Abstract.
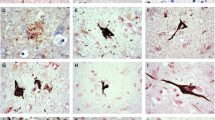
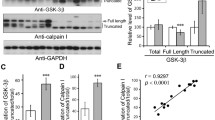
Tau phosphorylation was examined in Alzheimer's disease (AD), Pick's disease (PiD), progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) using phospho-specific tau antibodies recognizing the phosphorylated form of Ser202, Ser214 and Ser 396, and antibodies to non-phosphorylated glycogen synthase kinase-3α/β (GSK-3α/β), which regulates phosphorylation at these specific sites on tau and phosphorylated GSK-3βSer9 (GSK-3β-P); this antibody is directed to the inactive form of GSK-3β. Phospho-specific tau antibodies recognized disease-specific band patterns on Western blots of sarcosyl-insoluble fractions: four bands of 73, 68, 64 and 60 kDa in AD, two bands of 68 and 64 kDa in PSP and CBD, and two bands of 64 and 60 kDa in PiD. Moreover, anti-phospho-tau Ser202, Ser214 and Ser369 decorated neurons with neurofibrillary tangles, dystrophic neurites of senile plaques, neuropil threads, Pick bodies, astrocytes and oligodendrocytes with coiled bodies. No differences in the expression of GSK-3α/β were seen between neurons with and without neurofibrillary tangles. GSK-3α/β was enriched in sarcosyl-insoluble fractions, suggesting association of this kinase with tau hyperphosphorylation. In addition, strong expression of the phosphorylated form of GSK-3β was found in a subpopulation of neurons with neurofibrillary tangles, and in dystrophic neurites of senile plaques, neuropil threads, Pick bodies, tau-containing astrocytes and coiled bodies in AD, PiD, PSP and CBD. This was not due to cross-reactivity between GSK-3 and phospho-tau. Specific bands differing from those of phospho-tau were seen on Western blots of sarcosyl-insoluble fractions processed for GSK-3α/β and GSK-3β-P. Double-labeling immunohistochemistry discloses that GSK-3β-P co-localizes with abnormal tau in about 50% of neurons with neurofibrillary tangles, and in neuronal processes, astrocytes and oligodendrocytes in various tauopathies. The present results support a pivotal role for GSK-3 in tau phosphorylation in neurons and glial cells. Moreover, the elevated number of tau-containing cells stained with anti-GSK-3β-P antibodies suggests a partial inactivation of the kinase, or sequestration of the phosphorylated form, which may contribute to the regulation of the cascade of tau hyperphosphorylation in tauopathies, and to protect tau-containing cells from apoptosis.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Ferrer, .I., Barrachina, .M. & Puig, .B. Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 104, 583–591 (2002). https://doi.org/10.1007/s00401-002-0587-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00401-002-0587-8