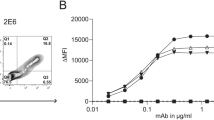
Abstract
Serum autoantibodies targeting the nicotinic acetylcholine receptor (AChR) in patients with autoimmune myasthenia gravis (MG) can mediate pathology via three distinct molecular mechanisms: complement activation, receptor blockade, and antigenic modulation. However, it is unclear whether multi-pathogenicity is mediated by individual or multiple autoantibody clones. Using an unbiased B cell culture screening approach, we generated a library of 11 human-derived AChR-specific recombinant monoclonal autoantibodies (mAb) and assessed their binding properties and pathogenic profiles using specialized cell-based assays. Five mAbs activated complement, three blocked α-bungarotoxin binding to the receptor, and seven induced antigenic modulation. Furthermore, two clonally related mAbs derived from one patient were each highly efficient at more than one of these mechanisms, demonstrating that pathogenic mechanisms are not mutually exclusive at the monoclonal level. Using novel Jurkat cell lines that individually express each monomeric AChR subunit (α2βδε), these two mAbs with multi-pathogenic capacity were determined to exclusively bind the α-subunit of AChR, demonstrating an association between mAb specificity and pathogenic capacity. These findings provide new insight into the immunopathology of MG, demonstrating that single autoreactive clones can efficiently mediate multiple modes of pathology. Current therapeutic approaches targeting only one autoantibody-mediated pathogenic mechanism may be evaded by autoantibodies with multifaceted capacity.




Similar content being viewed by others
References
Allen NM, O’Rahelly M, Eymard B, Chouchane M, Hahn A, Kearns G et al (2023) The emerging spectrum of foetal acetylcholine receptor antibody-associated disorders (FARAD). Brain. https://doi.org/10.1093/brain/awad153
Beeson D, Amar M, Bermudez I, Vincent A, Newsom-Davis J (1996) Stable functional expression of the adult subtype of human muscle acetylcholine receptor following transfection of the human rhabdomyosarcoma cell line TE671 with cDNA encoding the epsilon subunit. Neurosci Lett 207:57–60. https://doi.org/10.1016/0304-3940(96)12488-5
Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C et al (2009) Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol 66:617–629. https://doi.org/10.1002/ana.21802
Brochet X, Lefranc MP, Giudicelli V (2008) IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res 36:W503-508. https://doi.org/10.1093/nar/gkn316
Cardona A, Pritsch O, Dumas G, Bach JF, Dighiero G (1995) Evidence for an antigen-driven selection process in human autoantibodies against acetylcholine receptor. Mol Immunol 32:1215–1223. https://doi.org/10.1016/0161-5890(95)00101-8
Chang CC, Lee CY (1963) Isolation of neurotoxins from the venom of bungarus multicinctus and their modes of neuromuscular blocking action. Arch Int Pharmacodyn Ther 144:241–257
Changeux JP, Kasai M, Lee CY (1970) Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A 67:1241–1247. https://doi.org/10.1073/pnas.67.3.1241
Cho MJ, Lo AS, Mao X, Nagler AR, Ellebrecht CT, Mukherjee EM et al (2014) Shared VH1-46 gene usage by pemphigus vulgaris autoantibodies indicates common humoral immune responses among patients. Nat Commun 5:1–11
Cotzomi E, Stathopoulos P, Lee CS, Ritchie AM, Soltys JN, Delmotte FR et al (2019) Early B cell tolerance defects in neuromyelitis optica favour anti-AQP4 autoantibody production. Brain 142:1598–1615. https://doi.org/10.1093/brain/awz106
Drachman DB, Angus CW, Adams RN, Michelson JD, Hoffman GJ (1978) Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med 298:1116–1122
Dunand CJH, Wilson PC (2015) Restricted, canonical, stereotyped and convergent immunoglobulin responses. Philosoph Trans Royal Soc Biol Sci 370:20140238
Engel AG, Fumagalli G (1982) Mechanisms of acetylcholine receptor loss from the neuromuscular junction. Ciba Found Symp. https://doi.org/10.1002/9780470720721.ch12
Farrar J, Portolano S, Willcox N, Vincent A, Jacobson L, Newsom-Davis J et al (1997) Diverse Fab specific for acetylcholine receptor epitopes from a myasthenia gravis thymus combinatorial library. Int Immunol 9:1311–1318. https://doi.org/10.1093/intimm/9.9.1311
Fichtner ML, Hoehn KB, Ford EE, Mane-Damas M, Oh S, Waters P et al (2022) Reemergence of pathogenic, autoantibody-producing B cell clones in myasthenia gravis following B cell depletion therapy. Acta Neuropathol Commun 10:154. https://doi.org/10.1186/s40478-022-01454-0
Fichtner ML, Jiang R, Bourke A, Nowak RJ, O’Connor KC (2020) Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology. Front Immunol. https://doi.org/10.3389/fimmu.2020.00776
Fostieri E, Beeson D, Tzartos SJ (2000) The conformation of the main immunogenic region on the alpha-subunit of muscle acetylcholine receptor is affected by neighboring receptor subunits. FEBS Lett 481:127–130. https://doi.org/10.1016/s0014-5793(00)01980-3
Fostieri E, Tzartos SJ, Berrih-Aknin S, Beeson D, Mamalaki A (2005) Isolation of potent human Fab fragments against a novel highly immunogenic region on human muscle acetylcholine receptor which protect the receptor from myasthenic autoantibodies. Eur J Immunol 35:632–643. https://doi.org/10.1002/eji.200425671
Gilhus NE (2016) Myasthenia Gravis. N Engl J Med 375:2570–2581. https://doi.org/10.1056/NEJMra1602678
Gomez CM, Richman DP (1983) Anti-acetylcholine receptor antibodies directed against the alpha-bungarotoxin binding site induce a unique form of experimental myasthenia. Proc Natl Acad Sci USA 80:4089–4093. https://doi.org/10.1073/pnas.80.13.4089
Graus YF, de Baets MH, Parren PW, Berrih-Aknin S, Wokke J, van Breda Vriesman PJ et al (1997) Human anti-nicotinic acetylcholine receptor recombinant Fab fragments isolated from thymus-derived phage display libraries from myasthenia gravis patients reflect predominant specificities in serum and block the action of pathogenic serum antibodies. J Immunol 158:1919–1929
Gu Y, Forsayeth JR, Verrall S, Yu XM, Hall ZW (1991) Assembly of the mammalian muscle acetylcholine receptor in transfected COS cells. J Cell Biol 114:799–807
Guigou V, Emilie D, Berrih-Aknin S, Fumoux F, Fougereau M, Schiff C (1991) Individual germinal centres of myasthenia gravis human thymuses contain polyclonal activated B cells that express all the Vh and Vk families. Clin Exp Immunol 83:262–266. https://doi.org/10.1111/j.1365-2249.1991.tb05625.x
Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M et al (2001) The binding site of acetylcholine receptor as visualized in the X-Ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron 32:265–275. https://doi.org/10.1016/s0896-6273(01)00461-5
Howard JF Jr, Nowak RJ, Wolfe GI, Freimer ML, Vu TH, Hinton JL et al (2020) Clinical Effects of the Self-administered subcutaneous complement inhibitor zilucoplan in patients with moderate to severe generalized myasthenia gravis: results of a phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. JAMA Neurol 77:582–592. https://doi.org/10.1001/jamaneurol.2019.5125
Howard JF Jr, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I et al (2017) Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol 16:976–986. https://doi.org/10.1016/S1474-4422(17)30369-1
Howard JF Jr, Karam C, Yountz M, O’Brien FL, Mozaffar T, Group ftRS (2021) Long-term efficacy of eculizumab in refractory generalized myasthenia gravis: responder analyses. Annals Clin Translat Neurol 8:1398–1407. https://doi.org/10.1002/acn3.51376
Huijbers MG, Marx A, Plomp JJ, Le Panse R, Phillips WD (2022) Advances in the understanding of disease mechanisms of autoimmune neuromuscular junction disorders. Lancet Neurol 21:163–175. https://doi.org/10.1016/S1474-4422(21)00357-4
Jacobson L, Beeson D, Tzartos S, Vincent A (1999) Monoclonal antibodies raised against human acetylcholine receptor bind to all five subunits of the fetal isoform. J Neuroimmunol 98:112–120. https://doi.org/10.1016/s0165-5728(99)00086-7
Jiang R, Hoehn KB, Lee CS, Pham MC, Homer RJ, Detterbeck FC et al (2020) Thymus-derived B cell clones persist in the circulation after thymectomy in myasthenia gravis. Proc Natl Acad Sci USA 117:30649–30660. https://doi.org/10.1073/pnas.2007206117
Karlin A (2002) Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci 3:102–114. https://doi.org/10.1038/nrn731
Koers J, Derksen NIL, Ooijevaar-de Heer P, Nota B, van de Bovenkamp FS, Vidarsson G et al (2019) Biased N-Glycosylation site distribution and acquisition across the antibody v region during B cell maturation. J Immunol 202:2220–2228. https://doi.org/10.4049/jimmunol.1801622
Lang B, Richardson G, Rees J, Vincent A, Newsom-Davis J (1988) Plasma from myasthenia gravis patients reduces acetylcholine receptor agonist-induced Na+ flux into TE671 cell line. J Neuroimmunol 19:141–148. https://doi.org/10.1016/0165-5728(88)90043-4
Lee JY, Stathopoulos P, Gupta S, Bannock JM, Barohn RJ, Cotzomi E et al (2016) Compromised fidelity of B-cell tolerance checkpoints in AChR and MuSK myasthenia gravis. Ann Clin Transl Neurol 3:443–454. https://doi.org/10.1002/acn3.311
Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP et al (2008) IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis†. Brain 131:1940–1952. https://doi.org/10.1093/brain/awn092
Lozier BK, Haven TR, Astill ME, Hill HR (2015) Detection of acetylcholine receptor modulating antibodies by flow cytometry. Am J Clin Pathol 143:186–196. https://doi.org/10.1309/ajcpyeor6sge8zlu
Makino T, Nakamura R, Terakawa M, Muneoka S, Nagahira K, Nagane Y et al (2017) Analysis of peripheral B cells and autoantibodies against the anti-nicotinic acetylcholine receptor derived from patients with myasthenia gravis using single-cell manipulation tools. PLoS ONE 12:e0185976. https://doi.org/10.1371/journal.pone.0185976
Mandel-Brehm C, Fichtner ML, Jiang R, Winton VJ, Vazquez SE, Pham MC et al (2021) Elevated N-Linked Glycosylation of IgG V regions in myasthenia gravis disease subtypes. J Immunol 207:2005–2014. https://doi.org/10.4049/jimmunol.2100225
Mannara F, Radosevic M, Planagumà J, Soto D, Aguilar E, García-Serra A et al (2020) Allosteric modulation of NMDA receptors prevents the antibody effects of patients with anti-NMDAR encephalitis. Brain 143:2709–2720. https://doi.org/10.1093/brain/awaa195
Marx A, Pfister F, Schalke B, Saruhan-Direskeneli G, Melms A, Strobel P (2013) The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev 12:875–884. https://doi.org/10.1016/j.autrev.2013.03.007
Masi G, Li Y, Karatz T, Pham MC, Oxendine SR, Nowak RJ et al (2022) The clinical need for clustered AChR cell-based assay testing of seronegative MG. J Neuroimmunol 367:577850. https://doi.org/10.1016/j.jneuroim.2022.577850
Matthews I, Farrar J, McLachlan S, Rapoport B, Newsom-Davis J, Willcox N et al (1998) Production of Fab Fragments against the human acetylcholine receptor from myasthenia gravis thymus lambda and kappa phage libraries a. Anna NewYork Acad Sci 841:418–421
Matthews I, Sims G, Ledwidge S, Stott D, Beeson D, Willcox N et al (2002) Antibodies to acetylcholine receptor in parous women with myasthenia: evidence for immunization by fetal antigen. Lab Invest 82:1407–1417
Muppidi S, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I et al (2019) Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve 60:14–24. https://doi.org/10.1002/mus.26447
Noviello CM, Kreye J, Teng J, Prüss H, Hibbs RE (2022) Structural mechanisms of GABAA receptor autoimmune encephalitis. Cell 185(2469–2477):e2413
Obaid AH, Zografou C, Vadysirisack DD, Munro-Sheldon B, Fichtner ML, Roy B et al (2022) Heterogeneity of acetylcholine receptor autoantibody-mediated complement activity in patients with myasthenia gravis. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000001169
Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA et al (2001) Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Investig 108:1061–1070
Radosevic M, Planagumà J, Mannara F, Mellado A, Aguilar E, Sabater L et al (2022) Allosteric modulation of nmdars reverses patients’ autoantibody effects in mice. Neurol Neuroimmunol Neuroinflam 9:e1122. https://doi.org/10.1212/nxi.0000000000001122
Rahman MM, Teng J, Worrell BT, Noviello CM, Lee M, Karlin A et al (2020) Structure of the native muscle-type nicotinic receptor and inhibition by snake venom toxins. Neuron 106:952–962. https://doi.org/10.1016/j.neuron.2020.03.012
Rose N, Holdermann S, Callegari I, Kim H, Fruh I, Kappos L et al (2022) Receptor clustering and pathogenic complement activation in myasthenia gravis depend on synergy between antibodies with multiple subunit specificities. Acta Neuropathol 144:1005–1025. https://doi.org/10.1007/s00401-022-02493-6
Sims GP, Shiono H, Willcox N, Stott DI (2001) Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J Immunol 167:1935–1944. https://doi.org/10.4049/jimmunol.167.4.1935
Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R et al (2009) Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc 4:372–384. https://doi.org/10.1038/nprot.2009.3
Soltys J, Liu Y, Ritchie A, Wemlinger S, Schaller K, Schumann H et al (2019) Membrane assembly of aquaporin-4 autoantibodies regulates classical complement activation in neuromyelitis optica. J Clin Invest 129:2000–2013. https://doi.org/10.1172/JCI122942
Spatola M, Petit-Pedrol M, Simabukuro MM, Armangue T, Castro FJ, Barcelo Artigues MI et al (2017) Investigations in GABA(A) receptor antibody-associated encephalitis. Neurology 88:1012–1020. https://doi.org/10.1212/wnl.0000000000003713
Spreadbury I, Kishore U, Beeson D, Vincent A (2005) Inhibition of acetylcholine receptor function by seronegative myasthenia gravis non-IgG factor correlates with desensitisation. J Neuroimmunol 162:149–156. https://doi.org/10.1016/j.jneuroim.2005.01.009
Stathopoulos P, Chastre A, Waters P, Irani S, Fichtner ML, Benotti ES et al (2019) Autoantibodies against neurologic antigens in nonneurologic autoimmunity. J Immunol 202:2210–2219. https://doi.org/10.4049/jimmunol.1801295
Stathopoulos P, Kumar A, Nowak RJ, O’Connor KC (2017) Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight. https://doi.org/10.1172/jci.insight.94263
Sterz R, Hohlfeld R, Rajki K, Kaul M, Heininger K, Peper K et al (1986) Effector mechanisms in myasthenia gravis: end-plate function after passive transfer of IgG, Fab, and F(ab’)2 hybrid molecules. Muscle Nerve 9:306–312. https://doi.org/10.1002/mus.880090404
Su KY, Watanabe A, Yeh CH, Kelsoe G, Kuraoka M (2016) Efficient culture of human naive and memory B cells for Use as APCs. J Immunol 197:4163–4176. https://doi.org/10.4049/jimmunol.1502193
Takata K, Stathopoulos P, Cao M, Mané-Damas M, Fichtner ML, Benotti ES et al (2019) Characterization of pathogenic monoclonal autoantibodies derived from muscle-specific kinase myasthenia gravis patients. JCI Insight. https://doi.org/10.1172/jci.insight.127167
Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H (2008) Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods 329:112–124. https://doi.org/10.1016/j.jim.2007.09.017
Tindall RS (1981) Humoral immunity in myasthenia gravis: clinical correlations of anti-receptor antibody avidity and titer. Ann N Y Acad Sci 377:316–331. https://doi.org/10.1111/j.1749-6632.1981.tb33741.x
Toyka KV, Brachman DB, Pestronk A, Kao I (1975) Myasthenia gravis: passive transfer from man to mouse. Science 190:397–399. https://doi.org/10.1126/science.1179220
Tradtrantip L, Zhang H, Saadoun S, Phuan PW, Lam C, Papadopoulos MC et al (2012) Anti–Aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann Neurol 71:314–322
Tzartos S, Hochschwender S, Vasquez P, Lindstrom J (1987) Passive transfer of experimental autoimmune myasthenia gravis by monoclonal antibodies to the main immunogenic region of the acetylcholine receptor. J Neuroimmunol 15:185–194. https://doi.org/10.1016/0165-5728(87)90092-0
Tzartos SJ, Barkas T, Cung MT, Mamalaki A, Marraud M, Orlewski P et al (1998) Anatomy of the antigenic structure of a large membrane autoantigen, the muscle-type nicotinic acetylcholine receptor. Immunol Rev 163:89–120. https://doi.org/10.1111/j.1600-065x.1998.tb01190.x
Tzartos SJ, Lindstrom JM (1980) Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci USA 77:755–759. https://doi.org/10.1073/pnas.77.2.755
Tzartos SJ, Seybold ME, Lindstrom JM (1982) Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci 79:188–192
Tzartos SJ, Sophianos D, Efthimiadis A (1985) Role of the main immunogenic region of acetylcholine receptor in myasthenia gravis. An Fab monoclonal antibody protects against antigenic modulation by human sera. J Immunol 134:2343–2349
Verschuuren JJ, Huijbers MG, Plomp JJ, Niks EH, Molenaar PC, Martinez-Martinez P et al (2013) Pathophysiology of myasthenia gravis with antibodies to the acetylcholine receptor, muscle-specific kinase and low-density lipoprotein receptor-related protein 4. Autoimmun Rev 12:918–923. https://doi.org/10.1016/j.autrev.2013.03.001
Vincent A (2002) Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol 2:797–804. https://doi.org/10.1038/nri916
Vrolix K, Fraussen J, Losen M, Stevens J, Lazaridis K, Molenaar PC et al (2014) Clonal heterogeneity of thymic B cells from early-onset myasthenia gravis patients with antibodies against the acetylcholine receptor. J Autoimmun 52:101–112. https://doi.org/10.1016/j.jaut.2013.12.008
Vu T, Meisel A, Mantegazza R, Annane D, Katsuno M, Aguzzi R et al (2022) Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evid 1:2100066. https://doi.org/10.1056/EVIDoa2100066
Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC (2003) Predominant autoantibody production by early human B cell precursors. Science 301:1374–1377. https://doi.org/10.1126/science.1086907
Yamagami J, Payne AS, Kacir S, Ishii K, Siegel DL, Stanley JR (2010) Homologous regions of autoantibody heavy chain complementarity-determining region 3 (H-CDR3) in patients with pemphigus cause pathogenicity. J Clin Investig 120:4111–4117
Acknowledgements
We thank Dr. Philip Coish for manuscript editing and Dr. Bhaskar Roy for advice on statistical analysis. The AQP4-specific human mAb58 was generously provided by Dr. Jeffrey L. Bennett of the Departments of Neurology and Ophthalmology, Programs in Neuroscience and Immunology, University of Colorado Anschutz Medical Campus, Aurora CO.
Funding
Minh C. Pham was supported by the NIH T32 predoctoral training grant (award number T32AI007019-46). Gianvito Masi is an MGNet Scholar Awardee. MGNet is a member of the Rare Disease Clinical Research Network Consortium (RDCRN) NIH U54 NS115054. All RDCRN consortia are supported by the network’s Data Management and Coordinating Center (DMCC) (U2CTR002818). Funding support for the DMCC is provided by the National Center for Advancing Translational Sciences (NCATS) and the National Institute of Neurological Disorders and Stroke (NINDS). Rosa Patzina was supported, in part, by the Biomedical Education Program (BMEP) funded by the German Academic Exchange Service (DAAD). Kevin C. O’Connor is supported by the National Institute of Allergy and Infectious Diseases of the NIH under award numbers R01-AI114780 and R21-AI142198, through an award provided through the Rare Diseases Clinical Research Consortia of the NIH and MGNet (award number U54-NS115054), and a pilot award from MGFA (Myasthenia Gravis Foundation of America). The funders had no role in the decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
This study was originally conceived, initiated, and directed by KCO. MCP led the laboratory work at Yale, designed the study, optimized assays, performed experiments, interpreted data, and wrote the initial manuscript draft. All authors contributed to the acquisition, analysis, or interpretation of data. GM, RP, AHO, and SRO optimized assays, performed experiments, and interpreted data. RJN provided the clinical specimens and provided insight on the clinical and therapeutic relevance of the findings. SO and AP designed and optimized assays for subunit epitope mapping at the University of Pennsylvania. Figures were produced with GraphPad Prism, FlowJo, and BioRender by MCP. All authors contributed to the editing and revising of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Aimee S. Payne has received equity, research support, patent licensing and other payments from Cabaletta Bio; patent licensing payments from Novartis; and consultant fees from Janssen. Dr. Sangwook Oh has received patent licensing payments from Cabaletta Bio. Dr. Richard J. Nowak has received research support from the NIH, Genentech, Alexion Pharmaceuticals, argenx, Annexon Biosciences, UCB Ra Pharmaceuticals, Myasthenia Gravis Foundation of America, Momenta, Immunovant, Grifols, and Viela Bio, now (Horizon Therapeutics). RJN has served as consultant/ advisor for Alexion Pharmaceuticals, argenx, Cabaletta Bio, CSL Behring, Grifols, Ra Pharmaceuticals, now a part of UCB Pharma, Immunovant, Momenta, and Viela Bio, now a part of Horizon Therapeutics; Dr. Kevin C. O’Connor has received research support from Ra Pharma, now (UCB Pharma), Alexion, now (AstraZeneca), Viela Bio, now (Horizon Therapeutics), and argenx. KCO is a consultant and equity shareholder of Cabaletta Bio. KCO has served as a consultant/advisor for Alexion Pharmaceuticals, now (AstraZeneca), and Roche. The authors have no additional financial interests. All other authors declare no competing financial interests.
Human participants
Deidentified human-derived specimens were retrieved from a biorepository established at the Yale University School of Medicine under the approval of Yale University’s Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pham, M.C., Masi, G., Patzina, R. et al. Individual myasthenia gravis autoantibody clones can efficiently mediate multiple mechanisms of pathology. Acta Neuropathol 146, 319–336 (2023). https://doi.org/10.1007/s00401-023-02603-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-023-02603-y