Abstract
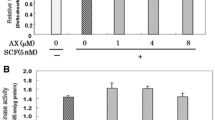
We have already reported that glucosamine (GlcN) distinctly abrogates the pigmentation of human epidermal equivalents stimulated by stem cell factor + endothelin-1 (SE). In this study, we characterized the molecular mechanism involved in the anti-melanogenic effects of GlcN using normal human melanocytes (NHMs) in culture. The SE-stimulated gene (12 h) and protein (24 h) expression levels of melanocyte-specific proteins (at the indicated times post-stimulation) were significantly abrogated by pretreatment with GlcN for 72 h. Western blotting analysis of the phosphorylation of intracellular signaling molecules in the MAPK pathway revealed that despite the significantly decreased level of total CREB protein at all times post-stimulation, the SE-stimulated phosphorylation of ERK, CREB and MITF is not attenuated at 15 min post-stimulation in GlcN-treated NHMs. However, the SE-stimulated protein expression level of total MITF at 2 and 6 h post-stimulation was significantly abrogated by 72 h pretreatment with GlcN. Consistently, pretreatment with GlcN for 72 h abrogated the stimulated gene and protein expression levels of MITF at 1 h and 2 h post-stimulation, respectively. Analysis of gene and protein expression levels also demonstrated that pretreatment with GlcN for 72 h significantly reduced the protein levels of CREB and MITF without affecting their gene expression levels prior to the SE stimulation. Silencing with a CREB siRNA distinctly abrogated the SE-stimulated expression of MITF (at 2 h post-stimulation) and melanocyte-specific proteins (at 24 h post-stimulation). Similarly, transfection of MITF siRNA markedly abrogated the SE-stimulated expression of MITF protein and melanocyte-specific proteins at 2 and 24 h post-stimulation, respectively. Finally, the decreased levels of CREB and MITF proteins induced by 72 h pretreatment with GlcN were abrogated by the co-addition of the proteosomal degradation inhibitor MG132. These findings suggest that the anti-melanogenic effect elicited by GlcN is mediated via the decreased expression of MITF which results from the attenuated transcriptional activity of CREB due to proteolytic degradation.









Similar content being viewed by others
Abbreviations
- GlcN:
-
Glucosamine
- EDN:
-
Endothelin
- SCF:
-
Stem cell factor
- MITF:
-
Microphthalmia-associated transcription factor
- CREB:
-
Cyclic AMP responsive element binding protein
- NHMs:
-
Normal human melanocytes
- TYR:
-
Tyrosinase
- TRP1:
-
Tyrosinase-related protein-1
- TRP2:
-
Tyrosinase-related protein-2 (or dopachrome tautomerase)
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- HEEs:
-
Human epidermal equivalents
References
Koch HU, Schwarz RT, Scholtissek C (1979) Glucosamine itself mediates reversible inhibition of protein glycosylation. A study of glucosamine metabolism at inhibitory concentrations in influenza-virus-infected cells. Eur J Biochem 94(2):515–522
Nakamura K, Compans RW (1978) Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology 84(2):303–319
Suh HN, Lee YJ, Kim MO, Ryu JM, Han HJ (2014) Glucosamine-induced Sp1 O-GlcNAcylation ameliorates hypoxia-induced SGLT dysfunction in primary cultured renal proximal tubule cells. J Cell Physiol 229(10):1557–1568
Imokawa G, Mishima Y (1982) Loss of melanogenic properties in tyrosinases induced by glucosylation inhibitors within malignant melanoma cells. Cancer Res 42(5):1994–2002
Imokawa G, Mishima Y (1984) Functional analysis of tyrosinase isozymes of cultured malignant melanoma cells during the recovery period following interrupted melanogenesis induced by glycosylation inhibitors. J Invest Dermatol 83(3):196–201
Imokawa G, Mishima Y (1985) Analysis of tyrosinases as asparagin-linked oligosaccharides by concanavalin A lectin chromatography: appearance of new segment of tyrosinases in melanoma cells following interrupted melanogenesis induced by glycosylation inhibitors. J Invest Dermatol 85(2):165–168
Imokawa G, Mishima Y (1986) Importance of glycoproteins in the initiation of melanogenesis: an electron microscopic study of B-16 melanoma cells after release from inhibition of glycosylation. J Invest Dermatol 87(3):319–325
Slominski A, Tobin DJ, Shibahara S, Wortsman J (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84(4):1155–1228
Imokawa G, Ishida K (2014) Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: perspective of anti-pigmenting agents. Int J Mol Sci 15(5):8293–8315
Slominski A, Zmijewski MA, Pawelek J (2012) L-tyrosine and L-dihyroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res 25(1):14–27
Wakabayashi Y, Nakajima H, Imokawa G (2013) Abrogating effect of N-linked carbohydrate modifiers on the stem cell factor and endothelin-1-stimulated epidermal pigmentation in human epidermal equivalents. J Dermatol Sci 69(3):215–228
Bentley NJ, Eisen T, Goding CR (1994) Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol 14(12):7996–8006
Bertolotto C, Buscà R, Abbe P, Bille K, Aberdam E, Ortonne JP, Ballotti R (1998) Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol Cell Biol 18(2):694–702
Du J, Miller AJ, Widlund HR, Horstmann MA, Ramaswamy S, Fisher DE (2003) MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol 163(1):333–343
Sato-Jin K, Nishimura EK, Akasaka E, Huber W, Nakano H, Miller A, Du J, Wu M, Hanada K, Sawamura D, Fisher DE, Imokawa G (2008) Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J 22(4):1155–1168
Nakajima H, Wakabayashi Y, Wakamatsu K, Imokawa G (2011) An extract of Melia toosendan attenuates endothelin-1-stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes. Arch Dermatol Res 303(4):263–276
Nakajima H, Fukazawa K, Wakabayashi Y, Wakamatsu K, Imokawa G (2012) Withania somnifera extract attenuates stem cell factor-stimulated pigmentation in human epidermal equivalents through interruption of ERK phosphorylation within melanocytes. J Nat Med 66(3):435–446
Nakajima H, Fukazawa K, Wakabayashi Y, Wakamatsu K, Senda K, Imokawa G (2012) Abrogating effect of a xanthophyll carotenoid astaxanthin on the stem cell factor-induced stimulation of human epidermal pigmentation. Arch Dermatol Res 304(10):803–816
Mizutani Y, Hayashi N, Kawashima M, Imokawa G (2010) A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch Dermatol Res 302(4):283–294
Newton RA, Cook AL, Roberts DW, Leonard JH, Sturm RA (2007) Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J Invest Dermatol 127(9):2216–2227
Imokawa G, Kobayasi T, Miyagishi M (2000) Intracellular signaling mechanisms leading to synergistic effects of endothelin-1 and stem cell factor on proliferation of cultured human melanocytes. Cross-talk via trans-activation of the tyrosine kinase c-kit receptor. J Biol Chem 275(43):33321–33328
Potterf SB, Furumura M, Dunn KJ, Arnheiter H, Pavan WJ (2000) Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum Genet 107(1):1–6
Wan P, Hu Y, He L (2011) Regulation of melanocyte pivotal transcription factor MITF by some other transcription factors. Mol Cell Biochem 354(1–2):241–246
Mascarenhas JB, Littlejohn EL, Wolsky RJ, Young KP, Nelson M, Salgia R, Lang D (2010) PAX3 and SOX10 activate MET receptor expression in melanoma. Pigment Cell Melanoma Res 23(2):225–237
Guan D, Wang H, Li VE, Xu Y, Yang M, Shen Z (2009) N-glycosylation of ATF6beta is essential for its proteolytic cleavage and transcriptional repressor function to ATF6alpha. J Cell Biochem 108(4):825–831
Azuma Y, Miura K, Higai K, Matsumoto K (2007) Protein O-N-acetylglucosaminylation modulates promoter activities of cyclic AMP response element and activator protein 1 and enhances E-selectin expression on HuH-7 human hepatoma cells. Biol Pharm Bull 30(12):2284–2289
Guinez C, Mir AM, Dehennaut V, Cacan R, Harduin-Lepers A, Michalski JC, Lefebvre T (2008) Protein ubiquitination is modulated by O-GlcNAc glycosylation. FASEB J 22(8):2901–2911
Han I, Kudlow JE (1997) Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol 17(5):2550–2558
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niwano, T., Terazawa, S., Sato, Y. et al. Glucosamine abrogates the stem cell factor + endothelin-1-induced stimulation of melanogenesis via a deficiency in MITF expression due to the proteolytic degradation of CREB in human melanocytes. Arch Dermatol Res 310, 625–637 (2018). https://doi.org/10.1007/s00403-018-1850-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-018-1850-8