Abstract
Purpose
To evaluate the clinical outcomes and prognosis of patients undergoing laparoscopic surgery for tubo-ovarian abscess (TOA) and identify risk factors for pelvic inflammatory disease (PID) recurrence.
Methods
We conducted a retrospective cohort analysis including 98 women who underwent laparoscopic surgery for TOA at the Department of Obstetrics and Gynecology at the Bern University Hospital from January 2011 to May 2021. The primary outcome studied was the recurrence of PID after TOA surgery. Clinical, laboratory, imaging, and surgical outcomes were examined as possible risk factors for PID recurrence.
Results
Out of the 98 patients included in the study, 21 (21.4%) presented at least one PID recurrence after surgery. In the univariate regression analysis, the presence of endometriosis, ovarian endometrioma, and the isolation of E. coli in the microbiology cultures correlated with PID recurrence. However, only endometriosis was identified as an independent risk factor in the multivariate analysis (OR (95% CI): 9.62 (1.931, 47.924), p < 0.01). With regard to the time of recurrence after surgery, two distinct recurrence clusters were observed. All patients with early recurrence (≤ 45 days after TOA surgery) were cured after 1 or 2 additional interventions, whereas 40% of the patients with late recurrence (> 45 days after TOA surgery) required 3 or more additional interventions until cured.
Conclusion
Endometriosis is a significant risk factor for PID recurrence after TOA surgery. Optimized therapeutic strategies such as closer postsurgical follow-up as well as longer antibiotic and hormonal therapy should be assessed in further studies in this specific patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endometriosis is a risk factor for recurrence of pelvic inflammatory disease after tubo-ovarian abscess surgery. Closer postsurgical follow-up and longer antibiotic or hormonal therapy may be considered for these patients. |
Introduction
Pelvic inflammatory disease (PID) is an infection affecting the uterus, ovaries, and fallopian tubes. This major global health problem leads to severe long-term complications such as chronic pelvic pain, infertility, and ectopic pregnancy [1, 2, 3]. It is estimated that 4% of women will have PID at some point in their lifetime and 1 in 8 women with a history of PID will experience difficulty getting pregnant [1]. In approximately one-third of the patients with PID, tubo-ovarian abscesses (TOA) occur, which are associated with higher morbidity and mortality [4, 5, 6]. Unfortunately, antibiotic therapy alone is not effective in many patients with TOA, and a surgical therapy is required [7, 8, 9].
The primary PID prevention strategies are well established [10]. Moreover, standardized therapeutic protocols for the management of PID exist, such as the Centers for Disease Controls and Prevention (CDC) guidelines [11, 12]. However, previous work has failed to address the problem of PID recurrence, which appears in 15–20% of the patients and is associated with an almost two-fold increase in infertility [5]. A first step for the establishment of prevention strategies to avoid recurrence is the identification of risk factors leading to PID recurrence.
The aim of this study, therefore, was to evaluate the clinical outcomes and prognosis of patients undergoing laparoscopic surgery for TOA and identify possible risk factors for PID recurrence.
Material and methods
Study population
A retrospective analysis of all laparoscopically treated TOA managed at the Department of Obstetrics and Gynecology at the Bern University Hospital from January 2011 to May 2021 was conducted. The primary outcome studied was the recurrence of PID after TOA surgery. Recurrence was defined as the occurrence of typical clinical signs such as fever or pelvic pain with increasing inflammatory markers or suspicion of recurrent tubo-ovarian complex in the imaging after TOA surgery (computer tomography (CT) scan or ultrasound (US)).
All patients with PID or TOA who received only a conservative antibiotic treatment without surgery were excluded. The clinical and imaging diagnostic standards for the diagnosis of TOA were in accordance with the BASHH Guidelines and the CDC Guidelines 2021 [12, 13]. TOA was confirmed intraoperatively by the presence of inflammatory exudate in the pelvis or the presence of pyosalpinx. The pre- and postsurgical course was documented in detail and Patients were divided into 2 groups (Group 1 = PID recurrence, Group 2 = non-recurrence).
Data collection
We extracted data from patients’ hospital admission records, laboratory and imaging scan reports, operation reports, and discharge letters, all of which were stored in the electronic medical record databases of the gynecological unit in our medical center. For the patients who were referred to us at the time of recurrence and had a previous TOA surgery in other hospitals, we reviewed the discharge letters and operation reports from the other hospitals. Records were reviewed by a single reviewer (M.Z.)
Data on patient characteristics (age, gravidity, parity, history of PID, mode of contraception), presence of clinical signs such as fever or lower abdominal pain, C reactive protein (CRP) serum level, white blood cell (WBC) count, and the dimension and side of the tubo-ovarian complex in the imaging at the time of admission were extracted. Leukocytosis was defined as a white blood cell count greater than 11,000/mm3 (11 × 109/l) [14, 15]. High cutoff levels of CRP (≥ 60 mg/L) were used to identify patients with severe pelvic inflammatory disease or TOA [16].
Data on medical and surgical treatment outcomes (antibiotic regimen and its duration, type of operation, intraoperative findings, need for a second operation or an imaging-guided drainage postoperatively) as well as other clinical outcomes such as the length of hospitalization at first TOA episode and the presence of complications were extracted. We also extracted laboratory findings, such as the microbiology results of the vaginal, cervical, IUD, and pelvic swabs, blood culture results, postoperative CRP and WBC levels, and the histology findings.
TOA management
Inpatient antibiotic regimens were administered empirically in accordance with the hospital’s internal guidelines. The most used antibiotic regimens were (a) ampicillin/clavulanic acid administered at a daily dose of 1gr every 8 h plus Doxycycline 100 mg daily every 12 h; (b) ampicillin/clavulanic acid plus Metronidazol in a daily dose of 500 mg every 8 h; and (c) a combination of ampicillin/clavulanic acid plus Doxycycline plus Metronidazol. An alternative regimen consisted of a combination of Doxycycline, Metronidazol, and ceftriaxone (2gr daily). Laparoscopic surgery took place after 24–48 h of intravenous antibiotic therapy while imaging-guided drainage was not considered as a primary management option.
All laparoscopic surgeries in our clinic were performed using a standard technique and were video recorded. The abscess was drained and an adhesiolysis and restoration of the pelvic anatomy was performed. Tube or ovarian removal was avoided. In the event of pyosalpinx, a small fenestration was placed on the antimesenteric side of the tube. Lavage of the pelvic and abdominal cavity with saline solution was performed, and an intra-abdominal drain was inserted in the pelvic cavity at the end of the surgery. According to the available surgical reports a similar surgical technique was followed for patients primarily treated in external clinics.
The diagnosis of endometriosis intraoperatively was based on the typical visual appearance and/or the results of histopathology.
The antibiotic regimens were adjusted postoperatively according to the microbiology results of the pelvic swabs and continued for at least 10 days.
In case of recurrence, antibiotic therapy, imaging-guided drainage (with CT or US) and laparoscopic or laparotomic surgery were considered as possible therapeutic interventions.
Follow-up data
The postoperative course was documented for all patients. The time and type of intervention at recurrence and the further course were documented. Due to the retrospective character of the study, the follow-up times varied, while the first follow-up appointment was mostly arranged 2–4 weeks after the first TOA surgery, when the patient was already discharged from the hospital.
Statistical analysis
An available case analysis was performed. Mean values ± standard deviation or median values and ranges were calculated for continuous variables and percentages for qualitative variables. The variables of patients with and without PID recurrence were analyzed and compared. The t test was used for continuous variables with normal distribution, the chi-square test or the Fisher’s exact test for categorical variables, and the Mann–Whitney U test for continuous variables with skewed distribution. Possible risk factors for PID recurrence were further assessed using a multivariate logistic regression analysis. A p value < 0.05 indicated statistical significance. Statistical analysis was performed using the SPSS 25.0 Software (SPSS USA).
Results
Between January 2011 and May 2021, 120 patients with TOA were treated in our clinic. Of these patients, 22 were excluded since they had received only an antibiotic conservative treatment and no surgery. From the 98 patients who underwent a laparoscopic surgery for TOA, 21 patients (21.4%) presented PID recurrence after surgery. Six of them (28.6%) underwent their first TOA surgery in another hospital in Switzerland and were referred to us at the time of recurrence. Thus, from the 92 patients who underwent their first surgery in our hospital, 14 patients (15.2%) presented a recurrence.
Risk factors for PID recurrence
In Table 1, we present the most important clinical outcomes and compare them in the two groups (patients with and without PID recurrence). The total number of patients differs in some cells due to missing (unknown) outcomes for some patients. Preoperative characteristics at the time of TOA diagnosis, such as age, parity, type of contraception, WBC count, CRP, clinical symptoms, and TOA size did not differ between the two groups. The most common symptoms were pelvic pain and fever, with most patients presenting a leukocytosis and a CRP ≥ 60 mg/l.
No differences between the two groups in the duration of surgery, intraoperative blood loss, and the quantity of saline solution used for lavage of the abdominal cavity were identified. However, the presence of endometriosis intraoperatively was more frequent in the recurrence group (13/21, 61.9% vs 12/77, 15.6%, p value < 0.001). In the most cases the presence of endometriosis was histologically confirmed, except from the cases with DIE (deep infiltrating endometriosis). In the last cases the diagnosis of endometriosis and the classification according to rASRM (revised American Society for Reproductive Medicine Score) or ENZIAN score has been made mainly during previous surgeries [17]. The incidence of an ovarian endometrioma was also higher in the recurrence group (Table 1). Nine patients from the recurrence group presented an endometrioma during TOA surgery. This involved 5 times the left ovary, one time the right ovary and 3 times both ovaries. In all cases with ovarian endometrioma, the TOA was developed on the side of the endometrioma. Furthermore, out of 98 patients, 57 patients (58%) in both groups presented a pyosalpinx intraoperatively. Most patients had a fertility sparing surgery (76%) at first episode due to the young age, as well their wishes for fertility preservation and hormonal preservation.
The mean CRP value on the second day after surgery remained high (≥ 60 mg/l in both groups), while the mean WBC count showed a slight decrease (< 11,000/mm3). Although these blood values were generally higher in the recurrence group, the difference did not reach statistical significance. Moreover, the differences between pre- and postsurgical WBC count and CRP were not statistically significantly different between groups. These results did not change even when only patients with early recurrence were analyzed.
E. coli isolated in the vaginal, pelvic swab, blood culture, or urine culture was the only bacterium that was significantly associated with recurrence (8/19, 42.1% in the recurrence group vs 15/75, 20% in the non-recurrence group, p value = 0.045). The mean length of hospital stay after the first TOA surgery was 9.6 ± 10.2 days in the recurrence group and 5.09 ± 2.07 days in the non-recurrence group (p value = 0.0005).
Endometriosis, ovarian endometrioma, and E. coli were further evaluated as possible independent risk factors for PID recurrence using a multivariate logistic regression analysis. Four patients were not included in the analysis due to missed values. The analysis confirmed a significant association of endometriosis with disease recurrence [OR (95% CI): 9.62 (1.931, 47.924), p < 0.01] (Table 2).
Complications
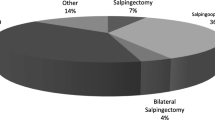
Complications during hospitalization at first PID episode occurred in 13/98 patients (13.2%). These were more frequent in the recurrence group compared to the non-recurrence group (6/21, 28.6% vs 7/77, 9.1%, p = 0.03, Table 1). The most significant complication was septic shock (positive shock index or SOFA Score > 2). E. coli was identified in the blood culture or in the pelvic swab in 4/6 cases of septic shock. Other complications included Covid-19-related pneumonia, pelvic hematoma, bowel perforation, and other complications such as pulmonary edema, left ovarian vein thrombosis, pleural effusion, and transfusion-related anemia (Fig. 1).
Time to recurrence and management
The mean time from TOA surgery to recurrence was 611 ± 1142 days. As seen in the scatter plot in Fig. 2, two different clusters of patients can be observed: the patients with early recurrence or persistence (up to 45 days after surgery) (N = 10) and the patients with late recurrence (> 45 days after surgery) (N = 10). For one patient the time of recurrence was unknown and therefore excluded from the further analysis.
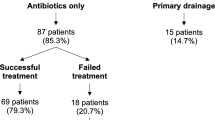
Half of the patients (5/10) with early recurrence after TOA surgery were first treated only with antibiotics or with antibiotics and an imaging-guided drainage. The other 5 patients with early recurrence and all patients with late recurrence were treated with antibiotics and a laparoscopic or laparotomic procedure (Table 3, Fig. 3). In total, 7 patients (5 patients from early recurrence group, and 2 patients from the late recurrence group) ultimately required a laparotomic intervention to be cured.
Type of intervention at first PID recurrence (early vs late recurrence). The figure shows the type of intervention, which had the patients at first PID recurrence after surgery. y-axis: number of patients, x-axis: type of intervention, blue color: patients with early recurrence or persistence (≤ 45 days), orange color: patients with late recurrence (> 45 days)
All patients with early recurrence were cured after 1 or 2 interventions (antibiotics only, antibiotics and imaging-guided drainage, antibiotics and laparoscopic or open surgery), whereas 40% of the patients with late recurrence required 3 or more of these interventions until cured (Fig. 4).
Total number of interventions at PID recurrence. This figure shows the total number of interventions (surgery, antibiotics, CT-guided drainage) after first PID recurrence in both groups (patients with early vs late recurrence) until cure. Blue refers to the group of patients with early recurrence or persistence (≤ 45 days), orange to the group of patients with late recurrence (after 45 days)
Discussion
Among the 98 patients with TOA requiring laparoscopic treatment, 21 (21.4%) presented a recurrence. Interestingly, the presence of endometriosis at the time of surgery was identified as the only independent risk factor for PID recurrence. The isolation of E. coli as well as the ovarian endometrioma were also associated with PID recurrence but were not statistically significant in the multivariate logistic regression analysis.
Our results are in line with Clarizia et al. demonstrating a four-fold higher prevalence of endometriosis found in PID patients when compared to the general population [18]. Furthermore, Tai et al. in a large cohort study demonstrated that patients with a PID had a three-fold increase in the risk of developing endometriosis compared with women without PID [19]. Women with endometriosis also seem to be at higher risk for developing a severe pelvic inflammatory disease, which involves antibiotic treatment failure and need for surgical intervention [20] while advanced stage of endometriosis (stage III and IV) is significantly associated with the occurrence of TOA [21]. It is also known that the incidence of TOA is higher in patients with ovarian endometrioma. Gao et al. reported that lower genital tract infection and rupture of ovarian endometriosis cysts are significantly associated with the development of TOA in women with ovarian endometriosis [22]. Furthermore, there are several case series, which have reported that IVF or oocyte retrieval are associated with the development of a TOA in patients with ovarian endometriosis [23, 24, 25].
Our study is the first to determine that endometriosis is not only a risk factor for PID but also a significant risk factor for PID recurrence after TOA surgery.
Regarding the pathogenic mechanisms leading to PID or TOA in patients with endometriosis, these include the static peritoneal milieu and cystic formation favorable to bacteria proliferation, the distorted anatomy of the ovaries and the tubes, an impairment in immune response [26], an increased inflammatory cytokines production [26, 27, 28] and alterations of the microbioma [27].
Other risk factors for PID or TOA are well recognized and described in previous studies. These include young age (< 25 years), multiple sexual partners, frequent change of sexual partner, previous history of PID, no history of contraceptive use, previous history of intrauterine device (IUD) insertion, drug consumption, and cigarette smoking [4, 5, 29, 30].
PID is usually polymicrobial with a mixture of aerobic, anaerobic, and facultative bacteria. The most frequent pathogens isolated from TOAs are E. coli (37%) and Bacteriodes Fragilis (22%) [4, 7, 31, 32]. Saini et al. showed in 2003 that the predominant aerobic bacteria isolated from Douglas specimens from women with PID was E. coli (33.2%) [33]. Furthermore, E. coli is one of the most common causative pathogens for severe infections and also the second most common cause of sepsis [34]. These results are in agreement with the results of our study in which E. coli was the predominant pathogen bacterium isolated in 42.1% of the patients in the recurrence group and in 4/6 blood cultures of patients with septic shock.
Referring to the management of TOA, the traditional treatment is the use of broad-spectrum antibiotics with a success rate of about 70% [35, 36, 37]. However, the recurrence rate after antibiotics alone is high. It has been shown, that the failure of antibiotic treatment and the need of surgical intervention is correlated with the size of the abscess [37]. More specifically, abscess size ≥ 5–6.5 cm is an independent risk factor for failure of antibiotic treatment and an independent predictor of patients requiring a surgical treatment [38, 39, 40, 41]. Nowadays, laparoscopic treatment, imaging-guided drainage (transvaginal ultrasound (US) or computerized tomography (CT)-guided) are established minimally invasive treatments in the management of TOA [42]. Comparing the three minimal invasive techniques, early laparoscopy has the key advantage that allows sampling of tissue for histopathology diagnosis, allows assessment of the intraoperative situs, and is associated with less complications and better clinical prognosis in the appropriate populations’ groups [39, 40, 43]. In our study, the mean size of abscess according to US or CT was 5.9 cm in both groups of patients. For these reasons, our first line therapy approach was the laparoscopy and not the imaging-guided drainage.
Interestingly, all patients with late recurrence (> 45 days) underwent a laparoscopic or open surgery. However, 50% of them needed 2 or more interventions until cure. On the contrary, patients with early recurrence were cured with 1 or 2 interventions. Obviously, the chronic tubo-ovarian abscesses with recurrent episodes and extensive adhesions are more challenging to manage, resulting in more interventions until cure [44].
In our study population, the PID recurrence rate after surgical therapy for PID was 21.4%, a little higher than the reported recurrence rate of PID in the literature [5]. This reflects the fact that our department is a tertiary referral center to which the more complex cases are referred. As a result, 6 of the 98 patients with PID recurrence were referred to us after first surgery in another hospital; this results in the overstatement of the recurrence rate. Excluding these patients and considering only the patients treated in our hospital, the recurrence rate was 15.2%. On the other hand, we might have missed patients with PID recurrence who were treated in other hospitals after TOA surgery in our department. However, since our department is the tertiary center in the broader area, we estimate this likelihood to be very small.
Some other potential limitations need to be considered. First, it is a single-center study, and consequently there were small numbers of events for some of the investigated outcomes; this resulted in a lower statistical power for these outcomes. Second, the design of this study was retrospective, with all the associated inherent limitations. On the other hand, the structured surgical approach to TOA and the relatively high data availability constitutes its strengths.
In conclusion, the laparoscopic management of TOA results in PID recurrence in more than 15% of the patients. This might be early or late recurrence, with the latter being more challenging, often requiring many interventions until cure. Endometriosis is a risk factor for recurrence and should therefore been taken into consideration when identified during TOA surgery. Optimized therapeutic strategies such as closer postsurgical follow-up as well as longer antibiotic and hormonal therapy should be assessed in further studies in this specific patient population.
References
Ford GW, Decker CF (2016) Pelvic inflammatory disease. Dis Mon 62(8):301–305. https://doi.org/10.1016/j.disamonth.2016.03.015
Trent M, Bass D, Ness RB, Haggerty C (2011) Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis 38(9):879–881. https://doi.org/10.1097/olq.0b013e31821f918c
Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE (1992) Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 19(4):185–92
Chappell CA, Wiesenfeld HC (2012) Pathogenesis, diagnosis, and management of severe pelvic inflammatory disease and tuboovarian abscess. Clin Obstet Gynecol 55(4):893–903. https://doi.org/10.1097/grf.0b013e3182714681
Siegenthaler F, Krause E, Mueller MD (2020) Management of pelvic inflammatory disease. Ther Umsch 77(4):164–170. https://doi.org/10.1024/0040-5930/a001171
Landers DV, Sweet RL (1985) Current trends in the diagnosis and treatment of tuboovarian abscess. Am J Obstet Gynecol 151(8):1098–1110. https://doi.org/10.1016/0002-9378(85)90392-8
Landers DV, Sweet RL (1983) Tubo-ovarian abscess: contemporary approach to management. Rev Infect Dis 5(5):876–884. https://doi.org/10.1093/clinids/5.5.876
Wiesenfeld HC, Sweet RL (1993) Progress in the management of tuboovarian abscesses. Clin Obstet Gynecol 36(2):433–444. https://doi.org/10.1097/00003081-199306000-00022
Hiller N, Sella T, Lev-Sagi A, Fields S, Lieberman S (2005) Computed tomographic features of tuboovarian abscess. J Reprod Med 50(3):203–208
Peterson HB, Galaid EI, Cates W Jr (1990) Pelvic inflammatory disease. Med Clin North Am 74(6):1603–1615. https://doi.org/10.1016/s0025-7125(16)30497-7
Workowski KA, Bolan GA (2015) Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64(Rr-3):1–137
Workowski KA et al (2021) Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 70(4):1–187. https://doi.org/10.15585/mmwr.rr7004a1
Jonathan Ross MC, Evans C, Lyons D, Dean G, Cousins D. PPI representative, 2018 United Kingdom National Guideline for the Management of Pelvic Inflammatory Disease. 2018, BASHH Guidelines.
Asadollahi K, Hastings IM, Beeching NJ, Gill GV, Asadollahi P (2011) Leukocytosis as an alarming sign for mortality in patients hospitalized in general wards. Iran J Med Sci 36(1):45–49
Shapiro MF, Greenfield S (1987) The complete blood count and leukocyte differential count. An approach to their rational application. Ann Intern Med 106(1):65–74. https://doi.org/10.7326/0003-4819-106-1-65
Miettinen AK, Heinonen PK, Laippala P, Paavonen J (1993) Test performance of erythrocyte sedimentation rate and C-reactive protein in assessing the severity of acute pelvic inflammatory disease. Am J Obstet Gynecol 169(5):1143–1149. https://doi.org/10.1016/0002-9378(93)90271-j
Keckstein J et al (2021) The #Enzian classification: a comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet Gynecol Scand 100(7):1165–1175
Clarizia R et al (2021) Inflammation calls for more: severe pelvic inflammatory disease with or without endometriosis. Outcomes on 311 laparoscopically treated women. J Gynecol Obstet Hum Reprod 50(3):101811. https://doi.org/10.1016/j.jogoh.2020.101811
Tai FW et al (2018) Association of pelvic inflammatory disease with risk of endometriosis: a nationwide cohort study involving 141,460 individuals. J Clin Med 7(11):379
Elizur SE et al (2014) Pelvic inflammatory disease in women with endometriosis is more severe than in those without. Aust NZ J Obstet Gynaecol 54(2):162–165. https://doi.org/10.1111/ajo.12189
Chen MJ et al (2004) Increased occurrence of tubo-ovarian abscesses in women with stage III and IV endometriosis. Fertil Steril 82(2):498–499
Gao Y et al (2021) Risk factors for the development of tubo-ovarian abscesses in women with ovarian endometriosis: a retrospective matched case–control study. BMC Womens Health 21(1):43
Padilla SL (1993) Ovarian abscess following puncture of an endometrioma during ultrasound-guided oocyte retrieval. Hum Reprod 8(8):1282–1283
Yaron Y et al (1994) Infected endometriotic cysts secondary to oocyte aspiration for in-vitro fertilization. Hum Reprod 9(9):1759–1760
Younis JS et al (1997) Late manifestation of pelvic abscess following oocyte retrieval, for in vitro fertilization, in patients with severe endometriosis and ovarian endometriomata. J Assist Reprod Genet 14(6):343–346
Ho HN, Wu MY, Yang YS (1997) Peritoneal cellular immunity and endometriosis. Am J Reprod Immunol 38(6):400–412
Chen C et al (2017) The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 8(1):875
Villette C et al (2016) Risks of tubo-ovarian abscess in cases of endometrioma and assisted reproductive technologies are both under—and overreported. Fertil Steril 106(2):410–415
Grimes DA (2000) Intrauterine device and upper-genital-tract infection. Lancet 356(9234):1013–1019. https://doi.org/10.1016/s0140-6736(00)02699-4
Kapustian V et al (2018) Is intrauterine device a risk factor for failure of conservative management in patients with tubo-ovarian abscess? An observational retrospective study. Arch Gynecol Obstet 297(5):1201–1204. https://doi.org/10.1007/s00404-018-4690-z
Granberg S, Gjelland K, Ekerhovd E (2009) The management of pelvic abscess. Best Pract Res Clin Obstet Gynaecol 23(5):667–678. https://doi.org/10.1016/j.bpobgyn.2009.01.010
Krivak TC, Cooksey C, Propst AM (2004) Tubo-ovarian abscess: diagnosis, medical and surgical management. Compr Ther 30(2):93–100. https://doi.org/10.1007/s12019-004-0003-5
Saini S, Gupta N, Aparna BG, Arora DR (2003) Role of anaerobes in acute pelvic inflammatory disease. Indian J Med Microbiol 21(3):189–92
Brook I (2008) Microbiology and management of abdominal infections. Dig Dis Sci 53(10):2585–2591. https://doi.org/10.1007/s10620-007-0194-6
Goharkhay N, Verma U, Maggiorotto F (2007) Comparison of CT- or ultrasound-guided drainage with concomitant intravenous antibiotics vs. intravenous antibiotics alone in the management of tubo-ovarian abscesses. Ultrasound Obstet Gynecol 29(1):65–9. https://doi.org/10.1002/uog.3890
McNeeley SG, Hendrix SL, Mazzoni MM, Kmak DC, Ransom SB (1998) Medically sound, cost-effective treatment for pelvic inflammatory disease and tuboovarian abscess. Am J Obstet Gynecol 178(6):1272–1278. https://doi.org/10.1016/s0002-9378(98)70333-3
Reed SD, Landers DV, Sweet RL (1991) Antibiotic treatment of tuboovarian abscess: comparison of broad-spectrum beta-lactam agents versus clindamycin-containing regimens. Am J Obstet Gynecol 164(6pt1):1556–61. https://doi.org/10.1016/0002-9378(91)91436-z
Topçu HO et al (2015) Risk factors for adverse clinical outcomes in patients with tubo-ovarian abscess. J Obstet Gynaecol 35(7):699–702. https://doi.org/10.3109/01443615.2014.991294
Jiang X et al (2019) Clinical value of early laparoscopic therapy in the management of tubo-ovarian or pelvic abscess. Exp Ther Med 18(2):1115–1122. https://doi.org/10.3892/etm.2019.7699
Chu L et al (2019) Effectiveness and adverse events of early laparoscopic therapy versus conservative treatment for tubo-ovarian or pelvic abscess: a single-center retrospective cohort study. Gynecol Obstet Invest 84(4):334–342. https://doi.org/10.1159/000493855
Kinay T et al (2016) The value of ultrasonographic tubo-ovarian abscess morphology in predicting whether patients will require surgical treatment. Int J Gynaecol Obstet 135(1):77–81. https://doi.org/10.1016/j.ijgo.2016.04.006
Silva F et al (2015) Minimally invasive approach of tubo-ovarian abscesses. Rev Bras Ginecol Obstet 37(3):115–118. https://doi.org/10.1590/so100-720320150005257
Brun JL et al (2016) Updated French guidelines for diagnosis and management of pelvic inflammatory disease. Int J Gynaecol Obstet 134(2):121–125. https://doi.org/10.1016/j.ijgo.2015.11.028
Nakayama K et al (2013) Surgical treatment outcomes of serious chronic tubo-ovarian abscess: a single-center series of 20 cases. Clin Exp Obstet Gynecol 40(3):377–380
Acknowledgements
We would like to thank all participants in our study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
ZTM: protocol and project development, data collection and management, data analysis, manuscript writing and editing. NK: protocol and project development, manuscript writing and editing. NS: data collection and management. IS: manuscript writing and editing. MMD: protocol and project development, manuscript writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
Authors A, B, and C declare they have no financial interests. Author D has received speaker honoraria from Company Bayer. Author E has received a research grant from Inno Suisse Foundation and speaker honoraria from Companies Bayer, Roche, and Storz.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The ethical approval was granted by the Ethics Committee of Switzerland, the IRB (Institutional Review Board for research ethics, Bern) and was registered with the number 2021-01194.
Informed consent
Due to the retrospective design of the study no informed consent was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zografou Themeli, M., Nirgianakis, K., Neumann, S. et al. Endometriosis is a risk factor for recurrent pelvic inflammatory disease after tubo-ovarian abscess surgery. Arch Gynecol Obstet 307, 139–148 (2023). https://doi.org/10.1007/s00404-022-06743-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06743-6