Abstract
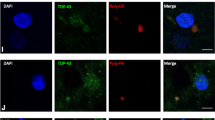
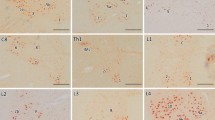
Pathological transactivation-responsive DNA-binding protein 43 (TDP-43) has been identified as a component of ubiquitinated inclusions in frontotemporal lobar degeneration with motor neuron disease, as well as in sporadic and some forms of familial amyotrophic lateral sclerosis. To clarify whether pathological TDP-43 is present in other neurodegenerative diseases involving the motor neuron system, we immunohistochemically examined the brain and spinal cord affected by two CAG repeat (polyglutamine) diseases, Machado–Joseph disease (MJD) and spinal and bulbar muscular atrophy (SBMA), using polyclonal antibody against TDP-43. In all the MJD cases, TDP-43-immunoreactive (ir) neuronal cytoplasmic inclusions (NCIs), although few in number, were found only in the lower motor neurons in the brainstem and spinal cord. TDP-43-ir NCIs appeared as linear wisp-like, skein-like, or thick, somewhat rod-like bodies. These inclusions were also visualized with antibodies against phosphoserines 409 and 410 of TDP-43, and ubiquitin, but were not recognized by antibody against expanded polyglutamine stretches or ataxin-3. The ultrastructure of the TDP-43-ir NCIs was similar to that of the inclusions seen in sporadic ALS, consisting of bundles of parallel filaments. None of the SBMA cases showed abnormal TDP-43 immunoreactivity in any of the regions examined. Immunoblot analysis failed to recognize hyperphosphorylated TDP-43 at ~23 kDa in two MJD cases examined. However, the immunohistochemical findings strongly suggested that in MJD, in addition to the polyglutamine-dependent disease process, TDP-43-related pathogenesis is associated with degeneration and death of the lower motor neurons.




Similar content being viewed by others
References
Amador-Ortiz C, Lin WL, Ahmed Z et al (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Arai T, Hasegawa M, Akiyama H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Cairns NJ, Neumann M, Bigio EH et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240
Daoud H, Valdmanis PN, Kabashi E et al (2009) Contribution of TARDBP mutations to sporadic amyotrophic lateral sclerosis. J Med Genet 46:112–114
Dickson DW, Josephs KA, Amador-Ortiz C (2007) TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol 114:71–79
Freeman SH, Spires-Jones T, Hyman BT, Growdon JH, Frosch MP (2008) TAR-DNA binding protein 43 in Pick disease. J Neuropathol Exp Neurol 67:62–67
Fujishiro H, Uchikado H, Arai T (2008) Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol 117:151–158
Geser F, Winton MJ, Kwong LK et al (2008) Pathological TDP-43 in parkinsonism–dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol 115:133–145
Gitcho MA, Baloh RH, Chakraverty S et al (2008) TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol 63:535–538
Hasegawa M, Arai T, Akiyama H et al (2007) TDP-43 is deposited in the Guam parkinsonism–dementia complex brains. Brain 13:1386–1394
Hasegawa M, Arai T, Nonaka T et al (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64:60–70
Hayashi M, Kobayashi K, Furuta H (2003) Immunohistochemical study of neuronal intranuclear and cytoplasmic inclusions in Machado–Joseph disease. Psychiatr Clin Neurosci 57:205–213
Hayashi Y, Kakita A, Yamada M et al (1998) Hereditary dentatorubral-pallidoluysian atrophy: ubiquitinated filamentous inclusions in the cerebellar dentate nucleus neurons. Acta Neuropathol 95:479–482
Inukai Y, Nonaka T, Arai T et al (2008) Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett 582:2899–2904
Kabashi E, Valdmanis PN, Dion P et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40:572–574
Kühnlein P, Sperfeld A-D, Vanmassenhove B et al (2008) Two German kindreds with familial amyotrophic lateral sclerosis due to TARDBP mutations. Arch Neurol 65:1185–1189
Lin WL, Dickson DW (2008) Ultrastructural localization of TDP-43 in filamentous neuronal inclusions in various neurodegenerative diseases. Acta Neuropathol 116:205–213
Mackenzie IRA, Bigio EH, Ince PG et al (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61:427–434
Miklossy J, Steele JC, Yu S et al (2008) Enduring involvement of tau, β-amyloid, α-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism–dementia complex of Guam (ALS/PDC). Acta Neuropathol 116:625–637
Mori F, Tanji K, Zhang HX et al (2008) Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 116:193–203
Nakashima-Yasuda H, Uryu K, Robinson J et al (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114:221–229
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Nishihira Y, Tan CF, Onodera O et al (2008) Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol 116:169–182
Piao YS, Wakabayashi K, Kakita A et al (2003) Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 12:10–22
Rutherford NJ, Zhang YJ, Baker M et al (2008) Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet 4:e1000193
Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL (2008) Colocalization of transactivation-responsive DNA-binding protein 43 and Huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol 67:1159–1165
Sreedharan J, Blair IP, Tripathi VB et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672
Suenaga T, Matsushima H, Nakamura S, Akiguchi I, Kimura J (1993) Ubiquitin-immunoreactive inclusions in anterior horn cells and hypoglossal neurons in a case with Joseph’s disease. Acta Neuropathol 85:341–344
Tan CF, Eguchi H, Tagawa A et al (2007) TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol 113:535–542
Uryu K, Nakashima-Yasuda H, Forman M et al (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67:555–564
Van Deelin VM, Leverenz JB, Bekris LM et al (2008) TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol 7:409–416
Yamada M, Piao YS, Toyoshima Y, Tsuji S, Takahashi H (2000) Ubiqutinated filamentous inclusions in cerebellar dentate nucleus neurons in dentatorubral-pallidoluysian atrophy contain expanded polyglutamine stretches. Acta Neuropathol 99:615–618
Yamada M, Sato T, Tsuji S, Takahashi H (2008) CAG repeat disorder models and human neuropathology: similarities and differences. Acta Neuropathol 115:71–86
Yamada M, Tan CF, Inenaga C, Tsuji S, Takahashi H (2004) Sharing of polyglutamine localization by the neuronal nucleus and cytoplasm in CAG-repeat diseases. Neuropathol Appl Neurobiol 30:665–675
Yamada M, Wood JD, Shimohata T et al (2001) Widespread occurrence of intranuclear atrophin-1 accumulation in the central nervous system neurons of patients with dentatorubral-pallidoluysian atrophy. Ann Neurol 49:14–23
Yokoseki A, Shiga A, Tan CF et al (2008) TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol 63:538–542
Acknowledgments
We thank C. Tanda, J. Takasaki, H. Saito and S. Egawa for their technical assistance, and M. Machida for help in preparing the manuscript. This work was supported by Grants-in-Aid for Scientific Research (17300109, 20240037) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and a grant from the Research Committee for Ataxic Diseases, the Ministry of Health, Labour and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, CF., Yamada, M., Toyoshima, Y. et al. Selective occurrence of TDP-43-immunoreactive inclusions in the lower motor neurons in Machado–Joseph disease. Acta Neuropathol 118, 553–560 (2009). https://doi.org/10.1007/s00401-009-0552-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0552-x