Abstract
In contrast to extracellular plaque and intracellular tangle pathology, the presence and relevance of intraneuronal Aβ in Alzheimer’s disease (AD) is still a matter of debate. Human brain tissue offers technical challenges such as post-mortem delay and uneven or prolonged tissue fixation that might affect immunohistochemical staining. In addition, previous studies on intracellular Aβ accumulation in human brain often used antibodies targeting the C-terminus of Aβ and differed strongly in the pretreatments used. To overcome these inconsistencies, we performed extensive parametrical testing using a highly specific N-terminal Aβ antibody detecting the aspartate at position 1, before developing an optimal staining protocol for intraneuronal Aβ detection in paraffin-embedded sections from AD patients. To rule out that this antibody also detects the β-cleaved APP C-terminal fragment (β-CTF, C99) bearing the same epitope, paraffin-sections of transgenic mice overexpressing the C99-fragment were stained without any evidence for cross-reactivity in our staining protocol. The staining intensity of intraneuronal Aβ in cortex and hippocampal tissue of 10 controls and 20 sporadic AD cases was then correlated to patient data including sex, Braak stage, plaque load, and apolipoprotein E (ApoE) genotype. In particular, the presence of one or two ApoE4 alleles strongly correlated with an increased accumulation of intraneuronal Aβ peptides. Given that ApoE4 is a major genetic risk factor for AD and is involved in neuronal cholesterol transport, it is tempting to speculate that perturbed intracellular trafficking is involved in the increased intraneuronal Aβ aggregation in AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic studies on familial Alzheimer’s disease (AD) patients have revealed mutations in genes linked to the beta amyloid (Aβ) generating cascade. Although this provides strong support for the amyloid hypothesis as the underlying pathological mechanism of AD [30], some controversies remain to be resolved. For instance, the accumulation of extracellular Aβ plaques does not correlate with the cognitive decline observed in AD patients [1]. In addition, a massive loss of neurons around plaques is not seen in human brain [54] and only rarely observed after the onset of Aβ plaque pathology in various different mouse models [22, 24].
One possible explanation is that, rather than extracellular Aβ plaque deposition, accumulation of Aβ inside neurons could be driving AD pathology, and may eventually lead to intracellular deficits in function and neuronal loss [25, 37, 68]. Intraneuronal Aβ has been reported to disrupt fast axonal transport (FAT) in isolated axoplasms [50], to impair multi vesicular body (MVB) sorting by inhibition of the ubiquitin–proteasome system [2] and to induce synaptic dysfunction leading to reduced PSD-95 and consequently GluR1 levels in synapses [3].
The accumulation of intraneuronal Aβ peptides in AD brain has been sporadically reported since the late 1980s [27]. However, initial problems with the inability of the antibodies to separate between full length amyloid precursor protein (APP) and Aβ itself founded a skepticism toward the presence of intraneuronal Aβ that has been difficult to eliminate [25, 37]. Despite the initial technical complications, several studies using Aβ40/42 end-specific antibodies have later reported the presence of intraneuronal Aβ in AD and healthy controls [17–19, 23, 26, 44, 46, 65], as well as in Down syndrome patients who are known to develop AD at an early age [29, 45]. One study isolated human hippocampal pyramidal neurons from the CA1 of AD patients by laser capture microdissection, determined Aβ peptide levels using ELISA quantification and reported an increased intraneuronal Aβ42/Aβ40 ratio in AD patients compared to controls [4]. Still, the presence and impact of intraneuronal Aβ in human AD tissue is a matter of controversial debate [22].
The data on intraneuronal Aβ in AD mouse models are quite consistent; intraneuronal Aβ was first reported in familial PS1 transgenic mice that also showed neurodegeneration but no plaques [15]. During the last years, it has been convincingly reported in several mouse models including APPSDLPS1M146L [69], APPSLPS1M146L [70], Tg2576 [60], 3xTg-AD [48], 5xFAD [47], APPArc [33, 40], APPT714I mice [63], and in APPSLPS1KIM233T,L235P mice [10] in which it was recently shown to correlate with neuron loss [8, 12, 14], as well as in a recently described mouse model expressing only pyroglutamate modified Aβ peptides within neurons [67].
Compared to mouse tissue, human tissue is more variable and factors like post-mortem delay, agonal state, and uneven tissue fixation, often of a prolonged duration, can interfere with immunohistochemical results. Although pH-dependent heat pretreatment is commonly used to reliably retrieve masked antigens from formalin-fixed, paraffin-embedded postmortem brain [41, 42, 58], most of the staining protocols for intracellular Aβ applied so far show considerable variation in use, duration and concentration of heat and formic acid (FA) treatment. Microwave heat pretreatment enhances the immunoreactive signal of intraneuronal Aβ as compared to conditions in which no or enzymatic pretreatment was applied [18]. FA is widely used to increase the staining of plaque pathology in AD; yet, the effect of FA on intraneuronal Aβ staining has been reported to be low and similar to the effect of heat [20], or even to counteract the enhancing effect of heat pretreatment on intraneuronal Aβ immunohistochemical detection [49]. On the other hand, formic acid pretreatment has been successfully used to improve aggregated α-synuclein staining in Lewy bodies and dystrophic neurites [61].
The present study optimized the staining protocol for intraneuronal Aβ using a highly specific N-terminal Aβ antibody on human AD tissue after testing various pretreatment conditions of heat and/or FA. The optimized protocol was then used to screen a series of sporadic AD cases and non-demented controls for the intensity of intraneuronal Aβ staining, which was then analyzed in a correlation analysis with patient data including ApoE genotype. A significant correlation between intraneuronal Aβ accumulation and the presence of at least one ApoE4 allele was established.
Materials and methods
AD brain tissue
Paraffin-embedded blocks from cortex and hippocampus of AD (n = 20) and control patients (n = 10) were acquired from the Netherlands Brain Bank (NBB, Amsterdam, The Netherlands) [51] that works with a rapid autopsy program and aims to keep postmortem delay (PMD) to a minimum. The NBB abides to all local ethical legislation, and permission was obtained for all brain autopsies and for the use of the tissues and clinical data for research purposes. To minimize variation as much as possible, control subjects were matched to the AD cases for age, sex, fixation time, pH of the cerebrospinal fluid, and PMD. Tissue of all subjects was investigated by a team of trained neuropathologists using a range of conventional and neuropathological stainings. The presence or absence of neuropathological changes was confirmed in the hippocampus and a range of other brain regions including the frontal and temporal cortex and the cerebellum. None of the control subjects had suffered from any primary neurological or psychiatric disease or brain metastases nor did they have a history of drug treatment or medication.
At autopsy, the hippocampus was dissected and fixed in 0.1 mol/L phosphate-buffered 4% formaldehyde (Sigma, St. Louis, MO, USA) solution (pH 7.2) for a period of approximately 4–5 weeks. Tissue was then dehydrated in graded ethanol and embedded in paraffin. 4-μm-thick tissue sections were cut for all patients from the midlevel of the hippocampus on a Leica microtome and mounted on SuperFrost Plus slides (Menzel-Gläser, Braunschweig, Germany). The following data were available for each patient: diagnosis, gender, age, post-mortem delay, disease duration, pH CSF, Braak stage, ApoE genotype, brain weight, as well as the medical history. In addition, information on extracellular plaque load was provided which contains a semi-quantitative score based on silver and 6F/3D (DAKO, Denmark, 1:400) immunostainings ranging from O (very few) to C (heavy plaque load) (Table 1).
The APOE genotyping of all patients was done according to standard protocols [66]. In brief, frozen human cortical samples were physically homogenized and powdered. DNA was then extracted and purified using standard methodology. Subsequently, parts of the APOE gene were amplified by means of PCR before the amplified DNA was fragmented using the restriction enzyme CfoI that is single nucleotide polymorphism (SNP) sensitive. Fragment size was then evaluated from their mobility in PolyNat gel system stained with SYBR-Green [31].
Immunohistochemistry on formalin-fixed, paraffin-embedded sections
Immunohistochemistry was performed as described previously [70]. In brief, sections were deparaffinized in xylene and rehydrated in a series of ethanol. After treatment with 0.3% H2O2 in PBS to block endogenous peroxidases, either no further treatment was applied or antigen retrieval was performed by boiling sections for 10 min in a microwave oven in 0.01 M citrate buffer pH 6.0 and/or incubating sections for 3 min in 88% FA. After long-term fixation, various antigens can be masked by formalin crosslinks which hamper immunocytochemical detection. For many antigens, this can be overcome by heat-induced antigen retrieval [42, 57, 62]. Non-specific binding sites were blocked by treatment with 4% skim milk and 10% fetal calf serum in PBS, prior to addition of the primary antibodies. Primary antibodies used were directed against N-terminal Aβ peptides [Aβ[N], IBL (rabbit polyclonal, catalog No. 18584), Hamburg, Germany, 1:200] [35], G2-10 (Aβ40, The Genetics Company, Switzerland, 1:500), fibrillar Aβ oligomers (OC, generous gift of C. Glabe and R. Kayed 1:200) [32], Aβ17–24 (4G8, Covance, 1:1,000), APP C-terminal (Synaptic Systems, Germany, 1:500) and glial fibrillary acidic protein (GFAP, Synaptic Systems, Germany, 1:1,000). All primary antibodies were incubated overnight in a humid chamber at room temperature. Single staining was visualized using the ABC method with Vectastain Kit (Vector Laboratories, Burlingame, USA) and 0.5 mg/mL DAB as chromogen providing a reddish brown color (10 min exposure for human tissue). In order to rule out a cross-reactivity of Aβ[N] with APP C-terminal fragments, SPA4CT mice overexpressing the β-cleaved APP C-terminal fragment under the control of the prion protein promoter [52], were stained with Aβ[N] and a C-terminal APP antibody (Supplementary Fig. 1).
Intraneuronal Aβ intensity was rated in the hippocampal region based on Aβ[N] staining intensity in CA4, CA3, and CA1 corresponding to either: no staining in any of the regions: 0 (−); weak staining in CA4 and CA3, but nothing in CA1: 1 (+); moderate staining in CA4 and CA3, but nothing or little in CA1: 2 (++); intense staining in all three regions: 3 (+++) (Fig. 4a–l).
Double immunocytochemical staining was visualized using the ABC method with DAB together with the HistoGreen kit (Linaris, Germany) providing a blue color. Counterstaining was carried out with hematoxylin.
Statistical analysis
Kolmogorov–Smirnov test was applied to examine which variables showed significant deviations from normal distribution. Most of the parameters, especially intraneuronal Aβ, showed significant deviation from normal distribution, and thus non-parametric tests were performed in the following analyses. Spearman rank correlations were calculated between intraneuronal Aβ and age, brain weight, extracellular plaque load, and post-mortem delay. Non-parametric Mann–Whitney U test was performed between intraneuronal Aβ and the factors gender, diagnosis, and number of ApoE 4 alleles. Non-parametric Kruskal–Wallis tests were computed between intraneuronal Aβ and Braak stage, where subgroup analysis between two groups was performed with Mann–Whitney U tests. As the study is explorative, the P values are given without Bonferroni adjustments. A binary logistic regression with dependent variable number of ApoE 4 alleles (0, ≥1) and independent variables intraneuronal Aβ, diagnosis, gender, age, and Braak stage was computed with the enter method, including all specified independent variables in the model. That way, the analysis on a correlation between intraneuronal Aß and ApoE 4 alleles was controlled for the indicated variables.
Results
Effect of formic acid and heat on staining of intraneuronal Aβ in AD patients
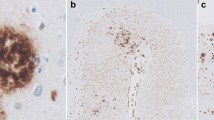
Optimization for intraneuronal Aβ staining was performed in hippocampal paraffin sections of sporadic AD cases using the Aβ N-terminal antibody detecting Aβ peptides starting with an aspartate at position 1. This antibody has been previously reported not to cross-react with full-length APP or β-C-terminal fragments [13, 35]. In addition, no cross-reactivity to APP C-terminal fragments could be demonstrated by staining SPA4CT mice overexpressing the β-cleaved APP C-terminal fragment C99. While strong immunoreactivity using a C-terminal APP antibody could be demonstrated in dentate gyrus granule cells and axonal fiber tracts like the corpus callosum, staining with the Aβ[N] antibody was consistently negative (Supplementary Fig. 1). Even without any antigen retrieval, a faint fairly homogenous intraneuronal Aβ1−x staining could be detected (Fig. 1a). Yet, 10-min heat treatment in 0.01 M citric acid buffer pH 6 dramatically increased the intraneuronal Aβ1−x staining showing a granular staining pattern and concentration around the nucleus (Fig. 1b). Compared to no pretreatment, 3-min FA pretreatment did not improve the staining of intracellular Aβ1−x (Fig. 1c), and actually very clearly counteracted the enhancing effect of the heat pretreatment on intraneuronal Aβ1−x staining (Fig. 1d). In addition to the fairly homogenous and punctate staining in the cytoplasm, some nuclei were observed to be surrounded by a highly granular Aβ staining pattern and were present in all three AD cases, and with all applied protocols (Fig. 1c). Double labeling for Aβ1−x and astrocytes using the Aβ[N] antibody visualized by DAB and the GFAP antibody visualized by Histogreen revealed that this highly abundant granular staining pattern in CA4 and CA3 of the hippocampal formation of many sporadic AD cases to be astrocytes accumulating Aβ (Fig. 2a, b, black arrows). In a few cases, astrocytes with no granular Aβ accumulation could be found in close proximity to neurons (Fig. 2c, d).
Optimization for intraneuronal Aβ1−x staining in the hippocampal formation of sporadic AD cases using the Aβ[N] antibody in paraffin-embedded sections. A faint homogenous intraneuronal Aβ1−x staining could be detected even without any antigen retrieval (a). However, 10-min heat pretreatment in citric acid buffer pH 6 dramatically increased the intraneuronal Aβ1−x staining that at higher magnification showed granularity and concentration around the nucleus (b). Compared to no treatment, 3-min formic acid (FA) pretreatment failed to improve intraneuronal Aβ1−x staining (c). In the combined treatment of heat and FA, FA actually counteracted the enhancing effect of the heat pretreatment (d). Besides the intraneuronal Aβ1−x staining, smaller nuclei surrounded by a highly granular Aβ staining pattern were observed with all the protocols (c, arrowheads). Scale bar 50 μm
Double labeling of Aβ1−x in reddish brown (DAB) using Aβ[N] antibody and astrocytes in blue (Histogreen, black arrowheads) using a GFAP antibody in paraffin-embedded sections. The highly granular staining pattern surrounding the smaller nuclei was due to astrocytes accumulating Aβ and was found in CA4 (a) as well as in CA3 (b) of many sporadic AD cases. However, in some AD cases, astrocytes without granular staining were found close to neurons in both CA4 (c) and CA3 (d). Scale bar 33 μm
The presence of intraneuronal Aβ in the hippocampal region of sporadic AD cases was confirmed qualitatively by staining using the OC antibody recognizing Aβ fibrils and fibrillar oligomers [32]. The OC antibody produced an intraneuronal staining much like that of Aβ[N] with heat pretreatment alone (Fig. 3a, b) as well as with combined heat and 3-min FA pretreatment. The latter was actually slightly more intense than that enhanced by heat alone (Fig. 3c, d).
Intraneuronal staining detected by OC and 4G8 antibodies in a sporadic AD brain. The OC antibody disclosed intraneuronal Aβ staining much like that of Aβ[N] with both 10-min heat pretreatment alone (a, b) and combined heat and 3-min formic acid (FA) treatment (c, d). With 10-min heat pretreatment, the 4G8 antibody produced a different, highly abundant and granular intracellular staining (e, f). Bottom left corners of a, c, and e show intracellular staining at a high magnification. Scale bar 50 μm
The widely used 4G8 antibody was applied to further confirm the presence of intraneuronal Aβ using heat pretreatment and was found to produce a prominent and intense granular staining. This pattern differed from that detected by OC or Aβ[N] antibodies (Fig. 3e, f), with the granules being much larger and surrounding the nucleus in a cap-like manner.
Intraneuronal Aβ staining in sporadic AD and control patients
Hippocampal sections from 10 controls and 20 sporadic AD patients were stained with the Aβ[N] antibody, and their intraneuronal Aβ1−x intensity was analyzed based on evaluation of the staining intensity in CA4, CA3, and CA1 (Fig. 4a–l). Four AD patients and two controls were found to accumulate the highest degree of intraneuronal Aβ1−x peptides, whereas nine AD patients and two controls accumulated a moderate amount of these Aβ peptides. Six AD patients and four controls accumulated low amount of Aβ peptides, whereas only one AD and two control patients were devoid of accumulation of Aβ1−x peptides (Table 1). By non-parametric statistical analysis, the accumulation of intraneuronal Aβ1−x was found to show no correlation with age, brain weight, post-mortem delay, gender, diagnosis, or Braak stage; however, a correlation between intraneuronal Aβ1−x accumulation and extracellular plaque load was established (P = 0.0138, r = 0.4446). Surprisingly, the ApoE genotype was found to strongly correlate with the presence of intraneuronal Aβ, where having one ApoE4 allele strongly correlated with increased intraneuronal Aβ1−x staining (Table 2; P = 0.002), which was also significant after Bonferroni adjustment (P = 0.023). Furthermore, from binary logistic regression with dependent variable ApoE 4 alleles and independent variables intraneuronal Aβ, diagnosis, gender, age, and Braak stage, there was only a significant influence of ApoE 4 alleles on intraneuronal Aβ (Wald statistic = 5.85; P = 0.016; odds-ratio = 16.38; 95% confidence interval = [1.70; 157.9]), while there was no significant influence of the other independent variables that the analysis was adjusted for. The rate of correct classifications for ApoE 4 alleles increased from 62 to 79% after the independent variables were entered to the model.
Rating of intraneuronal Aβ1−x staining intensity. +++ was assigned to cases with very strong intraneuronal Aβ staining in CA4, CA3, as well as in CA1 (a–c). ++ was assigned to cases with weaker but still obvious intracellular Aβ staining in CA4 and CA3 and low staining in CA1 (d–f). + was assigned to cases with a very faint intracellular Aβ staining in CA4 and CA3 and apparently no staining in CA1 (g–i). 0 was assigned to cases showing no intracellular Aβ staining either in CA4, CA3, or CA1 (j–l). Scale bar 50 μm
To confirm the results of the Aβ[N] staining in the detection of intraneuronal Aβ peptides in human post-mortem tissue, the same cohort of patients was stained using the commercially available antibody G2-10, which recognizes a neo-epitope at the C-terminus of Aβ40 peptides (Supplementary Fig. 2). A correlation analysis between the staining pattern of the two antibodies revealed a highly significant correlation (P = 0.002; r = 0.55), corroborating the validity of the staining protocol.
Discussion
The effect of heat and FA pretreatment was semi-quantitatively investigated in human AD tissue using a highly specific N-terminal-specific antibody detecting Aβ1−x . It has been previously reported that this antibody specifically recognizes Aβ peptides, without cross-reaction to APP or APP C-terminal fragments [13, 35]. The optimization was performed in paraffin-embedded hippocampal sections from sporadic AD cases by means of combinations of acid and heat pretreatments, essential conditions for antigen retrieval from brain tissue fixed for prolonged periods of time [42, 57, 62]. As the Aβ[N] antibody is directed against the aspartate at position 1 of Aβ, it might also detect APP C-terminal fragments bearing the same epitope. To overcome this problem, we stained tissue sections from a transgenic mouse model overexpressing the β-cleaved APP C-terminal fragment C99 (SPA4CT mice) [52], and found no evidence for any cross-reactivity to β-CTF in formalin-fixed and paraffin-embedded material. However, residual cross-reactivity using other techniques cannot be completely ruled out. Some intraneuronal Aβ1−x staining was evident even without any pretreatment, but heat dramatically increased the intraneuronal staining of smaller granules throughout the cytoplasm concentrating around the nucleus in all investigated hippocampal regions, especially in the CA4 region. In contrast, FA pretreatment did not increase the staining of intraneuronal Aβ1−x as compared to sections where no antigen retrieval was applied. In fact, FA even reduced the enhancing effect of heat; the combination of heat and FA only slightly increased the staining of Aβ1−x as compared to no pretreatment (Fig. 1d, h, l), resulting in much less retrieval than heat alone. The counteracting effect of FA on the heat-induced staining of intraneuronal Aβ1−x peptides corroborates findings of another study using an Aβ42(43) specific antibody (BC-05) and an autoclave heating protocol that reported a counteracting effect of FA on heat-induced Aβ42 staining [49] and is in line with previous studies showing that FA preferentially retrieves nuclear rather than cytoplasmic antigens [7, 57, 62]. Taken together, the strong improvement in intraneuronal Aβ immunoreactive signal after this pretreatment may explain discrepancies with older literature, where antigen retrieval was generally not applied.
These findings are in contrast to the observations in mouse models, where FA clearly enhanced staining of intraneuronal Aβ peptides with heat yielding no, or only a minor increase [13]. The difference in the effect of heat and FA between mouse and human tissue could be explained by differences in the Aβ species that accumulate in the cells. In the AD mouse models, much of the intraneuronal Aβ induced by FA pretreatment seemed to be aggregated peptides. Possibly, the intraneuronal Aβ1−x in AD tissue could reflect soluble oligomeric species that may be enhanced by heat but counteracted by FA pretreatment. In agreement, low-n oligomeric Aβ have been detected inside primary human neurons [64].
The presence of intraneuronal Aβ in sporadic AD tissue was further supported by staining with the polyclonal OC antibody recognizing fibrillar Aβ oligomers and Aβ fibrils [32]. An intense intraneuronal Aβ staining was observed after heat pretreatment as well as with additional FA pretreatment that enhanced intraneuronal OC staining. This is in contrast to the deleterious effects of FA pretreatment on the detection of Aβ1−x , but agrees with the suggestion that FA enhances the staining of aggregated Aβ fibrils recognized by the OC antibody. The staining retrieved by heat pretreatment could thus be due to detection of oligomeric Aβ fibrils by the OC antibody, which is consistent with two previous studies suggesting that Aβ oligomerization starts within neurons [59, 64].
It is known that besides Aβ peptides starting with an aspartate at position 1, a variety of different N-terminal truncated Aβ peptides have been identified in AD brain tissue [28, 53]. Especially compact Aβ deposits in human brain often contain pronounced N-terminal degradation and post-translational modifications [36]. While N-terminal modifications of Aβ have been identified to occur within neurons [8], it is likely that the majority of intraneuronal Aβ peptides start with the aspartic acid at position 1 as cleavage by BACE is the initial step for Aβ generation.
Intraneuronal Aβ originates exclusively from intraneuronal sources. Whether it can, in addition, be internalized from external sources is not yet clarified. Yet, much evidence supports the possibility of a reuptake of Aβ peptides into cells by endocytosis, and members of the lipoprotein receptor (LDLR) family [9], α7 nicotinic receptors [46], as well as scavenger receptor for advanced glycation end products (RAGE) [21, 56, 71] have all been reported to interact with Aβ and to be capable of internalization of extracellular Aβ peptides. In particular, RAGE-Aβ complexes have been shown to be internalized and co-localize with the lysosomal pathway in astrocytes in AD brains [56]. This is supported by our observation of astrocytes accumulating high amounts of Aβ granules.
The LDLR-related protein (LRP) is a member of the LDLR family and functions as an ApoE receptor. LRP has been shown to facilitate rapid endocytosis of APP promoting APP processing and thus Aβ generation. In addition to the effect on APP trafficking, LRP-induced rapid endocytosis also facilitates cellular uptake of Aβ peptides from the extracellular space, either directly through binding to Aβ or indirectly through interaction with ligands such as ApoE [9]. Accordingly, knock out of APOE in PDAPP transgenic mice reduced the accumulation of intracellular Aβ [72]. Given this possible role for ApoE in the intraneuronal Aβ accumulation, we correlated the intensity of the intraneuronal Aβ1−x staining in the hippocampal regions of 20 AD patients and 10 controls to ApoE genotype and found a highly significant correlation between having one ApoE4 allele and the intensity of Aβ1−x staining (P = 0.002). This is an interesting finding as ApoE4 plays an important role in neurodegeneration in general and in AD in particular (reviewed in [43]). The ε4 allele of the ApoE gene is the major known genetic risk factor for AD with a frequency of ~15% in general populations but >50% in AD patients [16]. Moreover, ApoE4 influences beta-amyloid degradation [73] brain and neuronal activity [39, 55] while senile plaques are more frequent in ApoE4-carriers compared to non-carriers. This was most evident during the age of 50–59 years, with ~41% of ε4-carriers bearing senile plaques, compared to only ~8% in non-carriers [34]. This suggests that there is a differential development of AD-associated changes in the brain of individuals having at least one ε4 allele, which starts already in middle age.
Crossing PDAPP mice with mice in which the endogenous mouse ApoE was substituted with human ApoE2, ApoE3 or ApoE4 resulted in a substantial and early increase in brain Aβ42 levels, prior to extracellular plaque deposition [5]. In addition, intraneuronal Aβ accumulation has been previously linked to ApoE. PDAPP mice overexpressing mLRP2 had higher hippocampal detergent-soluble Aβ42 levels than PDAPP wildtype mice. Furthermore, cortical intraneuronal Aβ42 was significantly reduced in PDAPP mice lacking ApoE, leading to the assumption that ApoE facilitates intraneuronal Aβ accumulation [72].
ApoE4-transgenic mice housed in an enriched environment showed increased levels of oligomerization and deposition of Aβ peptides in hippocampal neurons compared to ApoE3-transgenic mice housed under the same conditions [38]. Furthermore, inhibition of the Aβ-degrading enzyme neprilysin in ApoE3 and ApoE4 mice results in an ApoE4 isoform-specific degeneration of hippocampal CA1 neurons, which is accompanied by intracellular accumulation of Aβ and ApoE and lysosomal activation [6]. In good agreement with this lysosomal pathology, altered endocytic pathway activity is one of the earliest known intraneuronal changes occurring in sporadic AD, and it has been shown that the ApoE4 genotype promotes early-stage endocytic pathway activation [11]. In conclusion, having optimized techniques for reliable detection of intraneuronal Aβ, our subsequent analysis revealed a strong association between the ApoE4 genotype and the presence of intraneuronal Aβ. These results confirm recent data obtained in experimental settings and are consistent with an important role for intracellular Aβ metabolism, possibly mediated through ApoE4, in AD etiology.
References
Aizenstein HJ, Nebes RD, Saxton JA et al (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65:1509–1517
Almeida CG, Takahashi RH, Gouras GK (2006) Beta-amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system. J Neurosci 26:4277–4288
Almeida CG, Tampellini D, Takahashi RH et al (2005) Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis 20:187–198
Aoki M, Volkmann I, Tjernberg LO, Winblad B, Bogdanovic N (2008) Amyloid beta-peptide levels in laser capture microdissected cornu ammonis 1 pyramidal neurons of Alzheimer’s brain. Neuroreport 19:1085–1089
Bales KR, Liu F, Wu S et al (2009) Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci 29:6771–6779
Belinson H, Lev D, Masliah E, Michaelson DM (2008) Activation of the amyloid cascade in apolipoprotein E4 transgenic mice induces lysosomal activation and neurodegeneration resulting in marked cognitive deficits. J Neurosci 28:4690–4701
Boekhoorn K, Joels M, Lucassen PJ (2006) Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis 24:1–14
Breyhan H, Wirths O, Duan K, Marcello A, Rettig J, Bayer TA (2009) APP/PS1KI bigenic mice develop early synaptic deficits and hippocampus atrophy. Acta Neuropathol 117:677–685
Bu G, Cam J, Zerbinatti C (2006) LRP in amyloid-beta production and metabolism. Ann N Y Acad Sci 1086:35–53
Casas C, Sergeant N, Itier JM et al (2004) Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol 165:1289–1300
Cataldo AM, Petanceska S, Terio NB et al (2004) Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging 25:1263–1272
Christensen DZ, Bayer TA, Wirths O (2008) Intracellular Abeta triggers neuron loss in the cholinergic system of the APP/PS1KI mouse model of Alzheimer’s disease. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2008.07.022
Christensen DZ, Bayer TA, Wirths O (2009) Formic acid is essential for immunohistochemical detection of aggregated intraneuronal Abeta peptides in mouse models of Alzheimer’s disease. Brain Res 1301:116–125
Christensen DZ, Kraus SL, Flohr A, Cotel MC, Wirths O, Bayer TA (2008) Transient intraneuronal Abeta rather than extracellular plaque pathology correlates with neuron loss in the frontal cortex of APP/PS1KI mice. Acta Neuropathol 116:647–655
Chui DH, Tanahashi H, Ozawa K et al (1999) Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat Med 5:560–564
Corder EH, Saunders AM, Strittmatter WJ et al (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261:921–923
D’Andrea MR, Nagele RG, Gumula NA et al (2002) Lipofuscin and Abeta42 exhibit distinct distribution patterns in normal and Alzheimer’s disease brains. Neurosci Lett 323:45–49
D’Andrea MR, Nagele RG, Wang HY, Lee DH (2002) Consistent immunohistochemical detection of intracellular beta-amyloid42 in pyramidal neurons of Alzheimer’s disease entorhinal cortex. Neurosci Lett 333:163–166
D’Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH (2001) Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology 38:120–134
D’Andrea MR, Reiser PA, Polkovitch DA et al (2003) The use of formic acid to embellish amyloid plaque detection in Alzheimer’s disease tissues misguides key observations. Neurosci Lett 342:114–118
Deane R, Du Yan S, Submamaryan RK et al (2003) RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 9:907–913
Duyckaerts C, Potier MC, Delatour B (2008) Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol 115:5–38
Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S et al (2004) Intra- and extracellular Abeta and PHF in clinically evaluated cases of Alzheimer’s disease. Histol Histopathol 19:823–844
Games D, Buttini M, Kobayashi D, Schenk D, Seubert P (2006) Mice as models: transgenic approaches and Alzheimer’s disease. J Alzheimers Dis 9:133–149
Gouras GK, Almeida CG, Takahashi RH (2005) Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging 26:1235–1244
Gouras GK, Tsai J, Naslund J et al (2000) Intraneuronal Abeta42 accumulation in human brain. Am J Pathol 156:15–20
Grundke-Iqbal I, Iqbal K, George L, Tung YC, Kim KS, Wisniewski HM (1989) Amyloid protein and neurofibrillary tangles coexist in the same neuron in Alzheimer disease. Proc Natl Acad Sci USA 86:2853–2857
Guntert A, Dobeli H, Bohrmann B (2006) High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience 143:461–475
Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC (2001) Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med 125:489–492
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Hogh P, Garde E, Mortensen EL, Jorgensen OS, Krabbe K, Waldemar G (2007) The apolipoprotein E epsilon4-allele and antihypertensive treatment are associated with increased risk of cerebral MRI white matter hyperintensities. Acta Neurol Scand 115:248–253
Kayed R, Head E, Sarsoza F et al (2007) Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener 2:18
Knobloch M, Konietzko U, Krebs DC, Nitsch RM (2007) Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta mice. Neurobiol Aging 28:1297–1306
Kok E, Haikonen S, Luoto T et al (2009) Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol 65:650–657
Kokubo H, Kayed R, Glabe CG et al (2009) Amyloid beta annular protofibrils in cell processes and synapses accumulate with aging and Alzheimer-associated genetic modification. IJAD 2009:689285
Kuo YM, Kokjohn TA, Beach TG et al (2001) Comparative analysis of amyloid-beta chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer’s disease brains. J Biol Chem 276:12991–12998
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8:499–509
Levi O, Dolev I, Belinson H, Michaelson DM (2007) Intraneuronal amyloid-beta plays a role in mediating the synergistic pathological effects of apoE4 and environmental stimulation. J Neurochem 103:1031–1040
Lind J, Persson J, Ingvar M et al (2006) Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers. Brain 129:1240–1248
Lord A, Kalimo H, Eckman C, Zhang XQ, Lannfelt L, Nilsson LN (2006) The Arctic Alzheimer mutation facilitates early intraneuronal Abeta aggregation and senile plaque formation in transgenic mice. Neurobiol Aging 27:67–77
Lucassen PJ, Chung WC, Vermeulen JP, Van Lookeren Campagne M, Van Dierendonck JH, Swaab DF (1995) Microwave-enhanced in situ end-labeling of fragmented DNA: parametric studies in relation to postmortem delay and fixation of rat and human brain. J Histochem Cytochem 43:1163–1171
Lucassen PJ, Ravid R, Gonatas NK, Swaab DF (1993) Activation of the human supraoptic and paraventricular nucleus neurons with aging and in Alzheimer’s disease as judged from increasing size of the Golgi apparatus. Brain Res 632:105–113
Mahley RW, Weisgraber KH, Huang Y (2006) Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA 103:5644–5651
Mochizuki A, Tamaoka A, Shimohata A, Komatsuzaki Y, Shoji S (2000) Abeta42-positive non-pyramidal neurons around amyloid plaques in Alzheimer’s disease. Lancet 355:42–43
Mori C, Spooner ET, Wisniewsk KE et al (2002) Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid 9:88–102
Nagele RG, D’Andrea MR, Anderson WJ, Wang HY (2002) Intracellular accumulation of beta-amyloid(1-42) in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience 110:199–211
Oakley H, Cole SL, Logan S et al (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26:10129–10140
Oddo S, Caccamo A, Shepherd JD et al (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39:409–421
Ohyagi Y, Tsuruta Y, Motomura K et al (2007) Intraneuronal amyloid beta42 enhanced by heating but counteracted by formic acid. J Neurosci Methods 159:134–138
Pigino G, Morfini G, Atagi Y et al (2009) Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci USA 106:5907–5912
Ravid R, Swaab DF (1993) The Netherlands brain bank—a clinico-pathological link in aging and dementia research. J Neural Transm Suppl 39:143–153
Rutten BP, Wirths O, Van de Berg WD et al (2003) No alterations of hippocampal neuronal number and synaptic bouton number in a transgenic mouse model expressing the beta-cleaved C-terminal APP fragment. Neurobiol Dis 12:110–120
Saido TC, Yamao-Harigaya W, Iwatsubo T, Kawashima S (1996) Amino- and carboxyl-terminal heterogeneity of beta-amyloid peptides deposited in human brain. Neurosci Lett 215:173–176
Salehi A, Bakker JM, Mulder M, Swaab DF (1998) Limited effect of neuritic plaques on neuronal density in the hippocampal CA1 area of Alzheimer patients. Alzheimer Dis Assoc Disord 12:77–82
Salehi A, Dubelaar EJ, Mulder M, Swaab DF (1998) Aggravated decrease in the activity of nucleus basalis neurons in Alzheimer’s disease is apolipoprotein E-type dependent. Proc Natl Acad Sci USA 95:11445–11449
Sasaki N, Toki S, Chowei H et al (2001) Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer’s disease. Brain Res 888:256–262
Shi SR, Imam SA, Young L, Cote RJ, Taylor CR (1995) Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem 43:193–201
Shi SR, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741–748
Takahashi RH, Almeida CG, Kearney PF et al (2004) Oligomerization of Alzheimer’s beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci 24:3592–3599
Takahashi RH, Milner TA, Li F et al (2002) Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 161:1869–1879
Takeda A, Hashimoto M, Mallory M et al (1998) Abnormal distribution of the non-Abeta component of Alzheimer’s disease amyloid precursor/alpha-synuclein in Lewy body disease as revealed by proteinase K and formic acid pretreatment. Lab Invest 78:1169–1177
Taylor CR, Shi SR, Chaiwun B, Young L, Imam SA, Cote RJ (1994) Strategies for improving the immunohistochemical staining of various intranuclear prognostic markers in formalin-paraffin sections: androgen receptor, estrogen receptor, progesterone receptor, p53 protein, proliferating cell nuclear antigen, and Ki-67 antigen revealed by antigen retrieval techniques. Hum Pathol 25:263–270
Van Broeck B, Vanhoutte G, Pirici D et al (2008) Intraneuronal amyloid beta and reduced brain volume in a novel APP T714I mouse model for Alzheimer’s disease. Neurobiol Aging 29:241–252
Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ (2000) The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry 39:10831–10839
Wegiel J, Kuchna I, Nowicki K et al (2007) Intraneuronal Abeta immunoreactivity is not a predictor of brain amyloidosis-beta or neurofibrillary degeneration. Acta Neuropathol 113:389–402
Wenham PR, Price WH, Blandell G (1991) Apolipoprotein E genotyping by one-stage PCR. Lancet 337:1158–1159
Wirths O, Breyhan H, Cynis H, Schilling S, Demuth HU, Bayer TA (2009) Intraneuronal pyroglutamate-Abeta 3-42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol 118:487–496
Wirths O, Multhaup G, Bayer TA (2004) A modified beta-amyloid hypothesis: intraneuronal accumulation of the beta-amyloid peptide—the first step of a fatal cascade. J Neurochem 91:513–520
Wirths O, Multhaup G, Czech C et al (2001) Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci Lett 306:116–120
Wirths O, Multhaup G, Czech C et al (2002) Intraneuronal APP/A beta trafficking and plaque formation in beta-amyloid precursor protein and presenilin-1 transgenic mice. Brain Pathol 12:275–286
Yan SD, Chen X, Fu J et al (1996) RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 382:685–691
Zerbinatti CV, Wahrle SE, Kim H et al (2006) Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Abeta 42 accumulation in amyloid model mice. J Biol Chem 281:36180–36186
Zhao L, Lin S, Bales KR et al (2009) Macrophage-mediated degradation of beta-amyloid via an apolipoprotein E isoform-dependent mechanism. J Neurosci 29:3603–3612
Acknowledgments
We thank Petra Tucholla for excellent technical assistance. This work was supported by the Alzheimer Forschung Initiative e.V., Fritz Thyssen Foundation (to O.W.) and the European Commission, Marie Curie Early Stage Training, MEST-CT-2005-020013 (NEURAD), Alzheimer Ph.D. Graduate School (to O.W., P.J.L, T.A.B.), the Internationale Stichting Alzheimer Onderzoek (ISAO) and the Nederlandse Hersen Stichting to P.J.L).
Conflict of interest statement
Authors have no financial, personal or other conflict of interest to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Christensen, D.Z., Schneider-Axmann, T., Lucassen, P.J. et al. Accumulation of intraneuronal Aβ correlates with ApoE4 genotype. Acta Neuropathol 119, 555–566 (2010). https://doi.org/10.1007/s00401-010-0666-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0666-1