Abstract
Introduction
Distal radius fracture (DRF) is one of the three most common fractures of the human body with increasing incidences in all groups of age. Known causes of increasing incidence, such as ageing of the population or increased obesity, have been described and discussed. So far, literature reports ambivalent effects of body mass index (BMI) on bone physiology. It is worthwhile to examine the influence of BMI on the outcome of fractures more detailed. This study aims to investigate the influence of an abnormal BMI on fracture severity and treatment, as well as clinical, radiological, and functional outcome to improve clinical decision making.
Materials and methods
A retrospective observational study was conducted on data obtained from patients, who underwent open reduction and internal fixation (ORIF) of a DRF at a local Level 1 Trauma Center between May 2018 and October 2021. Follow-up examinations were performed approximately 1 year after surgical fracture treatment, during which various questionnaires and functional measurements (CMS, DASH, NRS, ROM) were applied. In addition, postoperative complications were recorded and radiological examinations of the affected hand were performed. After excluding incomplete data sets and applying set exclusion criteria, the complete data of 105 patients were analyzed.
Results
74 patients were female and 31 male with significant difference in mean BMI [p = 0.002; female: 23.8 (SD ± 3.3), men: 26.2 (SD ± 3.9)]. Patients with higher BMI had significantly more severe fractures (p = 0.042). However, there was no significant difference in surgery time for fracture management. At follow-up, patients with lower BMI showed a smaller difference in hand strength between the fractured and the other hand (p = 0.017). The BMI had no significant effect on the clinical and radiological outcome.
Conclusion
Despite the ambivalent effects of BMI on the skeletal system, our findings indicate that a higher BMI is associated with more severe DRF. Thereby BMI does not correlate with surgery time for fracture treatment. Furthermore, no evidence of an influence on the clinical and radiological outcome could be detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distal radius fracture (DRF) occurs in Germany with an incidence of 106 per 100,000 per year, making it one of the three most common fractures of the human musculoskeletal system. The treatment is an established part of everyday clinical practice in trauma surgery [1]. Two peaks in prevalence can be identified. One around the age of 10 and one at older ages around the age of 60 [2]. However, the incidence of DRF appears to be increasing in all groups of age. Different causes for this development are discussed [3]. It is known that environmental influences as adverse weather conditions and patient-related factors such as age, gender, and lifestyle have an impact on the incidence of DRF [4, 5]. Women are significantly more affected [1]. In this regard, lifestyle has a crucial influence on patients’ body weight. Both overweight and underweight are not only a risk factor for cardiometabolic diseases, but also significantly increase the risk for musculoskeletal diseases such as osteoporosis [6, 7].
The body mass index (BMI) has gained global acceptance as a valuable tool for evaluating and assessing underweight and overweight individuals. The classification of BMI for adults introduced by the World Health Organization (WHO) in 1995 is generally used in daily clinical practice. Simplified, the following categories are distinguished: BMI ≥ 30 kg/m2 obese, BMI > 25 kg/m2 overweight, 24.9–18.5 kg/m2 normal weight, < 18.5 kg/m2 underweight [8]. The influence of BMI on the risk of fracture is complex. It differs depending on the skeletal region and the associated bone mineral density. While an increased risk of hip and humerus fractures has been described for low BMI, a reduced risk has been described for distal forearm fractures, osteoporotic fractures and tibia and fibula fractures. An increased BMI was associated with an increased risk of humerus fractures and osteoporotic fractures [9]. With an increased BMI, in addition to putative benefits such as a protective soft tissue mantle or increased bone strength, greater mechanical stress on bone have been reported [10]. Underweight or lowered BMI is associated with soft tissue loss, muscle weakness (increased risk of falls), and often malnutrition [7]. As a result, altered bone structure and decreased bone mass or strength, which occurs in both overweight and underweight people, may lead to an increased risk of fracture [11].
Surgical treatment of DRF is an established procedure [12]. Several plate designs are available for surgical therapy. It is important to investigate whether BMI has an influence on the outcome or on the time of surgery. Recent studies have shown, that an elevated BMI leads to an elongated time of surgery. This has implications for cost and efficiency in the operating theatre [13]. In addition, longer operating times can increase morbidity and the risk of infection.
In summary, the purpose of this study is to investigate the influence of an abnormal BMI on fracture severity and surgery time in DRF using a retrospective cohort. All patients in this study received clinical and radiological re-examination after a 1-year follow-up and were rated using patient reported outcome measures (PROMs). The aim is to capture a comprehensive assessment of the impact of abnormal BMI on fracture entity, surgical challenges and healing process of a DRF.
Materials and methods
Ethics committee (Ethikkommission der Hamburger Ärztekammer) approval was given for retrospective registration (Reference number: WF-114/20). We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Study cohort
A retrospective observational study was conducted of all patient data from the local Level 1 Trauma Center who underwent ORIF of a DRF with locking plates between May 2018 and October 2021. Patients were excluded if they were under 18 years of age, had an open fracture, or previous surgery on the affected hand. Polytrauma patients were also excluded. All patients were examined one year after surgical treatment of the DRF. The distribution of dorsopalmar plate osteosynthesis were balanced in both groups and did not show a significant difference. All patients included in the study received the same standardized postoperative therapy regimen: wearing a self-removable wrist orthosis for 6 weeks after the operation, immediate start of physiotherapy—extension/flexion without strain and avoiding pronation/supination and weight-bearing activities. After 6 weeks, the wrist can be moved freely. Thus, our cohort consists of 105 patients with 108 DRFs who remained after application of the exclusion criteria and with complete adherence to the follow-up examinations (Fig. 1).
Patient-specific data
At time of diagnosis, demographic data (age, sex, height, weight), as well as fracture-related data (handedness, fracture side, concomitant wrist injuries and preoperative imaging) were obtained for each patient. Furthermore, the AO classification, including all subtypes, was determined and documented by the treating specialist and corrected by a senior surgeon. The BMI was calculated from the patient’s height and body weight. For better comparability of the results, the cohort was divided into two subgroups: BMI < 25: “no overweight” and BMI ≥ 25: “overweight”.
Clinical and patient-reported outcome measures
At 1-year follow-up, clinical outcome was evaluated after fracture healing was complete. For this purpose, a clinical examination with measurement of range of motion (ROM) was performed. In addition, strength was measured on both hands using a hydraulic hand dynamometer (kg, Sammons Preston, model: Jamar®). Patients were additionally asked to complete the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire. It is used to subjectively assess functional disability in daily life [14, 15]. Patients were also asked to complete the Numerical Rating Scale (NRS). It was used to assess pain at rest and on exertion. Complications and postoperative sequelae were recorded, as well as the duration of surgery (incision—suture time). Finally, radiographic examination of the affected hand was performed at the local radiology department.
Radiologic assessment
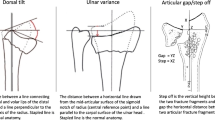
Two-plane radiographs were obtained immediately after surgical fracture treatment and at 1-year follow-up. The following measurements were obtained under the supervision of an attending physician: Radial height (mm), radial tilt (°), palmar tilt (°), ulnar variance (mm).
The 1-year radiograph was also used to assess complete consolidation of the fracture. The palmar plate position was evaluated using the Soong classification (Soong 0: dorsal to the watershed, Soong 1: volar to the watershed but proximal to the margin, Soong 2: volar to the watershed and on or distal to the volar margin) [16]. Furthermore, acceptable osteosynthesis was defined as ≤ 10° dorsal inclination, ≥ 15° radial inclination, < 2 mm ulnar variance, < 2 mm joint incongruence [17].
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD), while categorical variables are expressed as number and percentage The Shapiro–Wilk test was performed on all continuous variables to determine whether they were normally distributed. Based on the findings, either a parametric or non-parametric test was applied. To compare patients with an BMI < 25 and ≥ 25 in relation to continuous variables, Student’s t-test for independent samples was used for normally distributed data. For non-normally distributed data, the Mann–Whitney U test and for categorical variables, the Chi2 tests was applied. In order to compare the three AO classification subgroups, the one-way ANOVA for independent samples was conducted. SPSS statistical program 29.0 (SPSS, Chicago, IL) was used for all statistical analyses. The significance level was set at 0.05 for all statistical analyses. Exact p-values are reported unless p < 0.001.
Results
Demographics
The mean age within the cohort was approximately 59 years (± 16.1 years), with 74 (70.5%) patients being female and 31 (29.5%) patients being male. The majority of patients were right-handed and 6.7% were left-handed. 58% of patients had a BMI of < 25 (“no overweight”) and 42% had a BMI of ≥ 25 (“overweight”) (p = 0.083). Females having a mean BMI of 23.8 (± 3.3) and males of 26.2 (± 3.9) (p = 0.002). In addition to preoperative radiography, 78.7% of patients underwent computed tomography (CT), 4.6% underwent magnetic resonance imaging (MRI), and 2.8% patients underwent both CT and MRI. A detailed summary of the demographic data can be found in Table 1. Concomitant injuries were identified in approximately 52% of patients (Supp. Tab. 1).
Fracture severity
According to the international AO classification, 15.7% of our cohort had a type A fracture, 3.7% had a type B fracture, and 80.6% had a type C fracture. The type A fracture group showed a mean BMI of 22.7 (± 2.1), type B fractures of 23.6 (± 3), and type C fractures of 25 (± 3.8) (Fig. 2). There was a significant difference in BMI between these three AO subgroups (p = 0.042). Accordingly, fracture severity increases with higher BMI. However, patients with a higher BMI did not show significantly more concomitant bony injuries than patients with a low BMI (p = 0.714).
Surgery time
The average incision—suture time was 62.7 min (± 26.4 min; minimum: 20 min, maximum: 176 min). No significant difference regarding the duration of the operation was found between the two BMI subgroups (Fig. 3). Furthermore, there was no significant difference (p = 0.497) in incision—suture time for the treatment of a type A (55.6 ± 19.7 min), type B (45.8 ± 12.3 min), or type C fracture (64.8 ± 27.6 min).
Clinical and patient-reported outcome measures
The follow-up examinations of all 105 patients took place after a mean of 18.2 months and a standard deviation of 7 months. No significant differences were found between the two BMI subgroups with respect to CMS testing, DASH score, NR scale pain, or foreign body sensation (Tab. 2). However, patients with a higher DASH score reported significantly more severe pain on the NRS (r = 0.279, p = 0.004).
Hand strength showed a significant difference with a mean of 21.5 kg (± 9.97 kg) for the surgically treated hands and 25.4 kg (± 9.5 kg) for the unaffected hands, respectively (p < 0.001). Patients with higher DASH scores also had significantly lower hand strength in the operated hand (p < 0.001) compared to the unaffected hand. When comparing the difference in hand strength between the operated and unaffected hand in the BMI subgroups, the difference in hand strength was significantly less in the "no overweight" BMI subgroup (2.5 kg ± 4.8 kg) compared to the "overweight" subgroup (5.6 kg ± 7.0 kg) (p = 0.017). 24.1% (n = 26/108) of all operated hands showed peri- or postoperative complications. They manifested as tendon or nerve injury, revision surgery due to inadequate reduction or implant malpositioning, complex regional pain syndrome (CRPS), carpal tunnel syndrome, digitus saltans, and vascular injury. There was no significant difference in the complication rate between the BMI groups (“no overweight”: 15.7% and “overweight”: 8.3%). We found no significant difference in the ROM of the operated and non-operated hand of the BMI subgroups. The differences in each parameter between the operated and unoperated wrist in active and passive joint positions are shown in Table 2.
Radiographic measurements
The osteosynthesis location was palmar in 92 cases (86.8%), dorsal in 7 cases (6.6%), and dorsopalmar in 7 cases (6.6%). The locking plates used were 97.2% Medartis AG and 2.8% VariAx™. The plate position was classified as Soong 0 in 19.2% of patients, Soong 1 in 19.2% of patients and Soong 2 in 61.6% of patients. All fractures were considered to be fully consolidated. In 25 patients, there was no evidence that the osteosynthesis was acceptable in terms to the mentioned criteria. This was due to radial tilt of < 15° in 20 patients, dorsal tilt of > 10° in three patients. In one patient, there was both a pronounced ulnar variance and a pronounced radial tilt. In one patient there was a missed diagnosis of SL ligament dissociation (SLAC III, DISI). We could not demonstrate a significant correlation between radiographic measurements and BMI (radial height: p = 0.336; radial inclination: p = 0.155; volar tilt: p = 0.378; ulnar variance: p = 0.547). Also, the changes in radiologic measurements between preoperative imaging and follow-up examination showed no significant difference among the BMI subgroups (Table 3).
Discussion
This study analyzed clinical and radiological data from 105 patients with DRF to determine if there was an association between BMI and fracture severity, operative time, and clinical and radiological outcome. A significant relationship was found between BMI and fracture severity based on the AO classification. There seemed to be no effect of BMI on incision-suture time, clinical and radiological outcome. Functional outcome did not vary, except for a significantly smaller difference in hand strength in patients with lower BMI (“no overweight”).
For some other fractures than DRF, there is a consensus in literature postulating an increased BMI is associated with a significantly higher risk of a more severe fracture. For example, there is general agreement on the relationship between BMI and fracture severity when looking more closely at ankle and humerus fractures [9, 18].
For DRF, both Goodloe et al. and Montague et al. found a significant correlation between BMI and fracture severity. Both studies classified fracture severity according to the AO classification, as in this study. Goodloe et al. also reported an increased risk of intra-articular split fractures and intra-articular fragmentation [13]. Montague et al. demonstrated that the likelihood of a more complex DRF increased with each point increase in BMI [19]. In contrast, the study by Acosta-Olivo et al. did not show a significant increase in fracture severity, but did show a greater susceptibility to DRF in patients with higher BMI [20].
Some authors postulate that both a high BMI [21] and a low BMI protects against DRF. In their meta-analysis, Johansson et al. demonstrated a lower fracture rate in patients with a low BMI [9].
Similar to overweight, underweight is discussed in some studies as a risk factor for more severe forms of fracture. This is justified by cellular processes in bone metabolism that are affected by malnutrition and hormonal changes, and lean body mass [11]. Low BMI should therefore be discussed rather as a risk factor for more severe fractures [22,23,24].
A high BMI also influences the complex relationship between bone strain and bone strength. On the one hand, there are higher forces acting on the bone due to weight, which may explain the increase in fracture severity [25, 26]. On the other hand, higher body weight increases the mechanical stimuli on the bone, which enhances new bone growth and allows the bone to adapt to the increased body mass at the cellular level [27, 28]. However, this mechanism cannot be applied to the entire skeletal system.
The distal forearm, or the upper extremities in general, are not exposed to comparable mechanical stimuli as the lower extremities. For this reason, new bone formation on the distal forearm and the associated protective effect against fractures rather negligible.
Another protective mechanism mentioned in the literature is the soft tissue mantle formed by subcutaneous adipocytes, which may absorb impact forces and thus protect against fractures [29]. However, this cushioning does not provide uniform protection for all regions of the skeleton. In particular, at the forearm, the extent of the soft tissue mantle is very small and insufficient to reliably prevent the occurrence of fractures in general or to reduce the severity of fractures when a high force is applied [13, 29].
Thus, it can be concluded that increased body mass places greater stress on the bone, while protective mechanisms (bone strength and soft tissue cushioning) have little to no influence in DRF.
In addition to increased BMI, Ebinger et al. identified male sex and older age (over 50 years) as risk factors for complex fracture patterns [29]. The mean age of our cohort was 59 ± 16.1 years. It is possible that BMI and age may also have an additive effect on the likelihood of fracture severity.
Consistent with our findings, Zheng et al. describe optimal fracture prevention when both low and high BMI and early BMI loss can be avoided [30]. Our results underline that a BMI between 24.9–18.5 kg/m2, as recommended by the WHO, should be aimed for in order to avoid severe fractures [8].
An extension of surgery time of 0.38 min per BMI point has already been reported in the literature [13]. A similar correlation has been described in other surgical fields [31,32,33,34]. However, we were not able to demonstrate an increase in the duration of surgery due to a higher BMI. This may be due to the fact that there is less soft tissue surrounding the forearm. In addition, the treatment of an AO-A fracture took as long as the treatment of an AO-C fracture. Each operation could be performed with the same surgical efficiency regardless of BMI and fracture severity. In this way, cost inflation is controlled and staff capacity is conserved.
The complication rate of 24.1% of all patients that underwent surgery of distal radius fracture is comparable to other clinics. Other studies reported a postoperative complication rate of 39% after surgical treatment of distal radius fracture. The complications rate is increased due to a bias, as especially those patients presented to the follow-up who had complaints.
Common complications include tendinopathy, nerve injury, malposition, infection, healing in malposition, pseudarthrosis, chronic repetitive pain syndrome (CRPS) and compartment syndrome [35]. The complication rate had a high variability between different studies. McKay et al. found a complication rate varying from 6 to 80% [36]. Other studies have shown that obesity is associated with a higher risk for complications or revision surgery. In our study we were not able to show a significant association between the BMI and complication rate. The increased complication rate and rate for revision surgery in the study conducted by DeGeorge et al. was presented for patients with an BMI over 35 [37]. In our study we only analyzed the complication rate for patients with a BMI of over 25. The lower cut-off value of BMI in our study could explain the difference in both studies.
It is likely that increasing fracture severity with higher BMI also worsens functional outcome. However, in our cohort, all patients achieved good functional outcomes regardless of BMI. This fact has been confirmed in other studies [38, 39] and explains the high DASH score achieved by the patients in this study.
There was also no correlation between the radiological results and BMI. All fractures in our cohort are fully consolidated. We were not able to show that BMI has a negative effect on wound and bone healing processes in DRF or leads to a delay in these processes, as previously described for other fractures [40, 41]. This could be explained by the fact that we didn’t have an interim examination in which this was investigated.
This study is limited by the inaccurate estimation of the ratio of muscle to fat mass by BMI. More accurate results should be obtained in the future by detailed body fat measurement. This would also help to better classify the current health status of the patient. Another limitation is that especially those patients presented to the follow-up who had complaints. This could have led to a biased and increased complication rate. An additional limitation is the low number of patients included in the study, especially of those with an increased BMI.
Other parameters such as HBA1c should also be determined, as diabetes is also associated with impaired bone metabolism and a higher risk of osteoporosis. The risk of osteoporosis, and thus a correlation with BMI, could have been further supplemented with additional bone densitometry (DXA).
Conclusion
This study shows that patients with a higher BMI have more severe distal radius fractures according to the AO classification. BMI does not correlate with surgery time for fracture management. Neither clinical nor radiological outcome is influenced by BMI.
Data availability
All relevant data is displayed in the figures/tables of this manuscript.
References
Rupp M et al (2021) The incidence of fractures among the adult population of Germany-an analysis from 2009 through 2019. Dtsch Arztebl Int 118(40):665–669
Rueger JM et al (2014) Fractures of the distal radius. Unfallchirurg 117(11):1025–1034 (quiz 1035-6)
Porrino JA Jr et al (2014) Fracture of the distal radius: epidemiology and premanagement radiographic characterization. AJR Am J Roentgenol 203(3):551–559
MacIntyre NJ, Dewan N (2016) Epidemiology of distal radius fractures and factors predicting risk and prognosis. J Hand Ther 29(2):136–145
Stirling ERB, Johnson NA, Dias JJ (2018) Epidemiology of distal radius fractures in a geographically defined adult population. J Hand Surg Eur 43(9):974–982
Lin X, Li H (2021) Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne) 12:706978
Han S et al (2022) Incidence of hip fracture in underweight individuals: a nationwide population-based cohort study in Korea. J Cachexia Sarcopenia Muscle 13(5):2473–2479
Consultation WHOE (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363(9403):157–163
Johansson H et al (2014) A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 29(1):223–233
Parratte S, Pesenti S, Argenson JN (2014) Obesity in orthopedics and trauma surgery. Orthop Traumatol Surg Res 100(1 Suppl):S91–S97
Misra M, Klibanski A (2014) Anorexia nervosa and bone. J Endocrinol 221(3):R163–R176
Sander AL et al (2020) Epidemiology and treatment of distal radius fractures: current concept based on fracture severity and not on age. Eur J Trauma Emerg Surg 46(3):585–590
Goodloe JB et al (2020) Elevated BMI is associated with intra-articular comminution, prolonged operative time, and postoperative complications in distal radius fractures. Injury 51(11):2612–2616
Harth A et al (2007) DASH-Fragebogen zur Outcome-Messung an der oberen Extremität. Trauma und Berufskrankheit 10(1):84–89
Germann G, Wind G, Harth A (1999) The DASH (Disability of Arm-Shoulder-Hand) Questionnaire–a new instrument for evaluating upper extremity treatment outcome. Handchir Mikrochir Plast Chir 31(3):149–152
Creighton JJ 3rd, Jensen CD, Kaplan FTD (2020) Intrarater and interrater reliability of the soong classification for distal radius volar locking plate placement. Hand (N Y) 15(3):414–417
Quadlbauer S et al (2020) Functional and radiological outcome of distal radius fractures stabilized by volar-locking plate with a minimum follow-up of 1 year. Arch Orthop Trauma Surg 140(6):843–852
Boadi BI et al (2023) Patient obesity is associated with severity of proximal humerus fractures, not outcomes. Arch Orthop Trauma Surg 143(1):373–379
Montague MD et al (2019) Distal radius fractures: does obesity affect fracture pattern, treatment, and functional outcomes? Hand (N Y) 14(3):398–401
Acosta-Olivo C et al (2017) Correlation between obesity and severity of distal radius fractures. Orthop Traumatol Surg Res 103(2):199–202
Ong T et al (2014) A United Kingdom perspective on the relationship between body mass index (BMI) and bone health: a cross sectional analysis of data from the Nottingham Fracture Liaison Service. Bone 59:207–210
Fitzpatrick KK, Lock J (2011) Anorexia nervosa. BMJ Clin Evid 2011:1011
Robinson L, Micali N, Misra M (2017) Eating disorders and bone metabolism in women. Curr Opin Pediatr 29(4):488–496
Reid IR (2010) Fat and bone. Arch Biochem Biophys 503(1):20–27
Chiu J, Robinovitch SN (1998) Prediction of upper extremity impact forces during falls on the outstretched hand. J Biomech 31(12):1169–1176
Singhal V et al (2022) Load-to-strength ratio at the radius is higher in adolescent and young adult females with obesity compared to normal-weight controls. Bone 164:116515
Ehrlich PJ, Lanyon LE (2002) Mechanical strain and bone cell function: a review. Osteoporos Int 13(9):688–700
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42(4):606–615
Ebinger T et al (2016) Obesity increases complexity of distal radius fracture in fall from standing height. J Orthop Trauma 30(8):450–455
Zheng R et al (2021) Prior loss of body mass index, low body mass index, and central obesity independently contribute to higher rates of fractures in elderly women and men. J Bone Miner Res 36(7):1288–1299
Schuette HB et al (2020) The effect of obesity on operative time and postoperative complications for peritrochanteric femur fractures. Cureus 12(11):e11720
Raphael IJ et al (2013) Obesity and operative time in primary total joint arthroplasty. J Knee Surg 26(2):95–99
Gholson JJ et al (2016) Morbid obesity and congestive heart failure increase operative time and room time in total hip arthroplasty. J Arthroplasty 31(4):771–775
Traven SA et al (2021) Elevated BMI increases concurrent pathology and operative time in adolescent ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 29(12):4182–4187
Rosenauer R et al (2020) Complications after operatively treated distal radius fractures. Arch Orthop Trauma Surg 140:665–673
McKay SD et al (2001) Assessment of complications of distal radius fractures and development of a complication checklist. J Hand Surg 26(5):916–922
DeGeorge BR et al (2020) Incidence of complications following volar locking plate fixation of distal radius fractures: an analysis of 647 cases. Plast Reconstr Surg 145(4):969–976
Hall MJ et al (2019) The impact of obesity and smoking on outcomes after volar plate fixation of distal radius fractures. J Hand Surg Am 44(12):1037–1049
Ruckenstuhl P et al (2019) Influence of body mass index on health-related quality of life after surgical treatment of intra-articular distal radius fractures. A retrospective 7-year follow-up study. Hand Surg Rehabil 38(6):364–368
Ambrosi TH et al (2017) Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20(6):771-784.e6
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Anna Lena Kloberdanz: Study conception and planning, Literature search, Studies selection, Data extraction, Data analysis, Studies tables preparation, Manuscript writing. Jasmin Meyer, MD: Literature search, Studies selection, Data extraction, Flow chart figure preparation, Studies tables preparation, Manuscript writing. Kora Kammermeier: Studies selection, Data extraction, Data analysis, Studies tables preparation, Manuscript writing. André Strahl, PhD, MD, MSc: Data analysis, Studies tables preparation, Manuscript writing Carsten Schlickewei, MD: Studies selection, Data extraction, Data analysis, Studies tables preparation, Manuscript writing. Konrad Mader, MD: Manuscript writing. Karl-Heinz Frosch, MD: Manuscript writing, Project supervision. Sinef Yarar-Schlickewei, MD: Study conception and planning, Literature search, Data analysis, Statistical analysis, Studies tables preparation, Manuscript writing, Project supervision.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflict of interest to declare.
Ethical approval
Ethics committee (Ethikkommission der Hamburger Ärztekammer) approval was given for retrospective registration (Reference number: WF-114/20). We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kloberdanz, A.L., Meyer, J., Kammermeier, K. et al. Impact of body mass index on fracture severity, clinical, radiological and functional outcome in distal radius fractures: a retrospective observational study after surgical treatment. Arch Orthop Trauma Surg 144, 2915–2923 (2024). https://doi.org/10.1007/s00402-024-05391-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-024-05391-6