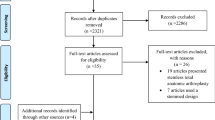
Abstract
Introduction
One current trend in the field of shoulder arthroplasty is a design shift to shorter and metaphyseal fixed humeral stem components. The aim of this investigation is to analyze complications resulting in revision surgery after anatomic (ASA) and reverse (RSA) short stem arthroplasty. We hypothesize that complications are influenced by the type of prosthesis and indication for arthroplasty.
Materials and methods
A total of 279 short stem shoulder prostheses were implanted by the same surgeon (162 ASA; 117 RSA), and 223 of these prostheses were implanted as primary procedures; in 54 cases, arthroplasty was performed secondary to prior open surgery. Main indications were osteoarthritis (OA) (n = 134), cuff tear arthropathy (CTA) (n = 74) and posttraumatic deformities (PTr) (n = 59). Patients were evaluated at 6 weeks (follow-up 1; FU1), 2 years (FU2) and the time span of the last follow-up defined as FU3 with a minimum FU of 2 years. Complications were categorized into early complications (within FU1), intermediate complications (within FU2) and late complications (> 2 years; FU3).
Results
In total, 268 prostheses (96.1%) were available for FU1; 267 prostheses (95.7%) were available for FU2 and 218 prostheses (77.8%) were available for FU3. The average time for FU3 was 53.0 months (range 24–95). A complication leading to revision occurred in 21 prostheses (7.8%), 6 (3.7%) in the ASA group and 15 (12.7%) in the RSA group (p < 0.005). The most frequent cause for revision was infection (n = 9; 42.9%). After primary implantation, 3 complications (2.2%) occurred in the ASA and 10 complications (11.0%) in the RSA group (p < 0.005). The complication rate was 2.2% in patients with OA, 13.5% in CTA and 11.9% in PTr.
Conclusions
Primary reverse shoulder arthroplasty had a significantly higher rate of complications and revisions than primary and secondary anatomic shoulder arthroplasty, respectively. Therefore, indications for reverse shoulder arthroplasty should be critically questioned in each individual case.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anatomic (ASA) and reverse (RSA) shoulder arthroplasty has become well-established and safe surgical procedures in the treatment of degenerative diseases of the glenohumeral joint [39]. One current trend in the field of shoulder arthroplasty is a design shift from cemented standard stem prostheses to cementless, shorter and metaphyseal fixed humeral stem components [20, 28]. Advantages of the new design are seen in the bone stock preserving implantation technique, especially in the event of revision [13, 21]. So far, clinical short- to midterm results for both short stem TSA and RSA are promising [11, 32, 34, 36, 44]. As a matter of concern, however, some authors reported the risk of stress shielding due to bone adaptions [29, 33, 35, 36, 38] and stem subsidence [43], which might facilitate stem loosening in the future.
Another trend is a more frequent use of RSA for primary joint replacement within the past 10 years. In the 2020 annual report of the Australian Orthopaedic Association National Joint Replacement Registry [1], one of the largest databases for shoulder arthroplasty worldwide, the proportion of RSA has increased from 42% in 2009 to 80.4% in 2019. In the latest report, which contains data up to December 2019, 44,561 total shoulder replacements were considered of which 32% were ASA and 68% were RSA, respectively. According to the German Shoulder Arthroplasty Registry (DVSE) report of 2020, comparable numbers can be found with 24% ASA and 76% RSA in 2020[20]. This new trend toward more frequent implantation of RSA was recently explained by a rising proportion of active elderly patients electing for RSA [20]. Another reason might be an expansion of indications for RSA from historical indications like cuff tear arthropathy (CTA) in elderly patients [42] and revision cases [15] to shoulders with B2 glenoid types according to the classification of Walch et al. [2, 8, 9, 18, 45, 46] and arthritic shoulders with an intact rotator cuff [47, 48].
The aim of this investigation is to record and analyze complications resulting in revision surgery after anatomic (ASA) and reverse (RSA) short stem arthroplasty. We hypothesize that complications are influenced by the type of prosthesis (ASA or RSA) and indication for joint replacement.
Methods
A retrospective comparative study was conducted on prospectively collected data of 162 short stem ASA and 117 short stem RSA performed at a single specialized shoulder center between January 2013 and December 2019. Institutional review board approval was obtained prior to the start of the study.
All surgeries were performed by the senior author (blinded for review). Inclusion criteria were osteoarthritis, OA; cuff tear arthropathy, CTA; posttraumatic deformities, PTr, rheumatoid arthritis, RA and avascular humeral head necrosis, AVN. Patients with revision surgery after prior arthroplasty were excluded from this study.
A cementless short stem system (AscendTM Flex, Wright Medical, Memphis, TN, USA) was used in all cases.
The implantation of ASA or RSA was defined as the index surgery. Osteoarthritis with an intact RC was the main indication for ASA in this series. In rare cases, elderly patients with OA and debatable compliance for complex rehabilitation and/or muscle atrophy were treated with RSA despite an intact rotator cuff. Glenoid deformity, according to Walch’s classification system [45], was not a criterium for RSA in any case. The distribution of glenoid types among 122 patients operated for primary OA with an intact rotator cuff was: Type A1: 3.3%, Type A2: 31.1%, Type B1: 48.4%, Type B2: 14.0%, Type A3: 0.8%, Type C: 2.5%.
All patients with cuff tear arthropathy were treated with RSA. In shoulders with posttraumatic sequelae (PTr), the decision for ASA or RSA was made depending on the type of deformities and the situation of the rotator cuff.
All procedures were further categorized into primary or secondary indications. Primary indications (PI) were defined as joint replacements without previous major operations. Patients with prior minor surgical procedures, such as arthroscopic decompression, rotator cuff or Bankart repair, were also classified as PI. Arthroplasties following prior major open procedures, such as bone reconstructive interventions, open stabilization procedures and open rotator cuff repair, were classified as secondary indications (SI).
All patients were followed up at six weeks (FU1) and two years (FU2) after the index surgery. Regular clinical controls (every 2 years) were recommended by the surgeon for each patient. Controls with a minimum FU of more than 2 years were defined as FU3. All patients who had missed their last regular checks were invited for clinical examination. Patients, who were not able to travel due to the COVID-19 pandemic, unacceptable distance to the hospital or due to their medical condition were called by phone and asked for complications or revisions by one of the authors (blinded for review).
All complications that were followed by revision surgery were analyzed. Complications were categorized into early complications (within the first 6 weeks; FU1), intermediate complications (within the first 2 years; FU2) and late complications (after more than two years; FU3). Cases of revision due to postoperative infections were categorized as early-onset (< 3 months)[23] and late-onset (> 3 months) infections [24]. The impact of type of prosthesis (anatomic or reverse) and indication (primary or secondary) on revision rates was analyzed. The Fisher–Boschloo test [25] was used for statistical evaluation. The Fisher–Boschloo’s test is a statistical hypothesis test for analyzing 2 × 2 contingency tables. It examines the association of two Bernoulli distributed random variables and is a uniformly more powerful alternative to Fisher's exact test.
Surgical technique and implant
Anatomic implant and technique: A deltopectoral approach was used in all cases. Resection of the humeral head was performed in a free-hand technique according to the individual conditions. The smallest fitting trial stem (compactor) was implanted into the metaphyseal cancellous bone and found appropriate in size when the surgeon was unable to rotate the stem with three fingers. An uncemented humeral short stem (Ascend Flex ™, Wright Medical, Memphis, TN, USA) was then implanted. A cemented keeled glenoid (Perform™ Glenoid, Wright Medical, Memphis, TN, USA) was used in all patients. In B1 or B2 glenoids, according to Walch’s classification [45], partial correction of retroversion was performed by restrained eccentric reaming. Posterior glenoid augmentation was not performed in any case.
Reverse implant and technique: The humeral stem was implanted in the same fashion as described above at 130° of inclination and 20° of retroversion. The platform was placed in the most medialized position and the optimal inlay size was individually selected. Based on the diameter of the resected humeral head, the size of the glenosphere was chosen. The glenoid implant was anchored to the bone with four screws. From 2013 until 2016, a standard glenosphere (Wright Medical, Memphis, TN, USA) was implanted and, since 2017, the Aequalis Perform Reversed Glenosphere (Wright Medical, Memphis, TN, USA) was used for all cases. The detailed surgical techniques have been described before [37].
Results
The overall cohort included in this study consisted of 279 prostheses: 162 ASA and 117 RSA (271 patients; 16 bilateral procedures). A total of 169 prostheses were used in females (61%), and 110 in males (39%). Mean age was 69 (range 34–92) years. The right shoulder joint was replaced in 186 cases (67%) and the left in 93 (33%) cases. The most common indication for arthroplasty was OA (n = 134; 48.0%) followed by CTA (n = 74; 26.4%) and PTr (n = 59; 21.1%). Arthroplasty secondary to major open procedures (secondary indication; SI) was performed in 54 cases (19.3%). OA was the main indication for primary ASA (88.9%) and CTA for primary RSA (81.3%). In 12 cases (10%), RSA was used for primary OA with an intact rotator cuff. Demographics and characteristics are demonstrated in Fig. 1.
Demographics and characteristics. ASA = anatomic total shoulder arthroplasty; RSA = reverse shoulder arthroplasty; OA = osteoarthritis; PTr = post-traumatic; RA = rheumatoid arthritis; AVN = avascular humeral head necrosis; CTA = cuff tear arthritis; PI = primary indication; SI = secondary indication
A total of 268 prostheses (96.1%) were available for FU1 (ASA 97.5%; RSA 94.0%) and 267 prostheses (95.7%) for FU2 (ASA 96.9%; RSA 94.0%); 217 prostheses (77.8%) had a minimum FU of two years (ASA 82.7%; RSA 71.0%) with a mean of 53.0 (range 24–95) months; 42 patients (15%) were unavailable via phone contact and 11 patients (3.9%) had died in the meantime.
Complications
The overall revision rate for the entire observation period was 3.7% (6 cases) in ASA and 12.7% (15 cases) in RSA. Infection was the most often reason for revision surgery (9 cases; 42.9%): late-onset infection occurred in six cases (29%) and early-onset infections occurred in three cases (14%). Proven bacteria were: Staphilococcus epidermidis (two cases), Staphilococcus capitis (one case), cutibacterium acnes (one case) and Pseudomonas aeruginosa (one case). The second most frequent complication was aseptic component loosening in three cases (14.3%), exclusively occurring in the RSA group. Detailed indications, complications and revisions are demonstrated in Tables 1 and 2.
Among 122 patients treated with an ASA for OA with an intact rotator cuff, revision was performed in just one case due to secondary rotator cuff deficiency.
No complications occurred among patients who received RSA for OA with an intact rotator cuff.
Early complications (< 6 weeks FU)
A total of 268 prostheses (96.1%) were available for FU1. Early complications occurred in three cases (1.1%), exclusively in the RSA group (Table 2).
Intermediate complications (6 weeks – 2 years FU)
A total of 267 prostheses (95.7%) were available for FU2. Intermediate complications occurred in 14 cases (5.2%). Four complications were found after ASA (2.5%) and 10 complications (9%) after RSA (Tables 1 and 2).
Late complications (> 2 years FU)
A total of 217 prostheses (77.8%) were available for FU3. Late complications occurred in four cases (1.8%). Two complications (1.5%) were found after ASA, and two complications (2.4%) were found after RSA (Tables 1 and 2).
Complications and revisions depending on indications for index surgery
OA was the indication for arthroplasty in 134 cases; 122 patients (91.1%) received ASA, and 12 patients (9%) received RSA. Three complications (2.5%) in the ASA group required revision surgery: a periprosthetic fracture, a secondary rotator cuff failure (M. subscapularis defect) and a late-onset infection.
In 74 cases, arthroplasty was performed for CTA and all these cases were treated with RSA. Ten complications (13.5%) required revision surgery: postoperative hematoma (n = 1), traumatic component dislocation (n = 1), early-onset infection (n = 2), late-onset infection (n = 3), stem loosening (n = 1), atraumatic glenosphere dissociation (n = 1) and glenosphere loosening (n = 1).
In 59 cases, arthroplasty was performed for PTr. 30 patients received ASA (50.8%) and 29 patients received RSA (49.2%). Three complications occurred in the ASA group: late-onset infection (n = 2) and secondary rotator cuff deficiency due to non-healing of the subscapularis tendon (n = 1). Four complications occurred in the RSA group: late infection (n = 1), periprosthetic fracture (n = 1), hematoma (n = 1) and acromion stress fracture (n = 1).
A total of 226 patients (80.7%) had primary indications (PI). Of those, 135 received ASA (59.7%) and 91 received RSA (40.3%). Three complications in the ASA group and 10 complications in the RSA group led to revision surgery.
A total of 53 patients (19.0%) had secondary indications (SI). Of those, 27 received ASA (51.0%) and 26 received RSA (49.0%). Three complications in the ASA and 5 complications in the RSA group led to revision surgery. In nine cases, arthroplasty was performed for patients with rheumatoid arthritis. ASA was used in seven (78%) and RSA in two cases (22%). One complication in the RSA group led to revision surgery. Three patients (1%) were indicated for ASA due to humeral head necrosis. After AVN, no complications were recorded.
Statistical analysis
Using the Fisher–Boschloo test, a significant difference was found between ASA and RSA. A complication requiring revision occurred in 6 ASA (3.7%) and in 15 RSA (12.7%) (p < 0.005). After primary implantation, three complications (2.2%) occurred in the ASA and 10 complications (11.0%) in the RSA group (p = 0.005). The difference between ASA and RSA could not be confirmed for secondary indications (SI). After SI, three complications (11.1%) occurred in the ASA and five complications (18.5%) in the RSA group (p = 0.649). The complication rate was 2.5% after OA, 13.5% after CTA, 11.9% after PTr and 14.8% after SI. Mean time until revision was 50.7 months for ASA and 53.4 months for RSA.
Discussion
A current trend in the field of shoulder arthroplasty is a design shift from cemented standard stem prostheses (length > 100 mm) to uncemented, metaphyseal fixed short humeral stem components [20, 28] with potential advantages in the event of revision due to a bone-preserving implantation technique [13, 21]. Promising clinical results for both short stem ASA and short stem RSA have been reported; however, only short- to medium-term data exist as short stem arthroplasty has only become popular recently [11, 32].
This study aimed to record and analyze complications resulting in revision surgery among 162 short stem ASA and 117 short stem RSA. Since the senior author performed all arthroplasties in this study, complications due to the bias by surgeons with different levels of experience, as previously shown [3], are most likely to be excluded.
The results demonstrate a revision rate of 3.7% (6 cases) after short stem ASA. These results are in line with a revision rate of 4.0% after short stem ASA reported by Schnetzke et al.[34] in a systematic review among ten included studies with an average follow-up of 20–64 months. Compared to reported revision rates after standard stem ASA, the complication rate after short stems is relatively low. Deshmukh et al.[10] described a revision rate of 22.0% after standard stem ASA at a minimum follow-up of 10 years and Gonzales et al. [16] reported a revision rate of 17.3% after ASA in their systematical review. The results of the current study imply a rather low revision rate after short stem arthroplasty, but due to a lower observation period, higher revision rates must be expected with longer follow-up.
Furthermore, the study found a revision rate of 12.7% (15 cases) after short stem RSA. Compared to a 4.9% revision rate after short stem RSA in a systematic review with an average follow-up of 20–99.6 months among 10 included studies, the revision rate in this study is somewhat higher [44]. Also, the reported revision rate in this study appears to be higher than revision rates after standard stem RSA [5, 14, 17, 22, 41, 50]. As an example, Boileau et al. [5] reported a revision rate of 4.0% after the implantation of 143 Grammont-style BIO (bio-increased offset) RSA at a mean observation time of 75 months and Sirveaux et al.[41] found a revision rate of 3.75% at a mean FU of 44 months in their multicenter study using the same type of prosthesis.
An explanation for higher revision rates among the present study cohort might be the inclusion of patients with posttraumatic sequelae and major open surgery before the index surgery, which made the cohort more heterogeneous than cohorts in other studies that predominantly included cuff tear arthropathy [26]. A study by Ascione et al. included various diagnoses and a comparable revision rate of 10.0% was found after the implantation of the same type of short stem RSA in 100 cases with a mean follow-up of 32.6 months. Also, the present analysis revealed that the mean time until revision was 53.4 months after the implantation of RSA, which is more than the maximum FU of the most published studies on short stem RSA [44].
In the past, high rates of bone adaptions [29, 33, 35, 36, 38] and stem subsidence [43, 49] have called into question the long-term stability of short stem prostheses. In the present study, stress shielding was not analyzed in detail, but no case of aseptic loosening of the humeral component was seen after ASA, and only two cases of stem loosening (1.7%) were found after RSA.
The present study is one of the first to compare revision rates of short stem ASA and short stem RSA. The analysis identified reverse shoulder arthroplasty as one risk factor for higher complication rates.
A complication requiring revision occurred significantly more often after short stem RSA compared to short stem ASA. Also, the revision rate after primary implantation of RSA was significantly higher compared to primary implantation of ASA. Among the study cohort, 54 patients were treated with an arthroplasty secondary to complex open procedures, such as bone reconstructive interventions, open stabilization procedures and open rotator cuff surgery.
Due to the heterogeneity in this group, a statistical comparison must be drawn with caution. However, the difference between ASA and RSA could not be confirmed for these secondary indications. Other studies in the past have shown comparable complication and revision rates for standard stem ASA and RSA [12, 19, 47].
As the results of this study imply higher revision rates after RSA, the increasing usage of RSA, as demonstrated in national and international shoulder arthroplasty registries [1, 20], should be critically discussed. Kircher et al. [20] explained the rising numbers of RSA with an increasing proportion of active elderly patients electing for RSA due to CTA, proximal humeral fractures and irreparable rotator cuff lesions.
Historically, the idea of RSA was to restore mobility and function in shoulders with cuff tear arthropathy [4, 42]. In this situation, the deltoid muscle is able to replace the rotator cuff to a large extent [6]. Nowadays, however, an increasing number of surgeons and authors consider RSA beneficial over ASA based on age of the patient and the likelihood of developing a rotator cuff deficiency in the future [47, 48]. Some authors considered secondary rotator cuff degeneration as the most common complication after ASA leading to glenoid loosening through the “rocking horse” mechanism [7, 47, 48]. Young et al. [48] reported a secondary rotator cuff dysfunction rate of 16.8% nine years after ASA. In their study, secondary rotator cuff dysfunction was associated with worse clinical outcomes; however, revision rates among the study cohort were not significantly different for patients with or without an intact rotator cuff.
On the other hand, Raval et al. [30] demonstrated that the preoperative presence of a partial rotator cuff tear was not associated with worse clinical outcomes 5.8 years after ASA. Moreover, the authors found a survival rate of over 90% at five years, which is comparable to other survival rates of ASA in the literature [40].
In the present study, secondary rotator cuff deficiency led to revision surgery in only one case, 25 months after ASA for OA with an intact rotator cuff. Another patient received ASA in a posttraumatic situation and was revised for rotator cuff deficiency due to non-healing of the subscapularis tendon 15 months after refixation.
Although evaluation of the rotator cuff before arthroplasty is crucial and especially fatty infiltration of the infraspinatus was shown to be predictive for a secondary rotator cuff failure [48], the authors of this article think that the implantation of RSA must be critically questioned when the rotator cuff is not torn by the time of arthroplasty. It should be reserved for patients with cuff tear arthropathy or osteoarthritis with massive rotator cuff tears.
Furthermore, controversy exists as to which type of arthroplasty is more useful in primary arthritis with an intact rotator cuff but biconcave glenoid deformities.
Walch et al. [46] reported a revision rate of 16.3% among 92 ASA implanted in shoulders with B1 and B2 glenoids at an average follow-up of 77 months, where revision was due to glenoid loosening, posterior instability or soft tissue problems. The authors thus recommended RSA as the preferred treatment option for biconcave glenoids [46].
Since then, in cases with biconcave glenoids in primary arthritis, the concept of RSA has competed with that of ASA with adjusted correction of the position of the glenoid component. In a recently published systematic review, Reahl et al. [31] compared the midterm clinical outcomes of ASA and RSA for B2 glenoids with an intact rotator cuff. Both groups showed improvement in patient-reported outcome scores and pooled complication rates (9% after ASA and 6% after RSA) as well as revision rates (2% after ASA and 1% after RSA). Single reports about good functional outcomes and low rates of glenoid component loosening after the implantation of RSA in shoulders with B2 glenoids and an intact rotator cuff exist [27]. However, randomized controlled trials are crucial to demonstrate the long-term superiority of RSA over ASA in these special cases.
As far as we know, no controlled comparative study has been performed about this issue and there is still no proof of lower complication and revision rates after RSA for arthritis with biconcave glenoids. The superiority of one of the two implants is therefore not demonstrated.
In fact, this study contributes nothing new to this question. It does, however, show a very low complication rate in cases with biconcave glenoids after restrained correction of the retroversion of the glenoid. On the other hand, the few cases in which an RSA was implanted in osteoarthritis with an intact rotator cuff did not show a higher complication rate.
However, it should be borne in mind that in the case of a glenoid failure, conversion from ASA to RSA is an easier retreat option than revision in a loosened glenosphere.
Limitations
The results of the two types of prostheses, ASA and RSA, used in this study are clearly not comparable. On the one hand, it is a retrospective analysis and, on the other hand, heterogeneous collectives and indications obviously lead to an indication bias. A multicenter prospective randomized study would have to be performed to clearly demonstrate the superiority of one of the systems.
Conclusion
Revision rates in short stem arthroplasty are generally low. Primary reverse shoulder arthroplasty was identified as a risk factor for complications requiring revision surgery. Therefore, indications for reverse shoulder arthroplasty should be critically questioned in each individual case.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Annual Reports 2020. 2020: Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Annual Reports 2020. https: //aoanjrr.sahmri.com/annual-reports-2020 (Accessed on 8 April 2022).
Alentorn-Geli E, Wanderman NR, Assenmacher AT, Cofield RH, Sanchez-Sotelo J, Sperling JW (2019) Reverse shoulder arthroplasty for patients with glenohumeral osteoarthritis secondary to glenoid dysplasia. Acta Orthop Belg 85(3):274–282
Barco R, Savvidou OD, Sperling JW, Sanchez-Sotelo J, Cofield RH (2016) Complications in reverse shoulder arthroplasty. EFORT Open Rev 1(3):72–80
Baulot E, Sirveaux F, Boileau P (2011) Grammont’s idea: The story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res 469(9):2425–2431
Boileau P, Morin-Salvo N, Bessière C, Chelli M, Gauci MO, Lemmex DB (2020) Bony increased-offset-reverse shoulder arthroplasty: 5 to 10 years’ follow-up. J Shoulder Elbow Surg 29(10):2111–2122
Boileau P, Watkinson DJ, Hatzidakis AM, Balg F (2005) Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 14(1 Suppl S):147S-161S
Chin PY, Sperling JW, Cofield RH, Schleck C (2006) Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg 15(1):19–22
Collin P, Hervé A, Walch G, Boileau P, Muniandy M, Chelli M (2019) Mid-term results of reverse shoulder arthroplasty for glenohumeral osteoarthritis with posterior glenoid deficiency and humeral subluxation. J Shoulder Elbow Surg 28(10):2023–2030
Denard PJ, Walch G (2013) Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg 22(11):1589–1598
Deshmukh AV, Koris M, Zurakowski D, Thornhill TS (2005) Total shoulder arthroplasty: Long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg 14(5):471–479
Dukan R, Bahman M, Rousseau MA, Boyer P (2020) Outcomes of reverse shoulder arthroplasty using a short stem through a superolateral approach. J Shoulder Elbow Surg 29(6):1197–1205
Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G (2004) Shoulder arthroplasty in patients with osteoarthritis and dysplastic glenoid morphology. J Shoulder Elbow Surg 13(1):1–4
Erickson BJ, Chalmers PN, Denard PJ, Gobezie R, Romeo AA, Lederman ES (2020) Current state of short-stem implants in total shoulder arthroplasty: a systematic review of the literature. JSES Int 4(1):114–119
Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D (2011) Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res 469(9):2469–2475
Gauci MO, Cavalier M, Gonzalez JF et al (2020) Revision of failed shoulder arthroplasty: epidemiology, etiology, and surgical options. J Shoulder Elbow Surg 29(3):541–549
Gonzalez J-F, Alami GB, Baque F, Walch G, Boileau P (2011) Complications of unconstrained shoulder prostheses. J Shoulder Elbow Surg 20(4):666–682
Gruber S, Schoch C, Geyer M (2017) The reverse shoulder arthroplasty Delta Xtend : Mid-term results. Orthopade 46(3):222–226
Holt AM, Throckmorton TW (2019) Reverse shoulder arthroplasty for B2 glenoid deformity. J Shoulder Elb Arthroplast 3:2471549219897661
Kiet TK, Feeley BT, Naimark M et al (2015) Outcomes after shoulder replacement: comparison between reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 24(2):179–185
Kircher J, Ohly B, Albers S, Kirchner F, Hudek R, Magosch P (2022) Versorgungsrealität in Deutschland: ein Auszug aus dem Schulterendoprothesenregister der Deutschen Vereinigung für Schulter- und Ellenbogenchirurgie e.V. (DVSE). Obere Extremität. https://doi.org/10.1007/s11678-022-00689-6
Langohr GDG, Reeves J, Roche CP, Faber KJ, Johnson JA (2020) The effect of short-stem humeral component sizing on humeral bone stress. J Shoulder Elbow Surg 29(4):761–767
Lehtimäki K, Rasmussen JV, Mokka J et al (2018) Risk and risk factors for revision after primary reverse shoulder arthroplasty for cuff tear arthropathy and osteoarthritis: a Nordic Arthroplasty Register Association study. J Shoulder Elbow Surg 27(9):1596–1601
Loew M (2010) Frühkomplikationen. In: Loew M (ed) AE-Manual der Endoprothetik Schulter. Springer Verlag, Heidelberg, pp 233–234
Loew M (2010) Spätkomplikationen. In: Loew M (ed) AE-Manual der Endoprothetik Schulter. Springer Verlag, Heidelberg, pp 241–242
Lydersen S, Fagerland MW, Laake P (2009) Recommended tests for association in 2 x 2 tables. Stat Med 28(7):1159–1175
Merolla G, Walch G, Ascione F et al (2018) Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg 27(4):701–710
Mizuno N, Denard PJ, Raiss P, Walch G (2013) Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am 95(14):1297–1304
Oh HK, Lim TK (2018) Short Humeral Stems in Shoulder Arthroplasty. Clin Shoulder Elb 21(2):105–110
Raiss P, Schnetzke M, Wittmann T et al (2019) Postoperative radiographic findings of an uncemented convertible short stem for anatomic and reverse shoulder arthroplasty. J Shoulder Elbow Surg 28(4):715–723
Raval P, Gibbs VN, Pandey R (2021) Preoperative partial-thickness rotator cuff tears do not compromise anatomic total shoulder replacement outcomes: medium-term follow-up. J Shoulder Elbow Surg 30(4):871–876
Reahl GB, Abdul-Rassoul H, Kim RL et al (2021) Anatomic vs. reverse shoulder arthroplasty for the treatment of Walch B2 glenoid morphology: a systematic review and meta-analysis. JSES Rev Rep Techn 1(4):317–328
Romeo AA, Thorsness RJ, Sumner SA, Gobezie R, Lederman ES, Denard PJ (2018) Short-term clinical outcome of an anatomic short-stem humeral component in total shoulder arthroplasty. J Shoulder Elbow Surg 27(1):70–74
Schnetzke M, Coda S, Raiss P, Walch G, Loew M (2016) Radiologic bone adaptations on a cementless short-stem shoulder prosthesis. J Shoulder Elbow Surg 25(4):650–657
Schnetzke M, Loew M, Raiss P, Walch G (2019) Short-stem anatomical shoulder replacement—a systematic review. Obere Extremität 14(2):139–148
Schnetzke M, Preis A, Coda S, Raiss P, Loew M (2017) Anatomical and reverse shoulder replacement with a convertible, uncemented short-stem shoulder prosthesis: first clinical and radiological results. Arch Orthop Trauma Surg 137(5):679–684
Schnetzke M, Rick S, Raiss P, Walch G, Loew M (2018) Mid-term results of anatomical total shoulder arthroplasty for primary osteoarthritis using a short-stemmed cementless humeral component. Bone Joint J 100-B(5):603–609
Schnetzke M, Sulzer S, Engelke J, Loew M (2021) Cemented all-polyethylene glenoid with standard or individualized backside curvature. Obere Extremität 16(1):68–74
Schnetzke M, Wittmann T, Raiss P, Walch G (2019) Short-term results of a second generation anatomic short-stem shoulder prosthesis in primary osteoarthritis. Arch Orthop Trauma Surg 139(2):149–154
Simovitch RW, Friedman RJ, Cheung EV et al (2017) Rate of improvement in clinical outcomes with anatomic and reverse total shoulder arthroplasty. J Bone Joint Surg Am 99(21):1801–1811
Singh JA, Sperling JW, Cofield RH (2011) Revision surgery following total shoulder arthroplasty: analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br 93(11):1513–1517
Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D (2004) Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 86(3):388–95
Smith CD, Guyver P, Bunker TD (2012) Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br 94(5):577–583
Tross AK, Lädermann A, Wittmann T et al (2020) Subsidence of uncemented short stems in reverse shoulder arthroplasty-a multicenter study. J Clin Med 9(10):3362. https://doi.org/10.3390/jcm9103362
Tross AK, Woolson TE, Nolte PC, Schnetzke M, Loew M, Millett PJ (2021) Primary reverse shoulder replacement with a short stem: A systematic literature review. JSES Rev Rep Techn 1(1):7–16
Walch G, Badet R, Boulahia A, Khoury A (1999) Morphologic study of the Glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 14(6):756–760
Walch G, Moraga C, Young A, Castellanos-Rosas J (2012) Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg 21(11):1526–1533
Wright MA, Keener JD, Chamberlain AM (2020) Comparison of clinical outcomes after anatomic total shoulder arthroplasty and reverse shoulder arthroplasty in patients 70 years and older with glenohumeral osteoarthritis and an intact rotator cuff. J Am Acad Orthop Surg 28(5):e222–e229
Young AA, Walch G, Pape G, Gohlke F, Favard L (2012) Secondary rotator cuff dysfunction following total shoulder arthroplasty for primary glenohumeral osteoarthritis: results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am 94(8):685–693
Zmistowski B, Carpenter DP, Chalmers PN, Smith MJ, Keener JD (2021) Symptomatic aseptic loosening of a short humeral stem following anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 30(12):2738–2744
Zumstein MA, Pinedo M, Old J, Boileau P (2011) Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 20(1):146–157
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: ML, AKN and MS. Methodology: ML; Data collection: AKN and SK; Formal analysis and investigation: ML, MS and TBr; Writing—original draft preparation: AKN; Writing—review and editing: ML and MS; Supervision: ML and MS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Markus Loew is a consultant for and receives royalties from Wright/Tornier Cie. Markus Loew receives royalties from Wright/Tornier Inc., which is related to the subject of this work. No company had any input into the review, data analysis, or manuscript preparation. The other authors, their immediate families and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Ethics approval
Institutional review board approval was obtained prior to the start of the study; Ethical Commission, University Clinic of Heidelberg (S-035/2022). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent to participate in the study was obtained from participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loew, M., Schnetzke, M., Kappes, S. et al. Complications and revisions in anatomic and reverse short stem shoulder arthroplasty. Arch Orthop Trauma Surg 143, 4853–4860 (2023). https://doi.org/10.1007/s00402-023-04802-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-04802-4