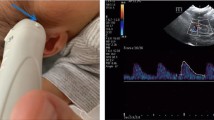
Abstract
Objective
To evaluate functional relationship between fetal circulatory response to intrauterine transfusion (IUT) as a circulatory challenge and appearance of second systolic peak (P2) in middle cerebral artery (MCA) based on hemodynamic principles.
Methods
According to the concept of pulse wave (PW) propagation and reflection in adults, PWs arrive twice at cerebral circulation, as primary wave caused by left ventricle ejection and secondary after reflection in peripheral arteries. Thus adults show a biphasic contour of systolic blood flow in cerebral arteries. Similar waveforms may appear in fetal MCA-Doppler, as a response to IUT as a circulatory challenge. This is a proof-of-principle study, applying classical hemodynamic principles to fetal circulation. Accordingly, appearance of MCA-P2 may indicate vasoconstriction with increased PW reflection and timing of P2(Δt) should agree with the additional PW travel time down to reflection and return (Tr). To test this agreement, we searched our database for IUTs performed for severe fetal anemia, and compared Δt, obtained by Doppler, with Tr, obtained by hemodynamic calculation using human fetal data. Level of agreement was assessed using Bland–Altman-Plots.
Results
We identified 21 fetuses with adequate Doppler quality for Δt evaluation. In four cases (19%) MCA-P2 was observed before the intervention, and in 17 interventions (81%) thereafter; a highly significant association between IUT and P2 appearance (p < 0.001). In these 17 interventions good agreement of P2 timing was found between Doppler assessment: Δt = 80 ± 8 ms, and hemodynamic calculation: Tr = 76 ± 4 ms.
Conclusion
P2 appearance in fetal MCA-Doppler seems to indicate PW reflection due to increased vasoconstriction after IUT. Thus hemodynamic considerations might enable Doppler monitoring of fetal vasoconstriction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A transient second systolic peak (P2) may appear in fetal middle cerebral artery (MCA) Doppler waveforms after intrauterine transfusion (IUT). In a classical paper on fetal cerebral arteries Doppler waveforms before and after IUT published in 1990 Mari et al. showed cerebral and especially MCA Doppler waveforms with a second systolic peak (MCA-P2), one in a fetus with severe anemia (Hb 4 g/dl), and another one 2 h after IUT [1]. However, in the detailed hemodynamic discussion this particular systolic waveform feature was not mentioned.
Fetal condition after IUT is transiently worsened as the transfused blood has very low pH (< 7.0) and high hematocrit (Hct). Both these conditions increase systematic vasoconstriction in animal models [2,3,4]. Recently evidence was found that appearance of a second systolic peak in MCA Doppler of human fetuses with severe anemia may indicate increased pulse wave (PW) reflection, with secondary transmission to head and cerebral circulation [5]. Furthermore, observed timing of reflection seems to minimize pulsatile energy consumption [6,7,8].
We speculate that IUT is associated with higher reflective conditions due to transiently increased fetal vasoconstriction. The aim of our study was to evaluate the functional relationship between the fetal circulatory response to the IUT and the appearance of a MCA-P2 based on a hemodynamic principles.
Patients and methods
This is a proof-of-principle study applying a classical hemodynamic principles to the fetal circulation.
The pulse wave model of hemodynamics
In hemodynamics the concept of PW propagation and reflection is well established: cardiac contraction generates a PW and accelerates blood flow. During propagation of the PW to the periphery, reflections in the arterial system occur. Apparently major reflected waves merge to a coherent reflected wave (RW), travelling back with an average velocity, c(Ao), along the aorta (Ao), and finally arriving at the left ventricle (LV) like an echo, after a definite time of return, the so-called reflection time, Tr [9,10,11].
Based on this reflection time Tr (i.e. the two-way travel time taken for travelling down to reflection and back (shown in Fig. 1)) and the average PW velocity in the aorta, c(Ao), the functional distance L to reflection may be obtained:
Hemodynamic model. Left: diagram of pulse wave propagation, reflection and cerebral transmission. Right: middle cerebral artery (MCA) Doppler waveform with the time interval Δt to MCA-P2 onset and reflection timing: Tr/T ≈ 0.2. Pulse waves reach twice to MCA: by direct transmission [1], and after reflection, return and subsequent transmission [2]. The time interval Δt from MCA waveform onset to MCA-P2 onset corresponds to the reflection time Tr, i.e. the two-way travel time needed for travelling down to reflection and back
This is the concept of the "effective length" L of the central arterial system in hemodynamics [12] and corresponds approximately to the path lengths of aorta plus common iliac artery (CIA) as anatomical surrogates, conveniently written as: L(Ao +)
Consequently, RW seems to return from a functional distance L(Ao +) corresponding to the pelvic region [6, 10, 12] and may arrive at the LV during ongoing systole [11].
After substituting "L" by "L(Ao +)" in Eq. 1, and rearranging, the following relation for Tr is obtained:
Like an echo time, the reflection time Tr is given by the two-way travel distance to reflection and back: 2∙L(Ao +), divided by the average PW velocity in the aorta, c(Ao). This relation is the key for the transfer of the classical PW model to fetal circulation.
At the level of the aortic arch, a fraction of the reflected wave RW is diverted cranially to the cerebral circulation as a forward wave, to create the second systolic peak, P2 [13, 14]. Hence this second wave arrives at the cerebral circulation after a temporal delay corresponding to the additional travel time down to reflection and back, and thus is given by the aforementioned reflection time, Tr [15,16,17].
Transfer of the pulse wave model to the fetal circulation
If we apply the same principles of PW propagation and reflection to the fetal circulation, then PWs should arrive twice at cerebral circulation too: first by direct transmission, and second after downstream propagation, reflection and subsequent transmission. This second wave accelerates ongoing flow and may create a second systolic peak in fetal cerebral waveforms, if PW reflection is strong enough. By analogy, the interval Δt until P2 onset (contour inflection point) on the fetal MCA waveform should coincide with the temporal delay of reflected PW arrival at the Doppler recording site, given by the reflection time, Tr. To our knowledge, reflection time is not known in the human fetus, but may approximately be calculated with [Eq. 3]: Tr = 2L(Ao +)/c(Ao).
Patients
In order to test the validity of the PW model in the fetal arterial system, IUT was chosen as an exemplary, reproducible, extreme fetal distress situation. We performed a preliminary search in our perinatal database for IUTs performed from 2006 to 2018 and reviewed the MCA Doppler waveforms obtained directly before and after IUT for appearance of MCA-P2. All cases with IUT performed for severe fetal anemia (fHb ≤ 0.55 MOM) with a transfusion coefficient ≥ 0.03 (IUT-volume [ml]/estimated fetal weight [g]) [18], and gestational age (GA) between 26 + 0 and 30 + 6 weeks were included if Doppler image quality was adequate for Δt evaluation (shown in Fig. 2).
The time interval (Δt) between MCA waveform onset and MCA-P2 onset was measured as shown in Fig. 1. Reflection time Tr was calculated according to Eq. 3 of the PW model: Tr = 2L(Ao +)/c(Ao) (shown in Fig. 1). The required factors: L(Ao +) = L(A0) + L(CIA), the anatomical surrogate of fetal functional distance to reflection, and c(Ao), the average PW velocity in the fetal aorta, were obtained based on human fetal data as follows:
-
For both anatomical parameters, L(Ao) and L(CIA), GA-adjusted values had been published, and the sum of the vascular lengths was calculated according to the formulas proposed by Szpinda et al. for fetal Ao [19] and CIA [20], yielding: L(fAo +) = L(Ao) + L(CIA)[mm] = 5.242∙GA(wks) − 48.36.
-
Similarly GA-adjusted values for the PW velocity in the fetal Ao, c(Ao), were obtained according the following formula: c(Ao) [cm/s] = 4.7∙GA(wks) + 125.1 [21]. In this study, c(Ao) was assessed from the longitudinal section of the descending fetal aorta at the level of the diaphragm.
The results of the Doppler-recorded time intervals Δt, were compared to the results of the model-calculated PW reflection time, Tr. Data coding and descriptive statistics were performed with SPSS version 26.0 (IBM, SPSS Inc., USA). To evaluate the agreement of the results obtained by both methods, Doppler assessment, Δt, and model calculation, Tr, a Bland–Altman (mean-difference or limits of agreement) plot was used. The intermethod average (Av) of each pair of results: Av(Δt, Tr) = (Δt + Tr)/2, was plotted against the intermethod difference: Diff(Δt, Tr) = (Δt − Tr), and the 95% limits of agreement (LA) between the results of both methods, Δt and Tr, were calculated.
The study was conducted in accordance with the approval of the local Ethics Commission (KEK-ZH. Nr.2020–01225).
Results
We could identify 21 interventions meeting all inclusion criteria and adequate Doppler quality for Δt evaluation. In 4 cases (19%) MCA-P2 was observed before the intervention, and in 17 interventions (81%) MCA-P2 was observed thereafter, thus indicating a highly significant association between IUT performed for severe anemia and observation of MCA-P2 (p < 0.001, Fisher’s exact test) after the intervention.
Thus 17 interventions remained for Δt measurements and these were performed on 14 fetuses (3 fetuses with repeated interventions). The underlying cause for anemia was maternal red cell alloimmunization in 10 cases, anti-Kell antibodies in 3 cases and fetal parvovirus B12 infection in one case. Mean GA (± SD) at IUT was 28 ± 1 weeks at a fetal weight of 1061 ± 358 g. Fetal hemoglobin measurements before IUT were 5.6 ± 1.4 g/l followed by IUT with a mean IUT-Volume of 42 ± 12 ml. Neonatal outcome data are presented in Table 1.
The mean value of the time intervals Δt, measured between MCA waveform onset and MCA-P2 onset in Doppler waveforms obtained in these cases after IUT was: Δt = 80 ± 8 ms.
The mean of the vascular path lengths obtained according Eq. 2: L(Ao +) = L(Ao) + L(CIA) using individual, GA-adjusted human fetal data on Ao and CIA lengths was: L(Ao +) = 9.7 ± 0.7 cm, and the mean of the aortic PW velocities obtained from individual, GA-adjusted fetal aortic velocity data [21] was: c(Ao) = 255 ± 6 cm/s.
Finally, we calculated the individual reflection times Tr according to Eq. 3: Tr = 2∙L(Ao +)/c(Ao), and obtained as mean value: Tr = 76 ± 4 ms (Table 2).
Good agreement between the results of both methods, Tr = 76 ± 4 ms and Δt = 80 ± 8 ms, was confirmed by the Bland–Altman plot: the intermethod difference between mean Δt (80 ms) and mean Tr (76 ms) was 4.8 ms, and the 95% limits of agreement (LA, ms) were [− 11.8; + 20.8] (shown in Fig. 3).
Discussion
This study provides evidence that a second systolic peak, P2, in MCA Doppler might indicate PW reflection due to increased fetal vasoconstriction after IUT. Accordingly, hemodynamic principles might enable Doppler monitoring of fetal vasoconstriction.
MCA Doppler has doubtlessly established its priority role in the diagnosis of fetal anemia [22,23,24]. In pregnancies complicated by maternal red cell alloimmunization, monitoring the MCA peak systolic blood flow velocity by Doppler assessment was shown to be reliable enough to replace invasive testing. The present study however, directs the focus to an additional, new tool of the MCA Doppler imaging, the second systolic peak, MCA-P2, which may identify increased fetal PW reflection as a marker of fetal distress. There are a number of aspects that deserve detailed consideration.
Fetal MCA Doppler waveforms mainly show a slightly convex [25] systolic downslope, sometimes changing from a more or less discernable systolic shoulder (MCA-S) to a clearly visible but less frequent second systolic peak (MCA-P2). This latter waveform pattern may appear in cerebral and especially in MCA Doppler waveforms after IUT, as shown in the classical paper by Mari [1] and occasionally in fetal MCA waveforms published by others [26,27,28]. We could observe that MCA-P2 was more frequently persisting in severe anemic fetuses after IUT with a significant transfusion volume, and we decided to study this so far unaddressed or overlooked phenomenon.
IUT performed as therapeutic intervention may transiently worsen the fetal condition due to low pH and high Hct of the transfused blood. Animal models revealed signs of systemic fetal vasoconstriction due to transient acidemia and hyperviscosity after IUT [2,3,4]. Thus, IUT constitutes a well-defined and quantifiable circulatory stress to the fetal cardiovascular system. In the human fetus IUT was found to increase blood pressure (BP) in the umbilical vein (UV) [29], but we could not localize any report on arterial BP measurements. Whereas in fetal lambs IUT was reported to increase arterial BP [30]. However, in the human fetus IUT was associated with a significant increase in Arginine vasopressin (AVP) levels [31], an antidiuretic hormone and potent vasoconstrictor, which increases peripheral vascular resistance and raises arterial BP in adults [32]. This supports the assumption that IUT may increase vasoconstriction in the human fetus too. According to our preliminary observations, major IUT challenge might be detected by the appearance of MCA-P2.
We looked for corresponding Doppler signs seen in cerebrovascular waveforms in adults, and found that similar systolic waveform modulations were attributed to PW propagation, reflection and cranial transmission [13, 15, 17, 33, 34]. Accordingly, systemic vasoconstriction modulates PW reflection and the shape of cerebrovascular waveforms [12, 13, 35]. Thus, we assumed that this hemodynamic approach might be applicable in fetuses too and be useful to describe the functional relationship between IUT as a circulatory challenge and MCA-P2 appearance as a result of PW reflection. A valid model should enable to describe the response of the fetal arterial system in a predictive way and this is what we could confirm with this study. The temporal delay Tr of reflected wave arrival to MCA, as calculated by the model: Tr = 2L(Ao +)/c(Ao), coincided with the clinically observed temporal delay Δt to MCA-P2 onset, the assumed sign of fetal PW reflection appearing in MCA Doppler waveforms. Thus, the result of the model-calculated PW reflection time, Tr = 76 ± 4 ms, is comparable to the results of the Doppler-recorded time interval Δt = 80 ± 8 ms. The Bland–Altman method confirmed good agreement between both results (shown in Fig. 3): within the 95% limits of agreement (LA) [− 11.8; + 20.8] (ms), indicating a mid-systolic event in fetuses between 26 and 32 weeks’ gestation, were mean ejection time (systole) was reported between 165 and 175 ms [36,37,38]. Finally the intermethod difference of 4.8 ms between mean Δt (= 80 ms) and mean Tr (= 76 m) is within the temporal resolution capacity of the Doppler method (~ 10 ms) [39].
The validity of the PW model in the human fetus is supported by recently published studies on optimal pulsatile timing in the mammalian cardiovascular system [6, 40]. Accordingly, energy consumption of pulsatile cardiac action is optimized when the (one-way) transit time to PW reflection takes about 10%, or equivalent, when the (2-way) transit time to PW reflection and return (= Tr) takes about 20% of the cardiac cycle T: Tr/T ≈ 0.2 [6,7,8]. Assuming a mean fetal heart rate of 150 bpm (i.e. cardiac cycle T = 400 ms), then with our study result: Tr = 76 ms, follows: Tr/T = 76 ms/400 ms = 0.19. This indicates nearly optimal pulsatile timing and seems to confirm the validity of the same PW optimization criterion in the fetus. Moreover, a simple look to the MCA waveform pattern allows a brief visual check of this 20% criterion (Tr/T ≈ 0.2, Fig. 1).
For ethical and clinical reasons we could not have direct access to the arterial system of the fetus, the subject of our study. This is a main limitation of our study, i.e. we could not measure vasoconstriction or arterial blood pressure to study the fetal circulatory response to IUT directly. Nevertheless, we could observe critical signs of PW reflection and these observations are based on a well-stablished hemodynamic model in (adult) physiology [10, 12, 13, 15, 33,34,35].
Conclusion
The transfer of the hemodynamic model of PW propagation and reflection to the fetus is feasible and provides a new, non-invasive access to the fetal cardiovascular system. Accordingly a second systolic peak in fetal MCA Doppler imaging, MCA-P2, seems to indicate increased fetal PW reflection, and may appear in particular circumstances after IUT, a therapeutic intervention associated with transient fetal circulatory distress. Thus consideration of a secondary systolic peak P2 in fetal MCA-Doppler waveforms might open a diagnostic window to observe signs of fetal vasoconstriction after IUT. Whether monitoring of this particular Doppler sign might be of clinical benefit in fetal treatment, remains to be evaluated in systematic clinical studies. Clearly, these hemodynamic considerations provide additional insight and may help to design such a study.
This hemodynamic model approach may also open a window to evaluate transplacental effects of vasoactive substances on fetal cardiovascular system, including anesthetics, tocolytics and antihypertensives [41]. In the meanwhile, the same hemodynamic principle of PW reflection from the arterial system and transmission to cerebral arteries became also the basis for the interpretation of maternal ophthalmic artery Doppler waveforms to predict and to monitor preeclampsia [42,43,44].
References
Mari G, Moise KJ Jr, Deter RL, Carpenter RJ Jr (1990) Flow velocity waveforms of the umbilical and cerebral arteries before and after intravascular transfusion. Obstet Gynecol 75(4):584–589
Brace RA (1989) ovine fetal cardiovascular response to packed red blood cell transfusion. Am J Obstet Gynecol 161:1367–1374
Fan FC, Chen RYZ, Chluessler GB, Chien S (1980) Effects of hematocrit variations on regional hemodynamics and oxygen transport in the dog. Am Physiol Soc. 238:545–522
Fumia FD, Edelstone DI, Holzmann IR (1884) Blood flow and oxygen delivery to fetal organs as function of fetal hematocrit. Am J Obstet Gynecol 150:274–282
Gonser M, Tavares M, Klee A, Hecher K (2018) A systolic shoulder in fetal middle cerebral artery Doppler wavefroms may indicate fetal pulse wave reflection and transmission to cerebral circulation—a contribution to fetal circulatory physiology. Ultraschall in Med 39:S1–S47
Pahlevan NM, Gharib M (2014) A wave dynamics criterion for optimization of mammalian cardiovascular system. J Biomech 47(7):1727–1732
Gonser M, Pahvelan NM, Gharib M (2020) Optimisation criterion for pulsatile timing: observation in the human fetus. Ultrsound Obst Gynecol 56(S1):197–198
Gonser M, Vonzun L, Tavares M, Ochsenbein-Koelble N, Hecher K, Zimmermann R (2020) Fetal pulse wave and pulsatile timing assessement in MCA-doppler of fetuses with severe anemia and after IUT. Ultrasound Obstet Gynecol 56(S1):135–136
O’Rourke MF (1982) Vascular impedance in studies of arterial and cardiac function. Physiol Rev 62(2):570–623
O’Rourke MF, Adji A, Safar ME (2018) Structure and function of systemic arteries: reflections on the arterial pulse. Am J Hypertens 31(8):934–940
Rodriguez C, Chi YY, Chiu KH, Zhai X, Lingis M, Williams RS et al (2018) Wave reflections and global arterial compliance during normal human pregnancy. Physiol Rep 6(24):e13947
Nichols WW, O’Rourke MF, Vlachopoulos C (eds) (2011) McDonald’s blood flow in arteries: theoretical, experimental and clinical principles, 6th edn. Hodder Arnold, London, pp 195–224 (Wave reflections)
Kim MO, Li Y, Wei F, Wang J, O’Rourke MF, Adji A et al (2017) Normal cerebral vascular pulsations in humans: changes with age and implications for microvascular disease. J Hypertens 35(11):2245–2256
Mynard JP, Kowalski R, Cheung MM, Smolich JJ (2017) Beyond the aorta: partial transmission of reflected waves from aortic coarctation into supra-aortic branches modulates cerebral hemodynamics and left ventricular load. Biomech Model Mechanobiol 16(2):635–650
Hashimoto J, Ito WB (2018) Carotid flow augementation, arterial aging, and cerebral white matter hyperintensities. Comparison with pressure aumentation. Arterioscler Thromb Vasc Biol 38:2843–2853
Heffernan KS, Lefferts WK, Augustine JA (2013) Hemodynamic correlates of late systolic flow velocity augmentation in the carotid artery. Int J Hypertens 2013:920605
Mills CJ, Gabe IT, Gault JH, Mason DT, Ross J Jr, Braunwald E et al (1970) Pressure-flow relationships and vascular impedance in man. Cardiovasc Res 4(4):405–417
Society for Maternal-Fetal Medicine (SMFM). Electronic address: pubs@smfm.org, Mari G, Norton ME, Stone J, Berghella V, Sciscione AC et al (2015) Society for Maternal-Fetal Medicine (SMFM) Clinical Guideline #8: the fetus at risk for anemia–diagnosis and management. Am J Obstet Gynecol 212(6):697–710
Szpinda M (2008) Length growth of the various aortic segments in human foetuses. Folia Morphol (Warsz) 67(4):245–250
Szpinda M, Szpinda A, Wozniak A, Daroszewski M, Mila-Kierzenkowska C (2012) The normal growth of the common iliac arteries in human fetuses—an anatomical, digital and statistical study. Med Sci Monit 18(3):BR109–BR116
Struijk PC, Migchels H, Mathews JV, Stewart PA, Clark EB, de Korte CL et al (2013) Fetal aortic distensibility, compliance and pulse pressure assessment during the second half of pregnancy. Ultrasound Med Biol 39(11):1966–1975
Mari G, Deter RL, Carpenter RL, Rahman F, Zimmerman R, Moise KJ Jr et al (2000) Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med 342(1):9–14
Kurmanavicius J, Streicher A, Wright EM, Wisser J, Muller R, Royston P et al (2001) Reference values of fetal peak systolic blood flow velocity in the middle cerebral artery at 19–40 weeks of gestation. Ultrasound Obstet Gynecol 17(1):50–53
Zimmerman R, Carpenter RJ Jr, Durig P, Mari G (2002) Longitudinal measurement of peak systolic velocity in the fetal middle cerebral artery for monitoring pregnancies complicated by red cell alloimmunisation: a prospective multicentre trial with intention-to-treat. BJOG 109(7):746–752
Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A (2006) Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension 48(4):595–601
Baschat AA (2011) Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet Gynecol 37(5):501–514
Brennand J (2009) Middle cerebral artery Doppler. Australas J Ultrasound Med 12(3):35–38
Cruz-Martinez R, Figueras F (2009) The role of Doppler and placental screening. Best Pract Res Clin Obstet Gynaecol 23(6):845–855
Nicolini U, Talbert DG, Fisk NM, Rodeck CH (1989) Pathophysiology of pressure changes during intrauterine transfusion. Am J Obstet Gynecol 160(5 Pt 1):1139–1145
Crosby WM, Brobmann GF, Chang AC (1970) Intrauterine transfusion and fetal death. Relationship of intraperitoneal pressure to umbilical vein blood flow. Am J Obstet Gynecol 108(1):135–138
Weiner CP, Smith F, Robillard JE (1989) Arginine vasopressin and acute, intravascular volume expansion in the human fetus. Fetal Ther 4(2–3):69–72
Wenzel V, Raab H, Dunser MW (2008) Role of arginine vasopressin in the setting of cardiopulmonary resuscitation. Best Pract Res Clin Anaesthesiol 22(2):287–297
Charlton PH, Mariscal Harana J, Vennin S, Li Y, Chowienczyk P, Alastruey J (2019) Modeling arterial pulse waves in healthy aging: a database for in silico evaluation of hemodynamics and pulse wave indexes. Am J Physiol Heart Circ Physiol 317(5):H1062–H1085
Curtis SL, Zambanini A, Mayet J, Mc GTSA, Foale R, Parker KH et al (2007) Reduced systolic wave generation and increased peripheral wave reflection in chronic heart failure. Am J Physiol Heart Circ Physiol 293(1):H557–H562
Mynard JP, Kondiboyina A, Kowalski R, Cheung MMH, Smolich JJ (2020) Measurement, analysis and interpretation of pressure/flow waves in blood vessels. Front Physiol 11:1085
Hernandez-Andrade E, Figueroa-Diesel H, Kottman C, Illanes S, Arraztoa J, Acosta-Rojas R, Gratacós E (2007) Gestationalage-adjusted reference values for the modified myocardial performance index for evaluation of fetal left cardiac function. Ultrasound Obstet Gynecol 29:321–325
Lobmaier SM, Cruz-Lemini M, Valenzuela-Alcaraz B, Ortiz JU, Martinez JM, Gratacos E et al (2014) Influence of equipment and settings on myocardial performance index repeatability and definition of settings to achieve optimal reproducibility. Ultrasound Obstet Gynecol 43(6):632–639
Mensah-Brown NA, Wakai RT, Cheulkar B, Srinivasan S, Strasburger JF (2010) Assessment of left ventricular pre-ejection period in the fetus using simultaneous magnetocardiography and echocardiography. Fetal Diagn Ther 28(3):167–174
Pozniak MAAP (2013) Clinical Doppler Ultrasound, 3rd edn. Churchill Livingstone, Edinburgh
Yigit B, Pekkan K (2016) Non-dimensional physics of pulsatile cardiovascular networks and energy efficiency. J R Soc Interface 13(114):20151019
Gonser M (2020) Different view on Doppler waveform—signs of pulse wave reflection in mother and (unborn) child (in Germen, engl. Abstract). Gynakologe 53:821–830. https://doi.org/10.1007/s00129-020-04712-1
Gonser M (2019) Hemodynamic relationship between ophthalmic artery and uterine artery in pre-eclampsia: pulse wave reflection and transmission might provide the missing link. Ultrasound Obstet Gynecol 53(1):135–136
Sapantzoglou I, Wright A, Arozena MG, Campos RV, Charakida M, Nicolaides KH (2021) Ophthalmic artery Doppler in combination with other biomarkers in prediction of pre-eclampsia at 19–23 weeks’ gestation. Ultrasound Obstet Gynecol 57(1):75–83
Sarno M, Wright A, Vieira N, Sapantzoglou I, Charakida M, Nicolaides KH (2020) Ophthalmic artery Doppler in prediction of pre-eclampsia at 35–37 weeks’ gestation. Ultrasound Obstet Gynecol 56(5):717–724
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Contributions
LV wrote the manuscript. The initial study outline was designed by MG, NO-K and LV. LV and DB collected data and performed its quality control. LV performed the data analysis. All authors participated in the drafting and/or revising of the manuscript and contributed to its intellectual content. The final version of the manuscript was approved by all authors prior to submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study was conducted in accordance with the approval of the local Ethics Commission (KEK-ZH. Nr.2020-01225).
Consent to participate
Written informed consent was obtained from all study participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vonzun, L., Ochsenbein-Kölble, N., Balsyte, D. et al. Second systolic peak in fetal middle cerebral artery Doppler after intrauterine transfusion. Arch Gynecol Obstet 307, 241–248 (2023). https://doi.org/10.1007/s00404-022-06517-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06517-0