Abstract
Secukinumab is a fully human IgG1 antibody that selectively binds to and neutralizes the proinflammatory cytokine interleukin-17A. Secukinumab is an effective and well-tolerated treatment for plaque psoriasis. There is a limited real-word evidence for dose optimisation of secukinumab based on clinical response. PURE is a multi-national, prospective, observational study in patients with moderate to severe chronic plaque psoriasis in Canada and Latin America, assessing the real-world safety and effectiveness of secukinumab and other indicated therapies. The aim of the current snapshot analysis was to evaluate the effectiveness and safety of on-label dose and updosed secukinumab in patients with plaque psoriasis enrolled in the PURE study. At the time of analysis, 676 patients received secukinumab, of which 84.6% (n = 572) remained on the on-label dose, while 15.4% (n = 104) were updosed. With on-label secukinumab, the absolute Psoriasis Area and Severity Index (PASI) score was reduced from 13.6 at baseline to 1.2 over 36 months, with treatment persistence of 73% at 40 months. At Month 36, 73.2% of the patients receiving on-label secukinumab achieved Investigator’s Global Assessment (IGA) 0/1. With updosed secukinumab (300 mg every 2 weeks, 300 mg every 3 weeks, 450 mg every 4 weeks, or 450 mg every 3 weeks), 57.9% of the patients showed improvement in the absolute PASI score at the first visit after updosing, with treatment persistence of 50% at 12 months after updosing. At Month 15, 40% of patients receiving updosed secukinumab achieved IGA 0/1. Patients with previous biologic exposure (odds ratio [OR]: 3.25; 95% confidence interval [CI]: 2.03, 5.18, p < 0.0001) were more likely to be updosed while those with a body weight < 90 kg (OR: 0.49; 95% CI [0.31, 0.77], p = 0.0019) were less likely to be updosed. Previous biologic exposure (HR [hazard ratio]: 1.47; 95% CI [1.24, 1.75], p < 0.0001) and current biologic exposure (secukinumab vs. other indicated therapies: HR 0.57; 95% CI [0.43, 0.75], p = 0.0001) were significantly associated with time to secukinumab updosing. No new or unexpected safety signals were observed with updosed secukinumab. Secukinumab updosing was efficacious and well-tolerated in patients with psoriasis who failed to respond to the approved on-label regimen, suggesting that updosing may be a useful therapeutic option for approved dose non-responders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis is a chronic, immune-mediated, inflammatory condition with manifestations that can involve the skin, nails, joints and other organ systems. With a worldwide prevalence ranging from 0.6 to 7% of the population, psoriasis affects up to 2–5% of adults in Western countries [1] and is associated with a high degree of morbidity. Biologics are primarily indicated for patients who are candidates for systemic therapy or phototherapy. The four main classes of biologics approved for the treatment of moderate to severe plaque psoriasis include the tumour necrosis factor alpha (TNF-α) inhibitors, interleukin (IL)-12/23 inhibitors, and IL-17 inhibitors [2].
Secukinumab, a fully human monoclonal antibody, selectively neutralises IL-17A, a cornerstone cytokine involved in the pathophysiology of psoriasis. The efficacy and safety of secukinumab in patients with moderate to severe chronic plaque psoriasis has been well-established in pivotal phase 3 clinical trials. These studies have demonstrated long‐lasting effects of secukinumab in treating the complete spectrum of psoriasis (including the scalp, nails, palms and soles), psoriatic arthritis, and ankylosing spondylitis [3,4,5,6]. Some patients may benefit from dose optimisation of the dosing regimen based on their individual responses. In recent years, considerable evidence on the effectiveness and safety of secukinumab has emerged from real-world experience through pharmaceutical company–sponsored or independent registries and post-marketing phase 4 studies [7,8,9]. Nonetheless, there is limited data examining the potential role of updosing secukinumab to optimise the treatment regimen for the desired efficacy outcomes of clear to almost clear skin.
PURE is a multinational, prospective, observational cohort study in patients with moderate to severe chronic plaque psoriasis in Canada and Latin America (Argentina, Brazil, Costa Rica, Guatemala, Mexico, Panama and Dominican Republic), assessing the real-world safety and effectiveness of secukinumab and other indicated therapies (NCT02786186). This report from the PURE registry provides an insight into the clinical outcomes and treatment persistence of secukinumab in patients who were updosed compared with those who were not until 05 June 2019.
Materials and methods
Study design
The PURE registry, an ongoing study, aimed to enroll approximately 2,500 adult patients from 81 community and hospital specialty sites across Canada and Latin America. The study comprises two different patient cohorts, 1250 patients each. At baseline, patients received treatment with either secukinumab (Cohort 1) or other indicated therapies (Cohort 2). Due to the longitudinal and observational nature of the study, treatment switch can occur. The decision to treat in any of these cohorts was reached prior to and independently of recruitment in the study. Symptomatic patients (≥ 18 years) diagnosed with moderate to severe chronic plaque psoriasis by a specialist were included in the study. All treatment decisions, including the need for updosing, were based on the clinical judgment of the treating physician. The study includes a 5-year follow-up at completion, with recommended assessments at enrolment, 3 and 6 months and every 6 months thereafter. At any time, patients were given the option to withdraw consent and discontinue the study. The treating physician could also decide to withdraw a patient from the study on their discretion at any given point. The study enrolment was completed on 31 December 2020. The detailed study design including eligibility criteria of the PURE registry have been previously described [10].
Outcome measures
Baseline demographics and disease characteristics were described for both the on-label and updosed secukinumab-treated patient populations. The treatment outcomes were evaluated for all patients at baseline, month 3, month 6 and every 6 months afterwards as per the study design. For the updosed secukinumab-treated patient population, treatment outcomes were evaluated before updosing and post-updosing (month 3 and every 6 months afterwards). Effectiveness assessment included Psoriasis Area and Severity Index (PASI) and Investigator’s Global Assessment (IGA) scores. Treatment persistence over time was also assessed. Safety was evaluated by measuring the number of total adverse events (AEs), total serious AEs (SAEs) and total severe AEs.
Statistical analysis
Since dose optimization is facilitated in Canada, all updosed patients in this analysis were from Canada. A multiple logistic regression was performed for patients who were updosed by employing baseline demographic and clinical characteristics including age, sex, biologic exposure, weight (≤ 90 kg vs. >90 kg) as well as PASI, IGA, and Dermatology Life Quality Index (DLQI) scores. These variables were also used to analyse the time to secukinumab updosing using a Cox proportional hazard analysis.
The modified intent-to-treat population (mITT) includes all enrolled participants with an assigned cohort at baseline that had at least one post-baseline visit or electronic patient-reported outcome (ePRO) submission and/or a post-baseline AE evaluation. All patients who started on secukinumab at any time point in this study were pooled regardless of their cohort at enrollment. Clinical characteristics of the patients were compared between treatment cohorts using the independent-samples t-test and Chi-square test for continuous and categorical variables, respectively. Data are reported as mean ± standard deviation (SD) or in the form of percentages. For both treatment cohorts, AEs and SAEs were assessed using the total number of events, the number of patients and percentage of patients who experienced at least one event within individual system organ class and within individual preferred term described in Medical Dictionary for Regulatory Activities (MedDRA).
Results
Baseline assessments
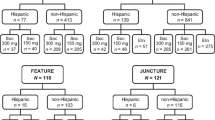
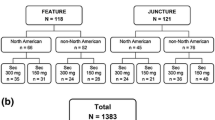
As of 05 June 2019, 1,773 patients (Cohort 1/Cohort 2: 720/1053) from Canada and Latin America were enrolled in the PURE registry, of whom 1,512 were in the mITT population (Cohort1/Cohort2: 635/877). Among the mITT population, 676 patients (Cohort1/Cohort2: 609/67) received secukinumab, where 84.6% (n = 572; Cohort 1/Cohort 2: 517/55) remained on the on-label dose and 15.4% (n = 104; Cohort 1/Cohort 2: 92/12) were updosed. During the observation period, only five (4.8%) of the total updosed patients discontinued the secukinumab treatment.
At baseline, the mean age of the updosed patients was similar to those on the on-label dose, but weight and proportion of patients with previous biologic exposure were both higher in updosed patients (mean [SD] weight, kg: 100.4 [26.1] vs. 90.9 [22.8]; previous biologic exposure: 64.4% vs. 36.0%; Table 1). Most of the updosed patients were administered a secukinumab regimen of 300 mg every 2 weeks (Q2W) (Table 2).
Treatment persistence
The treatment persistence for patients who were on the on-label dose of secukinumab was high (~ 84% at 12 months and 73% at 40 months; Fig. 1A). The median time patients remained on updosed secukinumab was shorter at 12.1 months (Fig. 1B).
Disease severity of patients on the on-label dose at enrolment and up to 36 months of follow-up
Overall, patients who remained on the on-label dose maintained low mean absolute PASI scores over time; those with available 36-month follow-up (n = 39) had a mean absolute PASI (SD) of 1.2 (1.8) (Fig. 2). The median absolute PASI score remained stable over time during the follow-up period up to 36 months, with values below 1 (range: 0.0-7.2). Overall, at 36 months, 73.2% of the patients achieved clear or almost clear skin (IGA 0/1) and 41.5% of the patients achieved clear skin (IGA 0) (Fig. 3).
Time to updosing
The time to updosing after updosed secukinumab initiation in the 104 patients is shown in Fig. 4. The overall mean time was 7 months (range: 1–36 months) (Fig. 4A). Both mean [SD] (9.8 [7.5] vs. 6.5 [5.8] months) and median times (7 [range 1–36] vs. 4 [1–19] months) to updose were slightly longer in patients from Cohort 1 than from Cohort 2 after secukinumab initiation (Fig. 4B).
Disease severity of patients who were updosed at enrolment and up to 30 months of follow-up
PASI score
Patient-level data analysis was performed for patients whose PASI scores were available after updosing. A total of 95 of the104 updosed patients had available PASI score measures. Among these, 89 patients who experienced a change in the PASI score after updosing of secukinumab, 55 of 95 (57.9%) patients benefited from updosing (as defined by improvement of their absolute PASI data after updosing) and experienced a further mean decrease of disease activity by 3.3 points for the absolute PASI score at first visit after updosing. The remaining 34 of 95 (35.8%) patients experienced further disease worsening, with a mean increase of 3.3 points on average for the absolute PASI score at first visit after updosing. Six patients had no change in PASI after updosing and data for nine patients were missing. In patients who were updosed, the mean PASI (SD) score remained stable from 5.0 (3.8; n = 101) at the visit before updosing to 3.8 (4.0; n = 20) at 15 months after updosing. However, of note, the maximum recorded PASI score in updosed patients decreased from 17.0 at the visit before updosing to 9.6 at 20 months after updosing.
IGA score
The mean change in IGA score from the visit before updosing in patients who were updosed remained stable (− 0.9 [1.0]; n = 100) to (− 1.6 [1.1]; n = 20) at 15 months after updosing. Moreover, 40% of the patients achieved IGA 0/1 and 20% of the patients achieved IGA 0 at 15 months after updosing, respectively (Fig. 5).
Factors associated with probability of updosing
Multiple logistic regression analysis, which included baseline demographics, clinical characteristics, and treatment parameters as covariates, revealed that prior biologic exposure and higher weight at baseline were significantly associated with updosing. Patients who were biologic-experienced were more than three times more likely to be updosed with secukinumab (odds ratio [OR]: 3.25, 95% confidence interval [CI] [2.03, 5.18]; p < 0.0001) than patients who were biologic-naive. Patients who had body weight ≤ 90 kg were less than half as likely to be updosed with secukinumab than patients with baseline body weight > 90 kg (OR: 0.49, 95% CI: [0.31, 0.77], p = 0.0019; Fig. 6A).
Factors associated with the time to secukinumab updosing
Cox proportional hazard analysis was conducted to examine factors influencing the time to secukinumab updosing. Previous biologic exposure (hazard ratio [HR]: 1.47, 95% CI [1.24, 1.75]; p < 0.0001) and current biologic exposure (secukinumab [cohort 1] vs. other indicated therapies [cohort 2]: HR 0.57, 95% CI: [0.43, 0.75]; p = 0.0001) were significantly associated with time to secukinumab updosing (Fig. 6B). There was a small but statistically significant negative relation between baseline PASI (estimate − 0.015, p = 0.0129) and DLQI (estimate − 0.0117, p = 0.0499) and the probability of updosing was of marginal significance.
Safety
Overall, secukinumab was well-tolerated and the safety profiles were comparable before and after updosing. There were no AEs leading to hospitalization and/or prolongation of existing hospitalization, and patients did not experience any SAEs after updosing with secukinumab (Table 3). One patient died after updosing due to subarachnoid hemorrhage; this AE was not suspected to be related to the study drug.
Discussion
The study results demonstrate real-world evidence of the effectiveness and safety of secukinumab updosing in patients with moderate to severe chronic plaque psoriasis in Canada. We sought to determine whether patients with an inadequate response to on-label secukinumab could further improve responses when treated with updosed secukinumab (300 mg Q2W, 300 mg every 3 weeks [Q3W], 450 mg every 4 weeks [Q4W], or 450 mg Q3W; based on clinical judgment of the treating physician) compared to when these patients were treated with on-label secukinumab (300 mg Q4W). The 84.6% of secukinumab-treated patients who continued with the on-label dose showed improvements in absolute PASI and IGA scores over a period of 36 months. Among secukinumab-treated patients who were updosed, 57.9% showed improvement in absolute PASI scores over 15 months. Half of the updosed secukinumab-treated patients remained on treatment 12.1 months after being updosed. Interestingly, the baseline PASI for patients who were updosed was slightly lower than patients receiving on-label dose. A previous study corroborates with this observation, where patients receiving high dose of secukinumab (300 mg Q2W) had low mean ± SD baseline PASI compared to patients receiving the on-label dose [11]. In this study, with over 36 months of treatment with the on-label dose, the mean baseline PASI value was reduced from 13.6 to 1.2 and treatment persistence was 73% at Month 40. These findings are similar to that observed in a previous real-world study, SERENA, where administration of secukinumab 300 mg Q4W for 36 months reduced a higher baseline PASI from 21.0 to 1.9 with a treatment persistence of 60.5% [7]. The mean baseline PASI in this study was also lower than the two pivotal phase 3 randomised controlled trials (RCTs) of secukinumab (ERASURE: 22.5; FIXTURE: 23.9) [3]. The reduction in the baseline PASI score at 36 months as reported is in line with that reported in the 3-year data of the ERASURE-FIXTURE extension study [12]. The study also demonstrated that with the on-label dose, consistent improvement in IGA 0/1 was observed from month 3 onwards. This finding is similar to that in the CLEAR study where rapid improvement in IGA 0/1 was observed at around month 3 with the on-label dose [13].
The ONDA study reported that, the efficacy of several biologics such as etanercept, adalimumab, infliximab and ustekinumab improved with dose adjustments in patients with psoriasis and psoriatic arthritis [14]. Studies conducted with SEC for updosing are generally small in scale, involving a limited number of patients. Nonetheless, these studies demonstrated a potential for improved outcomes with secukinumab updosing. The previous real-world studies conducted in Canada demonstrated that 40–46% of patients achieved IGA 0/1 with updosing of secukinumab for ~ 3 months [15, 16]. A retrospective chart review demonstrated that secukinumab updosing (300 mg Q3W, 300 mg Q2W, or 450 mg once weekly [QW]) led to improved clinical response with minimal AEs in 14 patients with moderate to severe plaque psoriasis [16]. The GAIN study also revealed that secukinumab 300 mg Q2W could be beneficial in patients with moderate to severe psoriasis with suboptimal response to 300 mg Q4W (IGA 0/1: 73.0% vs. 64.1%, p < 0.05) [17]. These findings indicate that updosing could be beneficial for patients who demonstrated inadequate treatment response to the approved on-label dose. In this study, 57.9% of the patients who received updosed secukinumab showed an improvement in PASI score with a treatment persistence of 50% at 12.1 months. Moreover, at 15 months, 40% of the patients also achieved IGA 0/1 with updosed secukinumab. Of note, 35.8% of the patients showed further disease worsening (measured by PASI scores) despite updosing of secukinumab. which was also observed in previous study [16].
Compared with the patients who remained on the on-label dosing, updosed patients were slightly heavier and had nearly double the exposure to prior biologics. Moreover, it is shown that higher body weight (≥ 90 kg) is associated with decrease in the mean concentration of secukinumab, impacting the treatment response to secukinumab [18]. Since weight and previous biologic exposure are known to affect the treatment response [19,20,21], these characteristics in updosed patients may explain the inadequate response to the on-label dose. Nonetheless, the updosing improved the response rates in this harder-to-treat population. These findings are in line with previous studies where updosing of secukinumab improved the therapeutic response in heavier patients with prior biologic exposure [22,23,24]. This study also conducted multivariate analyses to identify the factors that could predict the need for secukinumab updosing. The results further confirm previous findings that heavier body weight ≥90 kg and biologic-experience were independent factors significantly associated with updosing [19,20,21]. Patients in Cohort 1 were less likely to be updosed as they were comparatively lighter and had less biologic exposure compared to those in Cohort 2. The OPTIMISE study reported that heavy patients (≥ 90 kg) who did not achieve a PASI 90 response at Week 24 with secukinumab 300 mg Q4W may experience benefit with secukinumab 300 mg Q2W [25].
Secukinumab updosing was well-tolerated in patients with moderate to severe chronic plaque psoriasis. The safety findings of this study are in line with those reported in secukinumab phase 3 RCTs [6,7,8,9, 12] and there were no new or unexpected AEs. Moreover, these safety finding are consistent with previous studies evaluating the safety profile of higher doses of secukinumab [17, 25].
In August 2022, the Canadian product monograph of secukinumab was revised. It now provides an option for dose optimisation from 300 mg Q4W to 300 mg Q2W in adult patients with plaque psoriasis with a body weight ≥ 90 kg [26]. We would like to highlight that our results in patients with plaque psoriasis from Canada, which were collected approximately 3 years prior to the aforementioned date for the product monograph revision, is aligned with the dose adjustments suggested by the Canadian product monograph.
A major limitation of the study is the low number of patients who were updosed. As an interim analysis, the number of patients with evaluable data at the time of data cut-off was low. In addition, we report pooled effectiveness and safety data of the updosed secukinumab whereas the effectiveness and safety profile for each individual updosing regimen (i.e., 300 mg Q2W and Q3W; 450 mg Q3W and Q4W) is not available. However, as the PURE registry is still ongoing, further data will be collected to assess effectiveness and safety profile of updosed secukinumab.
Conclusion
This analysis from the PURE registry provides evidence that updosing of secukinumab can be beneficial and well tolerated in patients with moderate to severe chronic plaque psoriasis who do not respond adequately to the approved on-label dose. No safety concerns were identified during this analysis.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data provided are de-identified/anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data may be requested from the corresponding author of the manuscript.
References
Parisi R, Iskandar IY, Kontopantelis E, Augustin M, Griffiths CE, Ashcroft DM (2020) National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. ;369
Sbidian E, Chaimani A, Guelimi R, Garcia-Doval I, Hua C, Hughes C, Naldi L, Kinberger M, Afach S, Le Cleach L (2023) Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 7(7):CD011535
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E (2014) Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med 371(4):326–338
Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, Deodhar A, Porter B, Martin R, Andersson M, Mpofu S (2015) Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 373(26):2534–2548
Armstrong AW, Vender R, Kircik L (2016) Secukinumab in the treatment of palmoplantar, nail, scalp, and pustular psoriasis. J Clin Aesthetic Dermatol 9(6 Suppl 1):S12
Bissonnette R, Luger T, Thaçi D, Toth D, Messina I, You R, Guana A, Fox T, Papavassilis C, Gilloteau I, Mrowietz U (2017) Secukinumab sustains good efficacy and favourable safety in moderate-to‐severe psoriasis after up to 3 years of treatment: results from a double‐blind extension study. Br J Dermatol 177(4):1033–1042
Augustin M, Sator PG, von Kiedrowski R, Conrad C, Rigopoulos D, Romanelli M, Ghislain PD, Torres T, Ioannides D, Aassi M, Schulz B (2022) Secukinumab demonstrated sustained retention, effectiveness and safety in a real-world setting in patients with moderate‐to‐severe plaque psoriasis: long‐term results from an interim analysis of the SERENA study. J Eur Acad Dermatol Venereol 36(10):1796–1804
Yiu ZZ, Becher G, Kirby B, Laws P, Reynolds NJ, Smith CH, Warren RB, Griffiths CE, Browne F, Evans I, Kleyn E (2022) Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatology 158(10):1131–1141
Strober BE, Germino R, Guana A, Greenberg JD, Litman HJ, Guo N, Lebwohl M (2019) US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatological Treat 31(4):333–341
Papp KA, Gooderham M, Beecker J, Lynde CW, Delorme I, Dei-Cas I, Albrecht L, Rampakakis E, Sampalis JS, Vieira A, Hussein S (2019) Rationale, objectives and design of PURE, a prospective registry of patients with moderate to severe chronic plaque psoriasis in Canada and Latin America. BMC Dermatol 19:1–7
Augustin M, Reich K, Yamauchi P, Pinter A, Bagel J, Dahale S, You R, Bruin G, Djimopoulos J, Paguet B, Charef P (2022) Secukinumab dosing every 2 weeks demonstrated superior efficacy compared with dosing every 4 weeks in patients with psoriasis weighing 90 kg or more: results of a randomized controlled trial. Br J Dermatol 186(6):942–954
Langley RG, Sofen H, Dei-Cas I, Reich K, Sigurgeirsson B, Warren RB, Paul C, Szepietowski JC, Tsai TF, Hampele I, You R (2023) Secukinumab long-term efficacy and safety in psoriasis through to year 5 of treatment: results of a randomized extension of the phase III ERASURE and FIXTURE trials. Br J Dermatol 188(2):198–207
Thaçi D, Puig L, Reich K, Tsai TF, Tyring S, Kingo K, Ziv M, Pinter A, Vender R, Lacombe A, Xia S (2019) Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol 81(6):1405–1409
Esposito M, Gisondi P, Conti A, Giunta A, Del Giglio M, Di Mercurio M, Veneziano L, Ferrucci G, Bianchi L, Chimenti S, Girolomoni G (2017) Dose adjustment of biologic therapies for psoriasis in dermatological practice: a retrospective study. J Eur Acad Dermatol Venereol 31(5):863–869
Phung M, Georgakopoulos JR, Ighani A, Giroux L, Yeung J (2018) Secukinumab dose optimization in adult psoriasis patients: a retrospective, multicenter case series. JAAD Case Rep 4(4):310–313
Phung M, Ighani A, Georgakopoulos JR, Vender R, Giroux L, Lansang P, Yeung J (2019) Off-label high-dose secukinumab for the treatment of moderate-to-severe psoriasis. J Cutan Med Surg 23(4):391–393
Reich K, Körber A, Mrowietz U, Sticherling M, Sieder C, Früh J, Bachhuber T (2021) Secukinumab 2-weekly vs. 4‐weekly dosing in patients with plaque‐type psoriasis: results from the randomized GAIN study. Br J Dermatol 184(5):849–856
Lee JE, Wang J, Florian J, Wang YM, Kettl D, Marcus K, Woitach A (2019) Effect of body weight on risk-benefit and dosing regimen recommendation of secukinumab for the treatment of moderate to severe plaque psoriasis. Clin Pharmacol Ther 106(1):78–80
Ger TY, Huang YH, Hui RC, Tsai TF, Chiu HY (2019) Effectiveness and safety of secukinumab for psoriasis in real-world practice: analysis of subgroups stratified by prior biologic failure or reimbursement. Therapeutic Adv Chronic Disease 10:2040622319843756
Kisielnicka A, Szczerkowska-Dobosz A, Nowicki R (2020) The influence of body weight of patients with chronic plaque psoriasis on biological treatment response. Adv Dermatology Allergology/Postępy Dermatologii i Alergologii 37(2):168–173
Pirro F, Caldarola G, Chiricozzi A, Burlando M, Mariani M, Parodi A, Peris K, De Simone C (2021) Impact of body mass index on the efficacy of biological therapies in patients with psoriasis: a real-world study. Clin Drug Investig 41:917–925
Thaci D, Humeniuk J, Frambach Y, Bissonnette R, Goodman JJ, Shevade S, Gong Y, Papavassilis C, STATURE Study Group (2015) Secukinumab in psoriasis: randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE). Br J Dermatol 173(3):777–787
Beecker J, Joo J (2018) Treatment of moderate to severe psoriasis with high-dose (450-mg) secukinumab: case reports of off-label use. J Cutan Med Surg 22(1):86–88
Yıldırım FE, Hapa FA (2022) Clinical efficacy and safety of secukinumab for psoriasis in a real-world setting in Turkey. J Dermatological Treat 33(3):1531–1537
Reich K, Puig L, Szepietowski JC, Paul C, Lacour JP, Tsianakas A, Sieder C, Rissler M, Pournara E, Orsenigo R (2020) Secukinumab dosing optimization in patients with moderate-to‐severe plaque psoriasis: results from the randomized, open‐label OPTIMISE study. Br J Dermatol 182(2):304–315
Cosentyx product monograph (2022) https://pdf.hres.ca/dpd_pm/00067634.PDF Accessed 23 March 2023
Acknowledgements
We thank Syreon Corporation, Canada for providing operational management/data management and statistical analysis services/other, which was paid for by Novartis Pharmaceuticals Canada. The authors thank Nivedita Jangale, PhD and Haroon Mohammad, PhD (Novartis Healthcare Pvt. Ltd., Hyderabad) for providing medical writing support, which was funded by Novartis Pharmaceuticals AG, in accordance with the Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
Funding
This study was funded by Novartis Pharmaceuticals AG and led by Novartis Pharmaceuticals Canada.
Author information
Authors and Affiliations
Contributions
KAP, MG, CL, DB, FAlM, VHP, ID, LB, RH, MSA, JB, SS, and RGL contributed to material preparation and data collection. AV and LR contributed to study development plans including data analysis, statistical plan. MSF and MM contributed to data analysis and discussions. Data analysis was performed by Syreon Corporation, Canada. All authors provided critical feedback on the manuscript, approved the final manuscript for submission and are accountable for the accuracy and integrity of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All patients provided informed consent to participate in the study. The study design was reviewed and approved by the ethics committees or institutional review boards, and the study was conducted in accordance with the principle of the Declaration of Helsinki.
Competing interests
Kim A. Papp serves as a consultant for AbbVie, Akros, Amgen, Arcutis, Atellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Can-Fite Biopharma, Coherus, Dermavant, Dermira, Dow Pharma, Eli Lilly and Company, Evelo, Galapagos, Galderma, Genentech, Incyte, Janssen, Kyowa Hakko Kirin, Leo Pharma A/S, Merck (MSD), Merck Serono, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Takeda and UCB; received research grants from Anacor, Gilead, GSK, MedImmune, Moberg Pharma and Sun Pharmaceuticals; is a scientific officer for Akros, Anacor, Arcutis, Dice Pharmaceuticals and Kyowa Hakko Kirin; is a consultant for Dice Pharmaceuticals, Meiji Seika Pharma and Mitsubishi Pharma; is a speaker, received honoraria and participated in steering committees and advisory boards for AbbVie, Amgen, Bausch Health/Valeant, Celgene, Eli Lilly and Company, Janssen, Merck (MSD), Novartis, Pfizer and Sanofi-Aventis/Genzyme. Melinda Gooderham serves as a speaker, an investigator or an advisory board member for AbbVie, Amgen, Akros, Arcutis, Arena, Aslan, AnaptysBio, Apogee, Aristea, Bausch, Boehringer Ingelheim, BMS, Celgene, Coherus, Dermira, Dermavant, Eli Lilly, Galderma, GSK, Incyte, Janssen, Kyowa Kirin, LEO Pharma, MedImmune, Merck, Meiji, Moonlake, Nimbus, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharmaceuticals, Takeda, Tarsus, Union, UCB and Ventyx. Charles Lynde serves as a speaker or consultant or advisor or investigator or received honoraria from AbbVie, Altius, Amgen, Aralez, Arcutis, Bausch Health, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cipher, Dermavant, Devonian, Eli Lilly, Fresnius Kabi, Galderma, GSK, Innovaderm, Intega Skin, Janssen, Kyowa Kirin, La Roche Posay, LEO Pharma, L’Oreal, Medexus, MedX, Merck, Novartis, P&G, Pediapharm, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sandoz, Sentrex, TEVA, Tribute, UCB, Valeant, Viatris, Volo Health. Danielle Brassard is a speaker and receives honoraria from AbbVie, Actelion, Bausch Health, Galderma, Janssen, Leo Pharma, Novartis, Pfizer and Sanofi; participates as a consultant for AbbVie, Amgen, Bausch Health, Celgene, Novartis and Pfizer; and acts as principal investigator for AbbVie, BMS, Bausch Health, Incyte, Leo Pharma, Novartis, Pfizer, Reistone and Sanofi. Faisal Al-Mohammedi is an investigator for Novartis, Jansen, Bausch, Abbvie and Incyte Pharma.Vimal H. Prajapati is an investigator for AbbVie, Amgen, AnaptysBio, Arcutis, Arena, Asana, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Concert, CorEvitas, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, Nimbus Lakshmi, Novartis, Pfizer, RAPT Therapeutics, Regeneron, Reistone, Sanofi Genzyme, Sun Pharma, Takeda, UCB Pharma, and Valeant; has served as a consultant, advisor and/or speaker for AbbVie, Actelion, Amgen, Apogee Therapeutics, Aralez, Arcutis, Aspen, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cipher, CorEvitas, Eli Lilly, Galderma, GlaxoSmithKline, Homeocan, Incyte, Janssen, LEO Pharma, L’Oreal, Medexus, Novartis, Pediapharm, Pfizer, Sanofi Genzyme, Sun Pharma, Tribute, UCB Pharma, and Valeant; received grants from AbbVie, Bausch Health, Celgene, Janssen, LEO Pharma, Novartis, and Sanofi Genzyme. Antonio Vieira and is a full-time employee at Novartis Pharmaceuticals Canada Inc. and Lenka Rihakova was a full-time employee at Novartis Pharmaceuticals Canada Inc. until final draft of this manuscript. Isabelle Delorme serves as a speaker or consultant, or received honoraria from Abbvie, Bausch Health, Amgen, Eli Lilly, Novartis, and Janssen. Lorne Albrecht serves as a speaker or consultant or advisor or investigator or received honoraria/grants from AbbVie; Amgen; Arcutis; Bausch Health; Bristol-Myers-Squibb; Celgene; Eli Lilly; Galderma; Janssen; LEO Pharma; Novartis; Pfizer; Sanofi; UCB. Richard Haydey has been a consultant and advisor and/or received speaking fees and/or grants and/or served as an investigator in clinical trials for the following companies: AbbVie, Amgen, Galderma, GSK, Leo, Lilly, Novartis, Merck, Pfizer, UCB and consulting fees from AbbVie, Amgen, Janssen, Galderma, GSK, Leo, Lilly, Novartis, Pfizer, Bausch-Health, Celgene and UCB. Maryam Shayesteh Alam have no conflict of interest. Jennifer Beecker serves as a consultant for AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Sun Pharma, and UCB; is a speaker for AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Sun Pharma, and UCB; and received research grants from AbbVie, Amgen, Concert, Evelo, Incyte, Janssen, Leo Pharma, Novartis, Janssen, Leo Pharma, Novartis. Sanjay Siddha serves as an investigator for AbbVie, Amgen, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Janssen, LEO Pharma, has served as a consultant, advisor and/or speaker for AbbVie, Actelion, Amgen, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, LEO Pharma, L’Oreal, Novartis, Pediapharm, Pfizer, Sanofi Genzyme, Sun Pharma, Tribute, UCB Pharma, and Valeant; received grants from Amgen, Astellas. Marie Maguin, Mahmoud S. Farag and Antonio Vierra are a full-time employee at Novartis Pharmaceuticals Canada Inc. Lenka Rihakova was a full-time employee at Novartis Pharmaceuticals Canada Inc. until the initial draft development of this manuscript. Richard Langley serves as an investigator, advisory board member, or speaker for AbbVie, Amgen, Bausch, Boehringer Ingelheim, BMS, Celgene, Eli Lilly, Galderma, GSK, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharmaceuticals, and UCB.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Papp, K.A., Gooderham, M., Lynde, C. et al. Effectiveness and safety of secukinumab updosing in patients with moderate to severe plaque psoriasis: data from the PURE registry. Arch Dermatol Res 316, 362 (2024). https://doi.org/10.1007/s00403-024-03122-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-024-03122-w