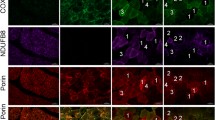
Abstract
The different histochemical ATPase properties of myosins separating the muscle fiber types have been utilized in diagnostic muscle biopsy routine for more than four decades. The ATPase staining method is rather laborious and has several disadvantages, such as weakening of staining over time and non-specific staining of capillaries, making the distinction of extremely atrophic muscle fibers difficult. We have developed a reliable and advanced immunohistochemical myosin double staining method for the identification of fiber types, including highly atrophic fibers in routine diagnostics. With this double staining method, we are able to distinguish among type I (ATPase type 1), IIA (ATPase type 2A), IIX (ATPase type 2B) and remodeled ATPase type 2C fibers expressing both fast and slow myosins using a one slide technique. Immunohistochemical double staining of myosin heavy chain isoforms can be used as an alternative for the conventional ATPase staining method in routine histopathology. The method provides even more detailed information of fast fiber subtypes and highly atrophic fibers on one single slide.




Similar content being viewed by others
References
Bancroft JD, Cook HC (1994) Manual of histological techniques and their diagnostic application. Churchill Livingstone, Edinburgh
Dye DE, Azzarelli B, Goebel HH, Laing NG (2006) Novel slow-skeletal myosin (MYH7) mutation on the original myosin storage myopathy kindred. Neuromuscul Disord 16:357–360
Harper PS (2001) Myotonic dystrophy. WB Sauders, London
Izumo S, Nadal-Ginard B, Mahadavi V (1986) All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science 231:597–600
Laing NG, Laing BA, Meredith C et al (1995) Autosomal dominant distal myopathy: linkage to chromosome 14. Am J Hum Genet 56:422–427
Liquori C, Ricker K, Moseley ML et al (2001) Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 293:864–867
Mahadevi V, Strehler EE, Periasamy M, Wieczorek DF, Izumo S, Nadal-Ginard B (1986) Sarcomeric myosin heavy chain gene family: organization and pattern of expression. Med Sci Sports Exerc 18:299–308
Martinsson T, Oldfors A, Darin N et al (2000) Autosomal dominant myopathy: missense mutation (Glu-706 → Lys) in the myosin heavy chain IIa gene. Proc Natl Acad Sci USA 97:14614–14619
Meredith C, Hermann R, Parry C et al (2004) Mutations in the slow muscle fiber myosin heavy chain gene (MYH7) cause Laing early-onset distal myopathy (MPD1). Am J Hum Genet 75:703–708
Oldfors A, Darin N, Martinsson T (2002) Autosomal dominant myosin heavy chain IIa myopathy. In: karpati G (ed) Structural and molecular basis of skeletal muscle diseases. ISN Neuropath Press, Basel, pp 85–87
Oldfors A, Tajsharghi H, Thornell LE (2005) Mutation of the slow myosin heavy chain rod domain underlies hyaline body myopathy. Neurology 64:580–581
Pette D, Vrbová G (1992) Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Rev Physiol Biochem Pharmacol 120:115–202
Rayment I, Holden HM, Whittaker M et al (1993) Structure of the actin complex and its implications for muscle contraction. Science 261:58–65
Ruppel KM, Spundich JA (1996) Structure–function analysis of the motor domain of myosin. Annu Rev Cell Dev Biol 12:543–573
Tajsharghi H, Oldfors A, Macleod DP, Swash M (1997) Homozygous mutation in MYH7 in myosin storage myopathy and cardiomyopathy. Neurology 68:962
Tajsharghi H, Thornell LE, Lindberg C, Lindvall B, Hendriksson KG, Oldfors A (2003) Myosin storage myopathy associated with a heterozygous missense mutation in MYH7. Ann Neurol 54:494–500
Udd B, Meola G, Krahe R et al (2006) 140th ENMC international workshop: myotonic dystrophy DM2/PROMM and other myotonic dystrophies with guidelines on management. Neuromuscul Disord 16:403–413
Udd B, Meola G, Krahe R et al (2003) Report of the 115th ENMC workshop: DM2/PROMM and other myotonic dystrophies. 3rd Workshop, 14–16 February 2003, Naarden, The Netherlands. Neuromuscul Disord 13:589–596
Udd B, Krahe R, Wallgren-Petterson C, Falkc B, Kalimo H (1997) Proximal myotonic dystrophy—a family with autosomal dominant muscular dystrophy, cataracts, hearing loss and hypogonadism: heterogeneity of proximal myotonic syndromes? Neuromuscl Disord 7:217–218
Vihola A, Bassez G, Meola G et al (2003) Histopathological differences of myotonic dystrophy type 1 (DM1) and PROMM/DM2. Neurology 60:1854–1857
Weiss A, McDonough D, Wertman B et al (1999) Organization of human and mouse skeletal myosin heavy chain gene clusters is highly conserved. Proc Natl Acad Sci USA 96:2958–2963
Acknowledgments
We thank Henna-Riikka Koskinen, Jaana Leppikangas and Satu Luhtasela for their expert technical assistance. This research was supported by funding from Tampere University Hospital Medical Research funds, The Folkhälsan Research Foundation and grants from the Liv&Hälsa Foundation.
Conflict of interest statement
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raheem, O., Huovinen, S., Suominen, T. et al. Novel myosin heavy chain immunohistochemical double staining developed for the routine diagnostic separation of I, IIA and IIX fibers. Acta Neuropathol 119, 495–500 (2010). https://doi.org/10.1007/s00401-010-0643-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0643-8