Abstract
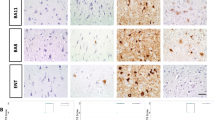
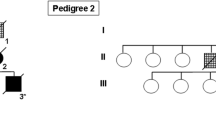
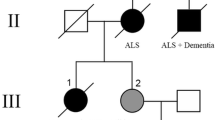
Tuberous sclerosis complex (TSC) is a neurogenetic disorder leading to epilepsy, developmental delay, and neurobehavioral dysfunction. The syndrome is caused by pathogenic variants in TSC1 (coding for hamartin) or TSC2 (coding for tuberin). Recently, we reported a progressive frontotemporal dementia-like clinical syndrome in a patient with a mutation in TSC1, but the neuropathological changes seen in adults with TSC with or without dementia have yet to be systematically explored. Here, we examined neuropathological findings in adults with TSC (n = 11) aged 30–58 years and compared them to age-matched patients with epilepsy unrelated to TSC (n = 9) and non-neurological controls (n = 10). In 3 of 11 subjects with TSC, we observed a neurofibrillary tangle-predominant “TSC tauopathy” not seen in epilepsy or non-neurological controls. This tauopathy was observed in the absence of pathological amyloid beta, TDP-43, or alpha-synuclein deposition. The neurofibrillary tangles in TSC tauopathy showed a unique pattern of post-translational modifications, with apparent differences between TSC1 and TSC2 mutation carriers. Tau acetylation (K274, K343) was prominent in both TSC1 and TSC2, whereas tau phosphorylation at a common phospho-epitope (S202) was observed only in TSC2. TSC tauopathy was observed in selected neocortical, limbic, subcortical, and brainstem sites and showed a 3-repeat greater than 4-repeat tau isoform pattern in both TSC1 and TSC2 mutation carriers, but no tangles were immunolabeled with MC1 or p62 antibodies. The findings suggest that individuals with TSC are at risk for a unique tauopathy in mid-life and that tauopathy pathogenesis may involve TSC1, TSC2, and related molecular pathways.




Similar content being viewed by others
References
Al-Saleem T, Wessner LL, Scheithauer BW, Patterson K, Roach ES, Dreyer SJ et al (1998) Malignant tumors of the kidney, brain, and soft tissues in children and young adults with the tuberous sclerosis complex. Cancer 83:2208–2216
Alquezar C, Schoch KM, Geier EG, Ramos EM, Scrivo A, Li KH et al (2021) TSC1 loss increases risk for tauopathy by inducing tau acetylation and preventing tau clearance via chaperone-mediated autophagy. Sci Adv 7:eabg3897. https://doi.org/10.1126/sciadv.abg3897
Braak H, Thal DR, Ghebremedhin E, Del Tredici K (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. https://doi.org/10.1097/NEN.0b013e318232a379
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. https://doi.org/10.1007/s00401-014-1349-0
Curatolo P, Moavero R, de Vries PJ (2015) Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol 14:733–745. https://doi.org/10.1016/S1474-4422(15)00069-1
de Vries PJ, Whittemore VH, Leclezio L, Byars AW, Dunn D, Ess KC et al (2015) Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr Neurol 52:25–35. https://doi.org/10.1016/j.pediatrneurol.2014.10.004
European Chromosome 16 Tuberous Sclerosis C (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75:1305–1315. https://doi.org/10.1016/0092-8674(93)90618-z
Feliciano DM (2020) The neurodevelopmental pathogenesis of tuberous sclerosis complex (TSC). Front Neuroanat 14:39. https://doi.org/10.3389/fnana.2020.00039
Grinberg LT, Wang X, Wang C, Sohn PD, Theofilas P, Sidhu M et al (2013) Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta Neuropathol. https://doi.org/10.1007/s00401-013-1080-2
Kerfoot C, Wienecke R, Menchine M, Emelin J, Maize JC Jr, Welsh CT et al (1996) Localization of tuberous sclerosis 2 mRNA and its protein product tuberin in normal human brain and in cerebral lesions of patients with tuberous sclerosis. Brain Pathol 6:367–375. https://doi.org/10.1111/j.1750-3639.1996.tb00866.x
Liu AJ, Lusk JB, Ervin J, Burke J, O’Brien R, Wang SJ (2022) Tuberous sclerosis complex is a novel, amyloid-independent tauopathy associated with elevated phosphorylated 3R/4R tau aggregation. Acta Neuropathol Commun 10:27. https://doi.org/10.1186/s40478-022-01330-x
Liu AJ, Staffaroni AM, Rojas-Martinez JC, Olney NT, Alquezar-Burillo C, Ljubenkov PA et al (2020) Association of cognitive and behavioral features between adults with tuberous sclerosis and frontotemporal dementia. JAMA Neurol 77:358–366. https://doi.org/10.1001/jamaneurol.2019.4284
McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I et al (2016) The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131:75–86. https://doi.org/10.1007/s00401-015-1515-z
McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH et al (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136:43–64. https://doi.org/10.1093/brain/aws307
Mizuguchi M, Ikeda K, Takashima S (2000) Simultaneous loss of hamartin and tuberin from the cerebrum, kidney and heart with tuberous sclerosis. Acta Neuropathol 99:503–510. https://doi.org/10.1007/s004010051152
Olney NT, Alquezar C, Ramos EM, Nana AL, Fong JC, Karydas AM et al (2017) Linking tuberous sclerosis complex, excessive mTOR signaling, and age-related neurodegeneration: a new association between TSC1 mutation and frontotemporal dementia. Acta Neuropathol 134:813–816. https://doi.org/10.1007/s00401-017-1764-0
Palmio J, Suhonen J, Keranen T, Hulkkonen J, Peltola J, Pirttila (2009) Cerebrospinal fluid tau as a marker of neuronal damage after epileptic seizure. Seizure 18:474–477. https://doi.org/10.1016/j.seizure.2009.04.006
Ramlaul K, Fu W, Li H, de Martin GN, He L, Trivedi M et al (2021) Architecture of the tuberous sclerosis protein complex. J Mol Biol 433:166743. https://doi.org/10.1016/j.jmb.2020.166743
Ramos EM, Dokuru DR, Van Berlo V, Wojta K, Wang Q, Huang AY et al (2019) Genetic screen in a large series of patients with primary progressive aphasia. Alzheimers Dement 15:553–560. https://doi.org/10.1016/j.jalz.2018.10.009
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Salminen A, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H, Alafuzoff I (2012) Emerging role of p62/sequestosome-1 in the pathogenesis of Alzheimer’s disease. Prog Neurobiol 96:87–95. https://doi.org/10.1016/j.pneurobio.2011.11.005
Sarnat HB, Flores-Sarnat L (2015) Infantile tauopathies: hemimegalencephaly; tuberous sclerosis complex; focal cortical dysplasia 2; ganglioglioma. Brain Dev 37:553–562. https://doi.org/10.1016/j.braindev.2014.08.010
Togo T, Akiyama H, Iseki E, Uchikado H, Kondo H, Ikeda K et al (2004) Immunohistochemical study of tau accumulation in early stages of Alzheimer-type neurofibrillary lesions. Acta Neuropathol 107:504–508. https://doi.org/10.1007/s00401-004-0842-2
van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S et al (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277:805–808. https://doi.org/10.1126/science.277.5327.805
Weaver CL, Espinoza M, Kress Y, Davies P (2000) Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiol Aging 21:719–727. https://doi.org/10.1016/s0197-4580(00)00157-3
Acknowledgements
We thank the NIH NeuroBioBank for providing the human brain tissue. This work was supported by the Rainwater Charitable Foundation, the Bluefield Project to Cure FTD, and NIH Grants P30AG062422, P01AG019724, and U01AG057195.
Author information
Authors and Affiliations
Contributions
WWS, AWK conceived and designed the project. JHH performed immunohistochemistry. CB performed western blotting. CA and EMR performed genetic analysis. WWS, OSP, SEG, and JHH wrote the paper. All authors read and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hwang, JH.L., Perloff, O.S., Gaus, S.E. et al. Tuberous sclerosis complex is associated with a novel human tauopathy. Acta Neuropathol 145, 1–12 (2023). https://doi.org/10.1007/s00401-022-02521-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02521-5