a population-based study Am J Obstet Gynecol 196 6 499 507
JG Martins JR Biggio A Abuhamad 2020 Society for Maternal-Fetal Medicine Consult Series# 52: diagnosis and management of fetal growth restriction:(replaces clinical guideline number 3, April 2012) Am J Obstet Gynecol 223 4 B2 B17
Obstetricians ACo, Gynecologists 2013 ACOG Practice bulletin no. 134: fetal growth restriction Obstet Gynecol 121 5 1122 1133
Fetal Growth Restriction 2021 ACOG Practice Bulletin, Number 227 Obstet Gynecol 137 2 e16 e28
M Anceschi A Ruozi-Berretta J Piazze 2004 Computerized cardiotocography in the management of intrauterine growth restriction associated with Doppler velocimetry alterations Int J Gynecol Obstet 86 3 365 370
M Verde La G Riemma M Torella 2021 Impact of Braxton-Hicks contractions on fetal wellbeing; a prospective analysis through computerised cardiotocography J Obstet Gynaecol 42 4 569 573
C Lees T Stampalija A Baschat 2020 ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction Ultrasound Obstet Gynaecol 56 2 298 312
F Figueras E Gratacós 2014 Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol Fetal Diagn Ther 36 2 86 98
F Figueras J Caradeux F Crispi 2018 Diagnosis and surveillance of late-onset fetal growth restriction Am J Obstet Gynecol 218 2S S790 S802
F Figueras E Gratacos 2017 An integrated approach to fetal growth restriction Best Pract Res Clin Obstet Gynaecol 38 48 58
M Verde La M Torella G Lanza 2021 Objective and quantitative evaluation of fetal hiccups by computerized cardiotocography: a prospective observational study [Article] Italian J Gynaecol Obstet 33 4 249 255
LM McCowan F Figueras NH Anderson 2018 Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy Am J Obstet Gynecol 218 2 S855 S868
JG Martins JR Biggio 2020 Society for maternal-fetal medicine consult series #52: diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012) Am J Obstet Gynecol 223 4 B2 B17
Z Alfirevic T Stampalija T Dowswell 2017 Fetal and umbilical Doppler ultrasound in high-risk pregnancies Cochrane Database Syst Rev 6 CD007529
T Stampalija J Thornton N Marlow 2020 Fetal cerebral Doppler changes and outcome in late preterm fetal growth restriction: prospective cohort study Ultrasound Obstet Gynecol 56 2 173 181
T Stampalija B Arabin H Wolf 2017 Is middle cerebral artery Doppler related to neonatal and 2-year infant outcome in early fetal growth restriction? Am J Obstet Gynecol 216 5 521
J Morales-Roselló A Khalil M Morlando 2014 Changes in fetal Doppler indices as a marker of failure to reach growth potential at term Ultrasound Obstet Gynecol 43 3 303 310
J Morales-Roselló A Khalil M Morlando 2015 Poor neonatal acid–base status in term fetuses with low cerebroplacental ratio Ultrasound Obstet Gynecol 45 2 156 161
J Morales-Roselló A Khalil J Alberola-Rubio 2015 Neonatal acid-base status in term fetuses: mathematical models investigating cerebroplacental ratio and birth weight Fetal Diagn Ther 38 1 55 60
K Flood J Unterscheider S Daly 2014 The role of brain sparing in the prediction of adverse outcomes in intrauterine growth restriction: results of the multicenter PORTO Study Am J Obstet Gynecol 211 3 288
SJ Roza EA Steegers BO Verburg 2008 What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population Am J Epidemiol 168 10 1145 1152
AA Khalil J Morales-Rosello M Morlando 2015 Is fetal cerebroplacental ratio an independent predictor of intrapartum fetal compromise and neonatal unit admission? Am J Obstet Gynecol 213 1 54
J Morales-Roselló A Khalil F Akhoundova 2017 Fetal cerebral and umbilical Doppler in pregnancies complicated by late-onset placental abruption J Matern Fetal Neonatal Med 30 11 1320 1324
R Morris R Say S Robson 2012 Systematic review and meta-analysis of middle cerebral artery Doppler to predict perinatal wellbeing Eur J Obstet Gynecol Reprod Biol 165 2 141 155
A Khalil J Morales-Roselló R Townsend 2016 Value of third-trimester cerebroplacental ratio and uterine artery Doppler indices as predictors of stillbirth and perinatal loss Ultrasound Obstet Gynecol 47 1 74 80
A Conde-Agudelo J Villar SH Kennedy 2018 Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: systematic review and meta-analysis Ultrasound Obstet Gynecol 52 4 430 441
A Khalil R Townsend J Morales-Roselló 2015 OC22. 06: Are fetal cerebroplacental ratio and impaired placental perfusion recorded in the third trimester predictors of stillbirth and perinatal loss? Ultrasound Obstet Gynecol 46 49 49
A Familiari C Scala M Morlando 2016 Mid-pregnancy fetal growth, uteroplacental Doppler indices and maternal demographic characteristics: role in prediction of stillbirth Acta Obstet Gynecol Scand 95 11 1313 1318
A Familiari A Bhide M Morlando 2016 Mid-pregnancy fetal biometry, uterine artery Doppler indices and maternal demographic characteristics: role in prediction of small-for-gestational-age birth Acta Obstet Gynecol Scand 95 2 238 244
R Allen M Morlando B Thilaganathan 2016 Predictive accuracy of second-trimester uterine artery Doppler indices for stillbirth: a systematic review and meta-analysis Ultrasound Obstet Gynecol 47 1 22 27
M Rio Del J Martinez F Figueras 2006 Reference ranges for Doppler parameters of the fetal aortic isthmus during the second half of pregnancy Ultrasound Obstet Gynecol 28 1 71 76
S Hidar R Zaafouri S Bouguizane 2004 Prognostic value of fetal aortic isthmus Doppler waveform in intrauterine growth retardation: prospective longitudinal study J Gynecol Obstet Biol Reprod 33 8 745 752
K Abdelrazzaq AÖ Yeniel AM Ergenoglu 2013 Fetal aortic isthmus Doppler measurements for prediction of perinatal morbidity and mortality associated with fetal growth restriction Acta Obstet Gynecol Scand 92 6 656 661
M Ropacka-Lesiak J Świder-Musielak GH Bręborowicz 2014 Retrograde diastolic blood flow in the aortic isthmus is not a simple marker of abnormal fetal outcome in pregnancy complicated by IUGR–a pilot study Ginekol Pol 85 7 509 515
M Rio Del J Martinez F Figueras 2008 Doppler assessment of the aortic isthmus and perinatal outcome in preterm fetuses with severe intrauterine growth restriction Ultrasound Obstet Gynecol 31 1 41 47
B Tantuway Y Mala A Garg 2018 Correlation of Doppler assessment of fetal aortic isthmus with perinatal outcome in intrauterine growth restriction Int J Reprod Contracept Obstet Gynecol 7 9 3780 3786
B Bhagat J Sheth 2018 Doppler assessment of the aortic isthmus and its utility in management of intrauterine growth restricted foetuses. J Evid Based Med Healthc 5(37):2661–2664. https://doi.org/10.18410/jebmh/2018/546
R Karakus AS Ozgu-Erdinc A Esercan 2015 Doppler assessment of the aortic isthmus in intrauterine growth-restricted fetuses Ultrasound Q 31 3 170 174
JC Fouron 2003 The unrecognized physiological and clinical significance of the fetal aortic isthmus Wiley Online Library
K Mäkikallio P Jouppila J Räsänen 2003 Retrograde aortic isthmus net blood flow and human fetal cardiac function in placental insufficiency Ultrasound Obstet Gynecol 22 4 351 357
J-C Fouron J Gosselin M-J Raboisson 2005 The relationship between an aortic isthmus blood flow velocity index and the postnatal neurodevelopmental status of fetuses with placental circulatory insufficiency Am J Obstet Gynecol 192 2 497 503
F Figueras A Benavides M Rio Del 2009 Monitoring of fetuses with intrauterine growth restriction: longitudinal changes in ductus venosus and aortic isthmus flow Ultrasound Obstet Gynecol 33 1 39 43
Mark Vrabel M (2015) editor Preferred reporting items for systematic reviews and meta-analyses. Oncology nursing forum. Oncology Nursing Society.
DF Stroup JA Berlin SC Morton 2000 Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group JAMA 283 15 2008 2012
E Ferrazzi M Bellotti H Galan 2001 Doppler investigation in intrauterine growth restriction—from qualitative indices to flow measurements: a review of the experience of a collaborative group Ann N Y Acad Sci 943 1 316 325
I Aditya V Tat A Sawana 2016 Use of Doppler velocimetry in diagnosis and prognosis of intrauterine growth restriction (IUGR): a review J Neonatal-perinatal Med 9 2 117 126
D Tynan J Alphonse A Henry 2016 The aortic isthmus: a significant yet underexplored watershed of the fetal circulation Fetal Diagn Ther 40 2 81 93
A Stang 2010 Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses Eur J Epidemiol 25 9 603 605
F Figueras E Meler A Iraola 2008 Customized birthweight standards for a Spanish population Eur J Obstet Gynecol Reprod Biol 136 1 20 24
P Yudkin M Aboualfa J Eyre 1987 New birthweight and head circumference centiles for gestational ages 24 to 42 weeks Early Human Dev 15 1 45 52
M Rio Del J Martinez F Figueras 2005 Doppler assessment of fetal aortic isthmus blood flow in two different sonographic planes during the second half of gestation Ultrasound Obstet Gynecol 26 2 170 174
L Maggio JD Dahlke H Mendez-Figueroa 2015 Perinatal outcomes with normal compared with elevated umbilical artery systolic-to-diastolic ratios in fetal growth restriction Obstet Gynecol 125 4 863 869
CC Lees N Marlow A Wassenaer-Leemhuis van 2015 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial The Lancet 385 9983 2162 2172
K Hecher C Bilardo R Stigter 2001 Monitoring of fetuses with intrauterine growth restriction: a longitudinal study Ultrasound Obstet Gynecol 18 6 564 570
A Baschat U Gembruch C Harman 2001 The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens Ultrasound Obstet Gynecol 18 6 571 577
TN Raju BM Mercer DJ Burchfield 2014 Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of child health and human development, Society for Maternal-Fetal medicine, American Academy of pediatrics, and American College of obstetricians and Gynecologists Am J Obstet Gynecol 210 5 406 417
Group E 2010 Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS) Acta Paediatr 99 7 978 992
J Caradeux R Martinez-Portilla T Basuki 2018 Risk of fetal death in growth-restricted fetuses with umbilical and/or ductus venosus absent or reversed end-diastolic velocities before 34 weeks of gestation: a systematic review and meta-analysis Am J Obstet Gynecol 218 2 S774 S782
CM Bilardo K Hecher GH Visser 2017 Severe fetal growth restriction at 26–32 weeks: key messages from the TRUFFLE study Wiley Online Library 285 290
R Hershkovitz J Kingdom M Geary 2000 Fetal cerebral blood flow redistribution in late gestation: identification of compromise in small fetuses with normal umbilical artery Doppler Ultrasound Obstet Gynecol 15 3 209 212
B Mylrea-Foley H Wolf T Stampalija 2021 Longitudinal Doppler assessments in late preterm fetal growth restriction Ultraschall Med 44 1 56 67
SM Lobmaier O Graupner JU Ortiz 2021 Perinatal outcome and its prediction using longitudinal feto-maternal doppler follow-up in late onset small for gestational age fetuses–a prospective cohort study Ultraschall Med https://doi.org/10.1055/a-1493-2367
Obstetricians RCo, Gynaecologists (2013) Small for gestational age fetus: investigation and management. Green-top Guideline 31
J-C Fouron A Skoll S-E Sonesson 1999 Relationship between flow through the fetal aortic isthmus and cerebral oxygenation during acute placental circulatory insufficiency in ovine fetuses Am J Obstet Gynecol 181 5 1102 1107
K Mäkikallio T Erkinaro N Niemi 2006 Fetal oxygenation and Doppler ultrasonography of cardiovascular hemodynamics in a chronic near-term sheep model Am J Obstet Gynecol 194 2 542 550
AM Rudolph 1985 Distribution and regulation of blood flow in the fetal and neonatal lamb Circ Res 57 6 811 821
Acknowledgements
We would like to thank Prof. Mariola Ropacka-Lesiak and Dr. Bhumika Bhagat for their contribution to this systematic review in terms of additional data supplied and support and thank the VALERE 2019 Program University of Campania L. Vanvitelli.
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection or management and data analysis were conducted by MLV, MM and GR. The first draft of the manuscript was written by PDF, NC and MT. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study does not involve human participants or animals.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
La Verde, M., Savoia, F., Riemma, G. et al. Fetal aortic isthmus Doppler assessment to predict the adverse perinatal outcomes associated with fetal growth restriction: systematic review and meta-analysis. Arch Gynecol Obstet 309, 79–92 (2024). https://doi.org/10.1007/s00404-023-06963-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-06963-4