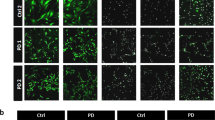
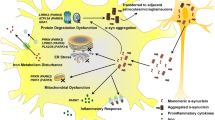
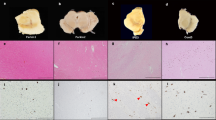
Abstract
Amyotrophic Lateral Sclerosis/Parkinsonism-Dementia Complex (ALS/PDC), a rare and complex neurological disorder, is predominantly observed in the Western Pacific islands, including regions of Japan, Guam, and Papua. This enigmatic condition continues to capture medical attention due to affected patients displaying symptoms that parallel those seen in either classical amyotrophic lateral sclerosis (ALS) or Parkinson’s disease (PD). Distinctly, postmortem examinations of the brains of affected individuals have shown the presence of α-synuclein aggregates and TDP-43, which are hallmarks of PD and classical ALS, respectively. These observations are further complicated by the detection of phosphorylated tau, accentuating the multifaceted proteinopathic nature of ALS/PDC. The etiological foundations of this disease remain undetermined, and genetic investigations have yet to provide conclusive answers. However, emerging evidence has implicated the contribution of astrocytes, pivotal cells for maintaining brain health, to neurodegenerative onset, and likely to play a significant role in the pathogenesis of ALS/PDC. Leveraging advanced induced pluripotent stem cell technology, our team cultivated multiple astrocyte lines to further investigate the Japanese variant of ALS/PDC (Kii ALS/PDC). CHCHD2 emerged as a significantly dysregulated gene when disease astrocytes were compared to healthy controls. Our analyses also revealed imbalances in the activation of specific pathways: those associated with astrocytic cilium dysfunction, known to be involved in neurodegeneration, and those related to major neurological disorders, including classical ALS and PD. Further in-depth examinations revealed abnormalities in the mitochondrial morphology and metabolic processes of the affected astrocytes. A particularly striking observation was the reduced expression of CHCHD2 in the spinal cord, motor cortex, and oculomotor nuclei of patients with Kii ALS/PDC. In summary, our findings suggest a potential reduction in the support Kii ALS/PDC astrocytes provide to neurons, emphasizing the need to explore the role of CHCHD2 in maintaining mitochondrial health and its implications for the disease.







Similar content being viewed by others
Data availability
Data and additional information are readily available from the authors upon reasonable request.
Change history
25 May 2024
The original article has been corrected. “Karyotype analysis” section head corrected to level 2 heading
References
Almad AA, Taga A, Joseph J, Gross SK, Welsh C, Patankar A et al (2022) Cx43 hemichannels contribute to astrocyte-mediated toxicity in sporadic and familial ALS Proc Natl Acad Sci 119(13):e2107391119. https://doi.org/10.1073/pnas.2107391119
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351(3):602–611. https://doi.org/10.1016/j.bbrc.2006.10.093
Aras S, Pak O, Sommer N, Finley R Jr, Hüttemann M, Weissmann N et al (2013) Oxygen-dependent expression of cytochrome c oxidase subunit 4–2 gene expression is mediated by transcription factors RBPJ, CXXC5 and CHCHD2. Nucleic Acids Res 41(4):2255–2266. https://doi.org/10.1093/nar/gks1454
Aras S, Bai M, Lee I, Springett R, Hüttemann M, Grossman LI (2015) MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism. Mitochondrion 20:43–51. https://doi.org/10.1016/j.mito.2014.10.003
Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D et al (2013) ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci 110(8):E736–E745. https://doi.org/10.1073/pnas.1222809110
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM et al (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium Nat Genet 25(1):25–29. https://doi.org/10.1038/75556
Bannwarth S, Ait-El-Mkadem S, Chaussenot A, Genin EC, Lacas-Gervais S, Fragaki K et al (2014) A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain 137(Pt 8):2329–2345. https://doi.org/10.1093/brain/awu138
Batulan Z, Taylor DM, Aarons RJ, Minotti S, Doroudchi MM, Nalbantoglu J et al (2006) Induction of multiple heat shock proteins and neuroprotection in a primary culture model of familial amyotrophic lateral sclerosis. Neurobiol Dis 24(2):213–225. https://doi.org/10.1016/j.nbd.2006.06.017
Baughman JM, Nilsson R, Gohil VM, Arlow DH, Gauhar Z, Mootha VK (2009) A computational screen for regulators of oxidative phosphorylation implicates SLIRP in mitochondrial RNA homeostasis. PLoS Genet 5(8):e1000590. https://doi.org/10.1371/journal.pgen.1000590Erratum.In:PLoSGenet6(3).10.1371/annotation/36fe7624-0904-46d4-a013-4be6195245c4
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL et al (2010) Arizona Parkinson’s Disease Consortium. Multi organ distribution of phosphorylated alpha synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119(6):689–702. https://doi.org/10.1007/s00401-010-0664-3
Bertrand E, Lewandowska E, Stepień T, Szpak GM, Pasennik E, Modzelewska J (2008) Amyloid angiopathy in idiopathic Parkinson’s disease. Immunohistochemical and ultrastructural study Folia Neuropathol 46(4):255–270 (PMID: 19169967)
Bhardwaj A, Myers MP, Buratti E, Baralle FE (2013) Characterizing TDP-43 interaction with its RNA targets. Nucleic Acids Res 41(9):5062–5074. https://doi.org/10.1093/nar/gkt189
Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD et al (2013) The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24(8):1250–1261. https://doi.org/10.1681/ASN.2012121216
Booth HDE, Hirst WD, Wade-Martins R (2017) The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci 40(6):358–370. https://doi.org/10.1016/j.tins.2017.04.001
Bordone MP, Salman MM, Titus HE, Amini E, Andersen JV, Chakraborti B et al (2019) The energetic brain - a review from students to students. J Neurochem 151(2):139–165. https://doi.org/10.1111/jnc.14829
Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L (2006) Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci 24(6):1687–1694. https://doi.org/10.1111/j.1460-9568.2006.05056.x
Bradley DP, Smith MI, Netsiri C, Smith JM, Bockhorst KH, Hall LD et al (2001) Diffusion-weighted MRI used to detect in vivo modulation of cortical spreading depression: comparison of sumatriptan and tonabersat. Exp Neurol 172(2):342–353. https://doi.org/10.1006/exnr.2001.7809
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS et al (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28(1):264–278. https://doi.org/10.1523/JNEUROSCI.4178-07.2008
Cao YL, Yang YP, Mao CJ, Zhang XQ, Wang CT, Yang J et al (2017) A role of BAG3 in regulating SNCA/α-synuclein clearance via selective macroautophagy. Neurobiol Aging 60:104–115. https://doi.org/10.1016/j.neurobiolaging.2017.08.023
Carra S, Seguin SJ, Lambert H, Landry J (2008) HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem 283(3):1437–1444. https://doi.org/10.1074/jbc.M706304200
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34(17):i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Choi HJ, Kang KS, Fukui M, Zhu BT (2011) Critical role of the JNK-p53-GADD45α apoptotic cascade in mediating oxidative cytotoxicity in hippocampal neurons. Br J Pharmacol 162(1):175–192. https://doi.org/10.1111/j.1476-5381.2010.01041.x
Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M et al (2013) Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155(1):160–171. https://doi.org/10.1016/j.cell.2013.08.032
Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M (1997) Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res Mol Brain Res 49(1–2):71–81. https://doi.org/10.1016/s0169-328x(97)00125-3
Dahl D, Rueger DC, Bignami A, Weber K, Osborn M (1981) Vimentin, the 57,000 molecular weight protein of fibroblast filaments, is the major cytoskeletal component in immature glia. Eur J Cell Biol 24:191–196 (PMID: 7285936)
Dal Canto MC, Gurney ME (1995) Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu, Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res 676(1):25–40. https://doi.org/10.1016/0006-8993(95)00063-v
Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F (1993) Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 52(1):1–6. https://doi.org/10.1016/0306-4522(93)90175-f
Darshi M, Mendiola VL, Mackey MR, Murphy AN, Koller A, Perkins GA et al (2011) ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function. J Biol Chem 286(4):2918–2932. https://doi.org/10.1074/jbc.M110.171975
Dermietzel R, Traub O, Hwang TK, Beyer E, Bennett MV, Spray DC et al (1989) Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci USA 86:10148–10152. https://doi.org/10.1073/pnas.86.24.10148
Dienel GA (2019) Brain Glucose Metabolism: Integration of Energetics with Function. Physiol Rev 99(1):949–1045. https://doi.org/10.1152/physrev.00062.2017
Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K (2007) Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci 10(5):608–614. https://doi.org/10.1038/nn1885
Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC (2008) Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell 3(6):637–648. https://doi.org/10.1016/j.stem.2008.09.017
Ding C, Wu Z, Huang L, Wang Y, Xue J, Chen S et al (2015) Mitofilin and CHCHD6 physically interact with Sam50 to sustain cristae structure. Sci Rep 4(5):16064. https://doi.org/10.1038/srep16064
Ebens A, Brose K, Leonardo ED, Hanson MG Jr, Bladt F, Birchmeier C et al (1996) Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron 17(6):1157–1172. https://doi.org/10.1016/s0896-6273(00)80247-0
Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24(3):312–325. https://doi.org/10.1038/s41593-020-00783-4
Friedman JR, Mourier A, Yamada J, McCaffery JM, Nunnari J (2015) MICOS coordinates with respiratory complexes and lipids to establish mitochondrial inner membrane architecture. Elife 28(4):e07739. https://doi.org/10.7554/eLife.07739
Funayama M, Ohe K, Amo T, Furuya N, Yamaguchi J, Saiki S et al (2015) CHCHD2 mutations in autosomal dominant late-onset Parkinson’s disease: a genome-wide linkage and sequencing study. Lancet Neurol 14(3):274–282. https://doi.org/10.1016/S1474-4422(14)70266-2
Furuta A, Rothstein JD, Martin LJ (1997) Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci 17(21):8363–8375. https://doi.org/10.1523/JNEUROSCI.17-21-08363.1997
Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 28(7):889–901. https://doi.org/10.1038/emboj.2009.29
Gaunt SJ, Krumlauf R, Duboule D (1989) Mouse homeo-genes within a subfamily, Hox-1.4, -2.6 and -5.1, display similar anteroposterior domains of expression in the embryo, but show stage- and tissue-dependent differences in their regulation. Development 107(1):131–141. https://doi.org/10.1242/dev.107.1.131
Gazea M, Tasouri E, Tolve M, Bosch V, Kabanova A, Gojak C et al (2016) Primary cilia are critical for Sonic hedgehog-mediated dopaminergic neurogenesis in the embryonic midbrain. Dev Biol 409(1):55–71. https://doi.org/10.1016/j.ydbio.2015.10.033
Gene Ontology Consortium (2021) The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res 49(D1):D325–D334. https://doi.org/10.1093/nar/gkaa1113
Genin EC, Plutino M, Bannwarth S, Villa E, Cisneros-Barroso E, Roy M et al (2016) CHCHD10 mutations promote loss of mitochondrial cristae junctions with impaired mitochondrial genome maintenance and inhibition of apoptosis. EMBO Mol Med 8(1):58–72. https://doi.org/10.15252/emmm.201505496
Gregory JM, Livesey MR, McDade K, Selvaraj BT, Barton SK, Chandran S et al (2020) Dysregulation of AMPA receptor subunit expression in sporadic ALS post-mortem brain. J Pathol 250(1):67–78. https://doi.org/10.1002/path.5351
Grossman LI, Purandare N, Arshad R, Gladyck S, Somayajulu M, Hüttemann M et al (2017) MNRR1, a biorganellar regulator of mitochondria. Oxid Med Cell Longev 2017:6739236. https://doi.org/10.1155/2017/6739236
Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y et al (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64(1):60–70. https://doi.org/10.1002/ana.21425
Hata Y, Ma N, Yoneda M, Morimoto S, Okano H, Murayama S et al (2018) Nitrative stress and tau accumulation in amyotrophic lateral sclerosis/parkinsonism-dementia complex (ALS/PDC) in the Kii Peninsula. Japan Front Neurosci 22(11):751. https://doi.org/10.3389/fnins.2017.00751
Hauge AW, Asghar MS, Schytz HW, Christensen K, Olesen J (2009) Effects of tonabersat on migraine with aura: a randomised, double-blind, placebo-controlled crossover study. Lancet Neurol 8(8):718–723. https://doi.org/10.1016/S1474-4422(09)70135-8
Hino S, Sasaki S (2015) Flail arm syndrome with cytoplasmic vacuoles in remaining anterior horn motor neurons: a peculiar variant of amyotrophic lateral sclerosis. Neuropathology 35(6):582–586. https://doi.org/10.1111/neup.12223
Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ et al (2000) Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol 166(1):127–135. https://doi.org/10.1006/exnr.2000.7483
Hwang EM, Kim E, Yarishkin O, Woo DH, Han KS, Park N et al (2014) A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat Commun 5:3227. https://doi.org/10.1038/ncomms4227
Ignatenko O, Malinen S, Rybas S, Vihinen H, Nikkanen J, Kononov A et al (2023) Mitochondrial dysfunction compromises ciliary homeostasis in astrocytes. J Cell Biol 222(1):e202203019. https://doi.org/10.1083/jcb.202203019
Ignatenko O, Nikkanen J, Kononov A, Zamboni N, Ince-Dunn G, Suomalainen A (2020) Mitochondrial spongiotic brain disease: astrocytic stress and harmful rapamycin and ketosis effect. Life Sci Alliance 3(9):e202000797. https://doi.org/10.26508/lsa.202000797
Ikeda A, Nishioka K, Meng H, Takanashi M, Hasegawa I, Inoshita T et al (2019) Mutations in CHCHD2 cause α-synuclein aggregation. Hum Mol Genet 28(23):3895–3911. https://doi.org/10.1093/hmg/ddz241
Ishikawa M, Aoyama T, Shibata S, Sone T, Miyoshi H, Watanabe H et al (2020) miRNA-Based Rapid Differentiation of Purified Neurons from hPSCs Advances towards Quick Screening for Neuronal Disease Phenotypes In Vitro. Cells 9(3):532. https://doi.org/10.3390/cells9030532
Ito D, Morimoto S, Takahashi S, Okada K, Nakahara J, Okano H (2023) Maiden voyage: induced pluripotent stem cell-based drug screening for amyotrophic lateral sclerosis. Brain 146(1):13–19. https://doi.org/10.1093/brain/awac306
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y et al (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236(2):313–322. https://doi.org/10.1006/bbrc.1997.6943
Jansen IE, Bras JM, Lesage S, Schulte C, Gibbs JR, Nalls MA et al (2015) CHCHD2 and Parkinson’s disease. Lancet Neurol 14(7):678–679. https://doi.org/10.1016/S1474-4422(15)00094-0
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28(1):27–30. https://doi.org/10.1093/nar/28.1.27
Kanehisa M (2019) Toward understanding the origin and evolution of cellular organisms. Protein Sci 28(11):1947–1951. https://doi.org/10.1002/pro.3715
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M (2023) KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res 51(D1):D587–D592. https://doi.org/10.1093/nar/gkac963
Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L (2004) Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med 10(4):402–405. https://doi.org/10.1038/nm1021
Kihira T, Sakurai I, Yoshida S, Wakayama I, Takamiya K, Okumura R et al (2015) Neutron activation analysis of scalp hair from ALS patients and residents in the Kii Peninsula. Japan Biol Trace Elem Res 164(1):36–42. https://doi.org/10.1007/s12011-014-0202-6
Kim Y, Griffin JM, Nor MNM, Zhang J, Freestone PS, Danesh-Meyer HV et al (2017) Tonabersat prevents inflammatory damage in the central nervous system by blocking connexin43 hemichannels. Neurotherapeutics 14(4):1148–1165. https://doi.org/10.1007/s13311-017-0536-9
Kleinsimon S, Longmuss E, Rolff J, Jäger S, Eggert A, Delebinski C et al (2018) GADD45A and CDKN1A are involved in apoptosis and cell cycle modulatory effects of viscumTT with further inactivation of the STAT3 pathway. Sci Rep 8(1):5750. https://doi.org/10.1038/s41598-018-24075-x
Kobayashi H, Ueda K, Morimoto S, Ishikawa M, Leventoux N, Sasaki R et al (2023) Protein profiling of extracellular vesicles from iPSC-derived astrocytes of patients with ALS/PDC in Kii peninsula. Neurol Sci 44(12):4511–4516. https://doi.org/10.1007/s10072-023-07000-7
Kokubo Y, Kuzuhara S (2004) Neurofibrillary tangles in ALS and Parkinsonism-dementia complex focus in Kii. Japan. Neurology 63(12):2399–2401. https://doi.org/10.1212/01.wnl.0000147241.52694.6a
Kokubo Y, Banack SA, Morimoto S, Murayama S, Togashi T, Metcalf JS et al (2017) β-N-methylamino-l-alanine analysis in the brains of patients with Kii ALS/PDC. Neurology 89(10):1091–1092. https://doi.org/10.1212/WNL.0000000000004310
Kokubo Y, Kuzuhara S (2003) Neuroradiological study of patients with amyotrophic lateral sclerosis and parkinsonism-dementia complex on the Kii peninsula of Japan. Arch Neurol 60(9):1257–1261. https://doi.org/10.1001/archneur.60.9.1257
Kokubo Y, Taniguchi A, Hasegawa M, Hayakawa Y, Morimoto S, Yoneda M et al (2012) α-Synuclein pathology in the amyotrophic lateral sclerosis/parkinsonism dementia complex in the Kii Peninsula. Japan J Neuropathol Exp Neurol 71(7):625–630. https://doi.org/10.1097/NEN.0b013e31825b9680
Koschmidder E, Weissbach A, Brüggemann N, Kasten M, Klein C, Lohmann K (2016) A nonsense mutation in CHCHD2 in a patient with Parkinson disease. Neurology 86(6):577–579. https://doi.org/10.1212/WNL.0000000000002361
Kuzuhara S (2021) “Endemic paraplegia of Koza in Kii” in Honcho Koji Innen Shu published in 1689 is probably the earliest description of amyotrophic lateral sclerosis of Kii Peninsula: Presentation of the original and investigation of factuality. Rinsho Shinkeigaku 61(12):815–824. https://doi.org/10.5692/clinicalneurol.cn-001688
Kuzuhara S, Kokubo Y, Sasaki R, Narita Y, Yabana T, Hasegawa M et al (2001) Familial amyotrophic lateral sclerosis and parkinsonism-dementia complex of the Kii Peninsula of Japan: clinical and neuropathological study and tau analysis. Ann Neurol 49(4):501–511. https://doi.org/10.1002/ana.100
Kuzuhara S, Kokubo Y (2005) Atypical parkinsonism of Japan: amyotrophic lateral sclerosis-parkinsonism-dementia complex of the Kii peninsula of Japan (Muro disease): an update. Mov Disord 20(Suppl 12):S108–S113. https://doi.org/10.1002/mds.20548
Lautrup S, Sinclair DA, Mattson MP, Fang EF (2019) NAD+ Cell Metab 30(4):630–655. https://doi.org/10.1016/j.cmet.2019.09.001
Lecat S, Belemnaba L, Galzi JL, Bucher B (2015) Neuropeptide Y receptor mediates activation of ERK1/2 via transactivation of the IGF receptor. Cell Signal 27(7):1297–1304. https://doi.org/10.1016/j.cellsig.2015.03.016
Leventoux N, Morimoto S, Hara K, Nakamura S, Ozawa F, Mitsuzawa S et al (2020) Generation of an ALS human iPSC line KEIOi001 A from peripheral blood of a Charcot disease-affected patient carrying TARDBP p. N345K heterozygous SNP mutation. Stem Cell Res 47:101896. https://doi.org/10.1016/j.scr.2020.101896
Leventoux N, Morimoto S, Imaizumi K, Sato Y, Takahashi S, Mashima K et al (2020) Human Astrocytes Model Derived from Induced Pluripotent Stem Cells. Cells 9(12):2680. https://doi.org/10.3390/cells9122680
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Liu Y, Han SS, Wu Y, Tuohy TM, Xue H, Cai J et al (2004) CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol 276(1):31–46. https://doi.org/10.1016/j.ydbio.2004.08.018
Liu Y, Zhang Y (2015) CHCHD2 connects mitochondrial metabolism to apoptosis. Mol Cell Oncol 2(4):e1004964. https://doi.org/10.1080/23723556.2015.1004964
Louie HH, Shome A, Kuo CY, Rupenthal ID, Green CR, Mugisho OO (2021) Connexin43 hemichannel block inhibits NLRP3 inflammasome activation in a human retinal explant model of diabetic retinopathy. Exp Eye Res 202:108384. https://doi.org/10.1016/j.exer.2020.108384
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):550. https://doi.org/10.1186/s13059-014-0550-8
Maquart FX, Bellon G, Pasco S, Monboisse JC (2005) Matrikines in the regulation of extracellular matrix degradation. Biochimie 87(3–4):353–360. https://doi.org/10.1016/j.biochi.2004.10.006
Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH (2008) Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3(6):649–657. https://doi.org/10.1016/j.stem.2008.10.001
Matsumoto T, Fujimori K, Andoh-Noda T, Ando T, Kuzumaki N, Toyoshima M et al (2016) Functional Neurons Generated from T Cell-Derived Induced Pluripotent Stem Cells for Neurological Disease Modeling. Stem Cell Reports 6(3):422–435. https://doi.org/10.1016/j.stemcr.2016.01.010
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62(6):670–684. https://doi.org/10.1007/s00018-004-4464-6
McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Ueda K et al (2002) TorsinA and heat shock proteins act as molecular chaperones: suppression of alpha-synuclein aggregation. J Neurochem 83(4):846–854. https://doi.org/10.1046/j.1471-4159.2002.01190.x
Mimoto T, Miyazaki K, Morimoto N, Kurata T, Satoh K, Ikeda Y et al (2012) Impaired antioxydative Keap1/Nrf2 system and the downstream stress protein responses in the motor neuron of ALS model mice. Brain Res 29(1446):109–118. https://doi.org/10.1016/j.brainres.2011.12.064
Mimuro M, Yoshida M, Kuzuhara S, Kokubo Y (2018) Amyotrophic lateral sclerosis and parkinsonism-dementia complex of the Hohara focus of the Kii Peninsula: a multiple proteinopathy? Neuropathology 38(1):98–107. https://doi.org/10.1111/neup.12434
Modjtahedi N, Tokatlidis K, Dessen P, Kroemer G (2016) Mitochondrial proteins containing coiled-coil-helix-coiled-coil-helix (CHCH) domains in health and disease. Trends Biochem Sci 41(3):245–260. https://doi.org/10.1016/j.tibs.2015.12.004
Morato Torres CA, Wassouf Z, Zafar F, Sastre D, Outeiro TF, Schüle B (2020) The role of alpha-synuclein and other Parkinson’s genes in neurodevelopmental and neurodegenerative disorders. Int J Mol Sci 21(16):5724. https://doi.org/10.3390/ijms21165724
Mori F, Tanji K, Zhang HX, Nishihira Y, Tan CF, Takahashi H et al (2008) Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 116(2):193–203. https://doi.org/10.1007/s00401-008-0396-9
Morimoto S, Takahashi S, Ito D, Daté Y, Okada K, Kato C, Nakamura S, Ozawa F, Chyi CM, Nishiyama A, Suzuki N, Fujimori K, Kondo T, Takao M, Hirai M, Kabe Y, Suematsu M, Jinzaki M, Aoki M, Fujiki Y, Sato Y, Suzuki N, Nakahara J; Pooled Resource Open-Access ALS Clinical Trials Consortium; Okano H (2023) Phase 1/2a clinical trial in ALS with ropinirole, a drug candidate identified by iPSC drug discovery. Cell Stem Cell 30(6):766-780.e9. https://doi.org/10.1016/j.stem.2023.04.017
Morimoto S, Ishikawa M, Watanabe H, Isoda M, Takao M, Nakamura S et al (2020) Brain transcriptome analysis links deficiencies of stress-responsive proteins to the pathomechanism of Kii ALS/PDC. Antioxidants (Basel) 9(5):423. https://doi.org/10.3390/antiox9050423
Morimoto S, Kuzuhara S, Kokubo Y (2009) Increased oxidative stress in patients with amyotrophic lateral sclerosis/Parkinsonism-dementia complex in the Kii peninsula. Japan. Mov Disord 24(1):123–126. https://doi.org/10.1002/mds.22362
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13. https://doi.org/10.1042/BJ20081386
Murru S, Hess S, Barth E, Almajan ER, Schatton D, Hermans S et al (2019) Astrocyte-specific deletion of the mitochondrial m-AAA protease reveals glial contribution to neurodegeneration. Glia 67(8):1526–1541. https://doi.org/10.1002/glia.23626
Nachman E, Wentink AS, Madiona K, Bousset L, Katsinelos T, Allinson K et al (2020) Disassembly of Tau fibrils by the human Hsp70 disaggregation machinery generates small seeding-competent species. J Biol Chem 295(28):9676–9690. https://doi.org/10.1074/jbc.RA120.013478
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H et al (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10(5):615–622. https://doi.org/10.1038/nn1876
Nagano T, Nakashima A, Onishi K, Kawai K, Awai Y, Kinugasa M et al (2017) Proline dehydrogenase promotes senescence through the generation of reactive oxygen species. J Cell Sci 130(8):1413–1420. https://doi.org/10.1242/jcs.196469
Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y et al (2009) Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 117(2):137–149. https://doi.org/10.1007/s00401-008-0477-9
Niewiadomska-Cimicka A, Doussau F, Perot JB, Roux MJ, Keime C, Hache A et al (2021) SCA7 mouse cerebellar pathology reveals preferential downregulation of key Purkinje cell-identity genes and shared disease signature with SCA1 and SCA2. J Neurosci 41(22):4910–4936. https://doi.org/10.1523/JNEUROSCI.1882-20.2021
Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45. https://doi.org/10.1007/978-1-61779-452-0_3
Okano H, Yamanaka S (2014) iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain 31(7):22. https://doi.org/10.1186/1756-6606-7-22
Okano H, Yasuda D, Fujimori K, Morimoto S, Takahashi S (2020) Ropinirole, a new ALS drug candidate developed using iPSCs. Trends Pharmacol Sci 41(2):99–109. https://doi.org/10.1016/j.tips.2019.12.002
Okano H, Morimoto S (2022) iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell 29(2):189–208. https://doi.org/10.1016/j.stem.2022.01.007
Orthmann-Murphy JL, Abrams CK, Scherer SS (2008) Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci 35(1):101–116. https://doi.org/10.1007/s12031-007-9027-5
Osellame LD, Blacker TS, Duchen MR (2012) Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab 26(6):711–723. https://doi.org/10.1016/j.beem.2012.05.003
Osterberg N, Wiehle M, Oehlke O, Heidrich S, Xu C, Fan CM et al (2011) Sim1 is a novel regulator in the differentiation of mouse dorsal raphe serotonergic neurons. PLoS ONE 6(4):e19239. https://doi.org/10.1371/journal.pone.0019239
Oyanagi K, Makifuchi T, Ohtoh T, Chen KM, Gajdusek DC, Chase TN (1997) Distinct pathological features of the gallyas- and tau-positive glia in the Parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. J Neuropathol Exp Neurol 56(3):308–316. https://doi.org/10.1097/00005072-199703000-00010
Ozaki S, Beppu H, Sonoda S, Okazaki H, Mizutani K, Itani Y et al (2007) Relationship between cytokine concentration and activities of daily living in rehabilitation patients with stroke. Rinsho Byori 55(6):522–527
Park MH, Jin HK, Bae JS (2020) Potential therapeutic target for aging and age-related neurodegenerative diseases: the role of acid sphingomyelinase. Exp Mol Med 52(3):380–389. https://doi.org/10.1038/s12276-020-0399-8
Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C (2017) Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14(4):417–419. https://doi.org/10.1038/nmeth.4197
Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V et al (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131(3):323–345. https://doi.org/10.1007/s00401-015-1513-1
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91(22):10625–10629. https://doi.org/10.1073/pnas.91.22.10625
Pemberton S, Madiona K, Pieri L, Kabani M, Bousset L, Melki R (2011) Hsc70 protein interaction with soluble and fibrillar alpha-synuclein. J Biol Chem 286(40):34690–34699. https://doi.org/10.1074/jbc.M111.261321
Peng AYT, Agrawal I, Ho WY, Yen YC, Pinter AJ, Liu J et al (2020) Loss of TDP-43 in astrocytes leads to motor deficits by triggering A1-like reactive phenotype and triglial dysfunction. Proc Natl Acad Sci 117(46):29101–29112. https://doi.org/10.1073/pnas.2007806117
Phatnani H, Maniatis T (2015) Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol 7(6):a020628. https://doi.org/10.1101/cshperspect.a020628
Philips T, Robberecht W (2011) Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol 10(3):253–263. https://doi.org/10.1016/S1474-4422(11)70015-1
Poewe W, Antonini A (2015) Novel formulations and modes of delivery of levodopa. Mov Disord 30(1):114–120. https://doi.org/10.1002/mds.26078
Qi C, Verheijen BM, Kokubo Y, Shi Y, Tetter S, Murzin AG et al (2023) Tau filaments from amyotrophic lateral sclerosis/parkinsonism-dementia complex adopt the CTE fold. Proc Natl Acad Sci USA 120(51):e2306767120. https://doi.org/10.1073/pnas.2306767120
Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI (2001) Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun Adhes 8(4–6):315–320. https://doi.org/10.3109/15419060109080745
Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H et al (2019) g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 47(W1):W191–W198. https://doi.org/10.1093/nar/gkz369
Reimand J, Isserlin R, Voisin V, Kucera M, Tannus-Lopes C, Rostamianfar A et al (2019) Pathway enrichment analysis and visualization of omics data using g: Profiler, GSEA. Cytoscape and EnrichmentMap Nat Protoc 14(2):482–517. https://doi.org/10.1038/s41596-018-0103-9
Ren R, Zhang L, Wang M (2018) Specific deletion connexin43 in astrocyte ameliorates cognitive dysfunction in APP/PS1 mice. Life Sci 1(208):175–191. https://doi.org/10.1016/j.lfs.2018.07.033
Rouach N, Avignone E, Même W, Koulakoff A, Venance L, Blomstrand F et al (2022) Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell 94(7–8):457–475. https://doi.org/10.1016/s0248-4900(02)00016-3
Sasaki R, Morimoto S, Ozawa F, Okano H, Yoshida M, Ishiura H et al (2022) Neurology 99(22):e2437–e2442. https://doi.org/10.1212/WNL.0000000000201156
Schmidt O, Pfanner N, Meisinger C (2010) Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol 11(9):655–667. https://doi.org/10.1038/nrm2959
Seo M, Lee WH, Suk K (2010) Identification of novel cell migration-promoting genes by a functional genetic screen. FASEB J 24(2):464–478. https://doi.org/10.1096/fj.09-137562
Shi CH, Mao CY, Zhang SY, Yang J, Song B, Wu P et al (2016) CHCHD2 gene mutations in familial and sporadic Parkinson’s disease. Neurobiol Aging 38:217.e9-217.e13. https://doi.org/10.1016/j.neurobiolaging.2015.10.040
Shibata S, Iseda T, Mitsuhashi T, Oka A, Shindo T, Moritoki N et al (2019) Large-area fluorescence and electron microscopic correlative imaging with multibeam scanning electron microscopy. Front Neural Circuits 8(13):29. https://doi.org/10.3389/fncir.2019.00029
Shinotoh H, Shimada H, Kokubo Y, Tagai K, Niwa F, Kitamura S et al (2019) Tau imaging detects distinctive distribution of tau pathology in ALS/PDC on the Kii Peninsula. Neurology 92(2):e136–e147. https://doi.org/10.1212/WNL.0000000000006736
Silva RM, Ries V, Oo TF, Yarygina O, Jackson-Lewis V, Ryu EJ et al (2005) CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem 95(4):974–986. https://doi.org/10.1111/j.1471-4159.2005.03428.x
Soneson C, Love MI, Robinson MD (2015) Differential analyses for RNA-seq: transcript level estimates improve gene level inferences. F1000Res 4:1521. https://doi.org/10.12688/f1000research.7563.1
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840. https://doi.org/10.1038/42166
Sun J, Osenberg S, Irwin A, Ma LH, Lee N, Xiang Y et al (2023) Mutations in the transcriptional regulator MeCP2 severely impact key cellular and molecular signatures of human astrocytes during maturation. Cell Rep 42(1):111942. https://doi.org/10.1016/j.celrep.2022.111942
Sung JY, Park SM, Lee CH, Um JW, Lee HJ, Kim J et al (2005) Proteolytic cleavage of extracellular secreted {alpha}-synuclein via matrix metalloproteinases. J Biol Chem 280(26):25216–25224. https://doi.org/10.1074/jbc.M503341200
Supakul S, Okano H, Maeda S (2021) Utilization of human induced pluripotent stem cells-derived in vitro models for the future study of sex differences in Alzheimer’s disease. Front Aging Neurosci 4(13):768948. https://doi.org/10.3389/fnagi.2021
Supakul S, Leventoux N, Tabuchi H, Mimura M, Ito D, Maeda S et al (2022) Establishment of KEIOi005-A iPSC line from urine-derived cells (UDCs) of a mild Alzheimer’s disease (AD) donor with multiple risk SNPs for sporadic Alzheimer’s disease (sAD). Stem Cell Res 62:102802. https://doi.org/10.1016/j.scr.2022.102802
Supakul S, Hatakeyama Y, Leventoux N, Itsuno M, Numata N, Hiramine H et al (2023) Urine-derived cells from the aged donor for the 2D/3D modeling of neural cells via iPSCs. Aging Brain 19(4):100101. https://doi.org/10.1016/j.nbas.2023.100101
Supakul S, Murakami R, Oyama C, Shindo T, Hatakeyama Y, Itsuno M et al (2023) Mutual interaction of neurons and astrocytes derived from iPSCs with APP V717L mutation developed the astrocytic phenotypes of Alzheimer’s disease. Inflammation and Regeneration. https://doi.org/10.1186/s41232-023-00310-5
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. https://doi.org/10.1016/j.cell.2007.11.019
Takahashi S (2021) Lactate and ketone bodies act as energy substrates as well as signal molecules in the brain. psychology and pathophysiological outcomes of eating. IntechOpen 01:00. https://doi.org/10.5772/intechopen.97035
Takahashi S (2020) Metabolic compartmentalization between astroglia and neurons in physiological and pathophysiological conditions of the neurovascular unit. Neuropathology 40(2):121–137. https://doi.org/10.1111/neup.12639
Takahashi S, Mashima K (2022) Neuroprotection and Disease Modification by Astrocytes and Microglia in Parkinson Disease. Antioxidants (Basel) 11(1):170. https://doi.org/10.3390/antiox11010170
Takeuchi H, Mizoguchi H, Doi Y, Jin S, Noda M, Liang J et al (2011) Blockade of gap junction hemichannel suppresses disease progression in mouse models of amyotrophic lateral sclerosis and Alzheimer’s disease. PLoS ONE 6:e21108. https://doi.org/10.1371/journal.pone.0021108
Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE (2007) Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol 67(13):1815–1829. https://doi.org/10.1002/dneu.20559
Tchieu J, Calder EL, Guttikonda SR, Gutzwiller EM, Aromolaran KA, Steinbeck JA et al (2019) NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat Biotechnol 37(3):267–275. https://doi.org/10.1038/s41587-019-0035-0
Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M et al (2011) Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci 14(4):452–458. https://doi.org/10.1038/nn.2778
Tomiyama H, Kokubo Y, Sasaki R, Li Y, Imamichi Y, Funayama M et al (2008) Mutation analyses in amyotrophic lateral sclerosis/parkinsonism-dementia complex of the Kii peninsula. Japan. Mov Disord 23(16):2344–2348. https://doi.org/10.1002/mds.22262
Vandoorne T, Veys K, Guo W, Sicart A, Vints K, Swijsen A et al (2019) Differentiation but not ALS mutations in FUS rewires motor neuron metabolism. Nat Commun 10(1):4147. https://doi.org/10.1038/s41467-019-12099-4
Velebit J, Horvat A, Smolič T, Prpar Mihevc S, Rogelj B, Zorec R et al (2020) Astrocytes with TDP-43 inclusions exhibit reduced noradrenergic cAMP and Ca2+ signaling and dysregulated cell metabolism. Sci Rep 10(1):6003. https://doi.org/10.1038/s41598-020-62864-5
Verheijen BM, Morimoto S, Sasaki R, Oyanagi K, Kokubo Y, Kuzuhara S et al (2020) Expression of mutant ubiquitin and proteostasis impairment in Kii amyotrophic lateral sclerosis/parkinsonism-dementia complex brains. J Neuropathol Exp Neurol 79(8):902–907. https://doi.org/10.1093/jnen/nlaa056
Walker AK, Daniels CM, Goldman JE, Trojanowski JQ, Lee VM, Messing A (2014) Astrocytic TDP-43 pathology in Alexander disease. J Neurosci 34(19):6448–6458. https://doi.org/10.1523/JNEUROSCI.0248-14.2014
Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG et al (2003) Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet 12(21):2753–2764. https://doi.org/10.1093/hmg/ddg312
Wang J, Martin E, Gonzales V, Borchelt DR, Lee MK (2008) Differential regulation of small heat shock proteins in transgenic mouse models of neurodegenerative diseases. Neurobiol Aging 29(4):586–597. https://doi.org/10.1016/j.neurobiolaging.2006.11.009
Wang Y, Wu Z, Liu X, Fu Q (2013) Gastrodin ameliorates Parkinson’s disease by downregulating connexin 43. Mol Med Rep 8(2):585–590. https://doi.org/10.3892/mmr.2013.1535
Watanabe H, Imaizumi K, Cai T, Zhou Z, Tomita T, Okano T (2021) Flexible and accurate substrate processing with distinct presenilin/γ-secretases in human cortical neurons. eNeuro 8(2):0500–0520. https://doi.org/10.1523/ENEURO.0500-20.2021
Weskamp K, Tank EM, Miguez R, McBride JP, Gómez NB, White M et al (2020) Shortened TDP43 isoforms upregulated by neuronal hyperactivity drive TDP43 pathology in ALS. J Clin Invest 130(3):1139–1155. https://doi.org/10.1172/JCI130988
Xu B, Zheng C, Chen X, Zhang Z, Liu J, Spencer P et al (2019) Dysregulation of Myosin Complex and Striated Muscle Contraction Pathway in the Brains of ALS-SOD1 Model Mice. ACS Chem Neurosci 10(5):2408–2417. https://doi.org/10.1021/acschemneuro.8b00704
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH et al (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11(3):251–253. https://doi.org/10.1038/nn2047
Yamazaki M, Hasegawa M, Mori O, Murayama S, Tsuchiya K, Ikeda K et al (2005) Tau-positive fine granules in the cerebral white matter: a novel finding among the tauopathies exclusive to parkinsonism-dementia complex of Guam. J Neuropathol Exp Neurol 64(10):839–846. https://doi.org/10.1097/01.jnen.0000182977.79483.89
Yasui M, Yase Y, Kihira T, Adachi K, Suzuki Y (1992) Magnesium and calcium contents in CNS tissues of amyotrophic lateral sclerosis patients from the Kii peninsula. Japan Eur Neurol 32(2):95–98. https://doi.org/10.1159/000116800
Yasui M, Ota K, Yoshida M (1997) Effects of low calcium and magnesium dietary intake on the central nervous system tissues of rats and calcium-magnesium related disorders in the amyotrophic lateral sclerosis focus in the Kii Peninsula of Japan. Magnes Res 10(1):39–50 (PMID: 9339837)
Yin GN, Lee HW, Cho JY, Suk K (2009) Neuronal pentraxin receptor in cerebrospinal fluid as a potential biomarker for neurodegenerative diseases. Brain Res 10(1265):158–170. https://doi.org/10.1016/j.brainres.2009.01.058
Yu WW, Cao SN, Zang CX, Wang L, Yang HY, Bao XQ et al (2018) Heat shock protein 70 suppresses neuroinflammation induced by α-synuclein in astrocytes. Mol Cell Neurosci 86:58–64. https://doi.org/10.1016/j.mcn.2017.11.013
Yun W, Hong W, Son D, Liu HW, Kim SS, Park M et al (2019) Generation of anterior hindbrain-specific, glial-restricted progenitor-like cells from human pluripotent stem cells. Stem Cells Dev 28(10):633–648. https://doi.org/10.1089/scd.2019.0033
Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD et al (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89(1):37–53. https://doi.org/10.1016/j.neuron.2015.11.013
Zhang Y, Yang X, Zhuang J, Zhang H, Gao C (2022) β-Amyloid activates reactive astrocytes by enhancing glycolysis of astrocytes. Mol Biol Rep 49(6):4699–4707. https://doi.org/10.1007/s11033-022-07319-y
Zhao W, Xu Z, Cao J, Fu Q, Wu Y, Zhang X et al (2019) Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J Neuroinflammation 16(1):230. https://doi.org/10.1186/s12974-019-1627-9
Zhou B, Zuo YX, Jiang RT (2019) Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci Ther 25(6):665–673. https://doi.org/10.1111/cns.13123
Zhou W, Ma D, Sun AX, Tran HD, Ma DL, Singh BK et al (2019) PD-linked CHCHD2 mutations impair CHCHD10 and MICOS complex leading to mitochondria dysfunction. Hum Mol Genet 28(7):1100–1116. https://doi.org/10.1093/hmg/ddy413
Zhu L, Gomez-Duran A, Saretzki G, Jin S, Tilgner K, Melguizo-Sanchis D et al (2016) The mitochondrial protein CHCHD2 primes the differentiation potential of human induced pluripotent stem cells to neuroectodermal lineages. J Cell Biol 215(2):187–202. https://doi.org/10.1083/jcb.201601061
Ziff OJ, Clarke BE, Taha DM, Crerar H, Luscombe NM, Patani R (2022) Meta-analysis of human and mouse ALS astrocytes reveals multi-omic signatures of inflammatory reactive states. Genome Res 32(1):71–84. https://doi.org/10.1101/gr.275939.121
Acknowledgements
We would like to extend our warmest thanks to the patients and families affected by Kii ALS/PDC whose generosity has enabled us to make a major advance in the mechanistic understanding of the pathology of this disease.
For their technical support, we would like to express our thanks to Masako Ichishi at the Department of Oncologic Pathology, Mie University Graduate School of Medicine, Mie, Japan, and Mitsutoshi Tano at the Mihara Memorial Hospital, Isesaki, Japan, for preparing the human spinal cord and brains samples. We would also like to thank Mari Fujiwara of the Core Facility, Collaborative Research Resources at Keio University for her precious support during image acquisition of human samples.
We also thank Profs. Shinya Yamanaka and Masato Nakagawa, CiRA, Kyoto University for providing the iPSCs (201B7).
For his great contribution to the study of Kii ALS/PDC, we acknowledge Professor Shigeki Kuzuhara (1944-2021).
Funding
S.M. reports grant supports from Japan Society for the Promotion of Science (JSPS) (KAKENHI Grant No. JP15J03921, JP18K07368, JP18KK0239, JP19K17002, JP19K08002, JP21H05278, JP22K07500, and JP22K15736), the Japan Agency for Medical Research and Development (AMED) (Grant No. JP23bm1123046, JP23kk0305024), Japan Intractable Diseases Research Foundation, The Kanae Foundation for the Promotion of Medical Science, The Uehara Memorial Foundation, THE YUKIHIKO MIYATA MEMORIAL TRUST FOR ALS RESEARCH, Okasan-Kato Foundation Research Grant and Yoshio Koide Grant, Japan ALS Association, Daiichi Sankyo Foundation of Life Science and UBE Academic Foundation during the conduct of the study. M.I. reports grants from AMED (Grant No. JP22bm0804023) and JSPS (KAKENHI Grant No. JP17K10083, JP20H03567, JP22K18388, and JP23H02882). S.T. reports a grant from JSPS (KAKENHI Grant No. JP22K07500). S.S. reports a grant from AMED (Grant No. JP22gm6510006). M.T. reports grants from AMED (Grant No. JP21wm0425019), Intramural funds from the National Center of Neurology and Psychiatry (NCNP), JSPS (KAKENHI Grant No. JP21K06417, JP18K06506, and JP22H04923), the Research Committee of Prion Disease and Slow Virus Infection, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health and Labour Sciences Research Grants, the Ministry of Health, Labour and Welfare (MHLW), Japan. Y.K. reports grant supports from the Mie Medical Fund, Japan Foundation for Neuroscience and Mental Health, the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases (H29-Nanchi-Ippan-085, collaborator, 2020–2022), the Research Committee of Muro Disease (Kii ALS/PDC) (Grant No. 21210301, Chair, 2009–2014), by MHLW, Japan, JSPS (KAKENHI Grant No. JP25305030, JP15K09364, JP17H01689, JP18K07514, JP18KK0239, and JP18K07368), and by a grant-in-aid of the Research Consortium of Kii ALS/PDC from AMED (Grant No. JP17ek0109139). H.O. reports grant supports from JSPS (KAKENHI Grant No. JP20H00485, JP21F21410, JP21H05273, and JP22KF0333) and AMED (Grant No. JP22bm0804003, JP20ek0109395, JP20ek0109329, JP21wm0425009, and JP23bm1423002). The human spinal cord samples were provided by Platform of Supporting Cohort Study and Biospecimen Analysis, Grant-in-Aid for Scientific Research on Innovative Areas—Platforms for Advanced Technologies and Research Resources, the Japanese MEXT (Grant No. JP16H06277) and Intramural Research Grant for Neurological Psychiatric Disorders from NCNP. The funding sources had no role in the analysis.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Satoru Morimoto, Hideyuki Okano, Yasumasa Kokubo, Nicolas Leventoux, Hirotaka Watanabe, Satoshi Okamoto and Zhi Zhou conceptualized the programme research and designed the methodology. Yasumasa Kokubo and Ikuko Aiba treated the patients at the hospital and provided the clinical data. Yasumasa Kokubo selected the donors. Mitsuru Ishikawa, Satoru Morimoto, Shiho Nakamura and Fumiko Ozawa generated the Kii-iPSC. Nicolas Leventoux and Sopak Supakul performed the three-germ layers experiments. Nicolas Leventoux and Satoru Morimoto differentiated the Kii-iPSC into astrocytes. Nicolas Leventoux and Sopak Supakul generated the iPSC-derived neurons and their coculture with astrocytes. RNA profiling and bioinformatics study were performed by Nicolas Leventoux, Satoru Morimoto, Chris Kato, Koji Yamanaka, Fumito Endo, Shiho Nakamura and Fumiko Ozawa. Staining was performed by Nicolas Leventoux, Satoru Morimoto and Reona Kobayashi. Glutamate uptake assays and metabolic profiling were performed by Nicolas Leventoux, Zhi Zhou and Satoru Morimoto. Post-mortem tissue preparation was performed by Yoshifumi Hirokawa, Masaki Takao, Mari Yoshida and Yasumasa Kokubo. Pictures were taken by Nicolas Leventoux, Hiroya Kobayashi and Satoru Morimoto. Electronic microscopy was performed by Satoru Morimoto and Shinsuke Shibata. Counts and statistics were performed by Nicolas Leventoux and Satoru Morimoto. Nicolas Leventoux, Satoru Morimoto, Hiroya Kobayashi and Hideyuki Okano wrote the first draft of the manuscript. Shinichi Takahashi and Mari Yoshida critically reviewed and modified the manuscript. Hideyuki Okano oversaw the whole research process. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
H.O. reports grants and personal fees from K Pharma, Inc. during the conduct of the study; personal fees from Sanbio Co. Ltd., outside the submitted work; In addition, H.O. has a patent on a therapeutic agent for amyotrophic lateral sclerosis and composition for treatment licensed to K Pharma, Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leventoux, N., Morimoto, S., Ishikawa, M. et al. Aberrant CHCHD2-associated mitochondriopathy in Kii ALS/PDC astrocytes. Acta Neuropathol 147, 84 (2024). https://doi.org/10.1007/s00401-024-02734-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00401-024-02734-w