Abstract
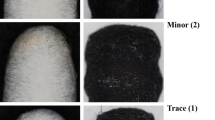
In this work, cleaning efficiency of aqueous solutions of four different surfactants was studied to remove the post soldering flux residue from printed circuit boards (PCBs). A systematic study is carried out to select surfactant system which includes dispersing efficiency, surface tension, contact angle and surface energy followed by actual cleaning test. Efficiency of the system was quantified by their solubilizing or dispersing capacity of the residue. Surface activity and wetting behavior were monitored by contact angle of the solutions on PCBs and their surface tension (ST). Polar and dispersive components of surface tension of solutions and surface free energy of PCB substrates were determined using OWRK (Owens, Wendt, Rabel and Kaelble) method. A representative cleaning test was carried out to correlate the experimental results and actual residue cleaning performance. Results suggested that HLB value is not the only criteria to select the surfactant in cleaner formulation. All the four surfactant solutions showed varying ability to disperse the selected residue. TMDE was found to be the best, and SPAN20 was the least effective in dispersing the residue. Findings of the study suggest that in order to be an efficient residue remover, a cleaning chemistry should have following three features — (a) able to solubilize or disperse the residue, (b) low ST and low contact angle on the substrate to be cleaned, and (c) polar and dispersive components of ST of the solution and those of SFE of the substrate should be a close match.







Similar content being viewed by others
References
Kotadia HR, Howes PD, Mannan SH (2014) A review: on the development of low melting temperature Pb-free solders. Microelectron Reliab 54:1253–1273
Mo J, Yang Q, Zhang N et al (2018) A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J Environ Manage 227:395–405
Ilankoon IMSK, Ghorbani Y, Chong MN et al (2018) E-waste in the international context – a review of trade flows, regulations, hazards, waste management strategies and technologies for value recovery. Waste Manag 82:258–275
Gao F, Bai R, Ferlin F et al (2020) Replacement strategies for non-green dipolar aprotic solvents. Green Chem 22:6240–6257. https://doi.org/10.1039/D0GC02149K
Calvo-Flores FG, Monteagudo-Arrebola MJ, Dobado JA, Isac-García J (2018) Green and bio-based solvents. Top Curr Chem 376:18. https://doi.org/10.1007/s41061-018-0191-6
Regula C, Carretier E, Wyart Y et al (2014) Chemical cleaning/disinfection and ageing of organic UF membranes: a review. Water Res 56:325–365
Kanegsberg B, Kanegsberg E (2011) Handbook for critical cleaning. CRC Press
Felty JR (1991) Defluxing for high reliability applications and general environmental issues. In: Hymes L. (ed) Cleaning printed wiring assemblies in today’s environment. Springer Netherlands, Dordrecht
Bixenman M, Chan J, Loy TC (2012) Removal of flux residues from highly dense assemblies. In: 2012 35th IEEE/CPMT International Electronics Manufacturing Technology Conference (IEMT). IEEE, pp 1–9
Mahdi FM, Record TE, Amadi CA et al (2015) Removal of submicron particles from solid surfaces using surfactants. Colloids Interface Sci Commun 6:13–16. https://doi.org/10.1016/j.colcom.2015.10.001
Guth LA (1991) Flux considerations with emphasis on low solids. In: Hymes L (ed) Cleaning printed wiring assemblies in today’s environment. Springer, Netherlands, Dordrecht, pp 26–64
Baglioni M, Guaragnone T, Mastrangelo R et al (2020) Nonionic surfactants for the cleaning of works of art: insights on acrylic polymer films dewetting and artificial soil removal. ACS Appl Mater & Interfaces 12:26704–26716. https://doi.org/10.1021/acsami.0c06425
Beaudoin S, Grant C, Carbonell R (1995) Removal of organic films from solid surfaces using aqueous solutions of nonionic surfactants. 1. Experiments Ind Eng Chem Res 34:3307–3317
Raudino M, Giamblanco N, Montis C et al (2017) Probing the cleaning of polymeric coatings by nanostructured fluids: a QCM-D study. Langmuir 33:5675–5684. https://doi.org/10.1021/acs.langmuir.7b00968
Vaziri Hassas B, Karakaş F, Çelik MS (2014) Ultrafine coal dewatering: relationship between hydrophilic lipophilic balance (HLB) of surfactants and coal rank. Int J Miner Process 133:97–104. https://doi.org/10.1016/j.minpro.2014.10.010
Yamaguchi S, Kunieda H (1997) Determination of a three-phase tie triangle (the hydrophile−lipophile balance plane) in a composition tetrahedron: evaluation of the composition of adsorbed mixed-surfactant and the monomeric solubilities of short-chain surfactant. Langmuir 13:6995–7002. https://doi.org/10.1021/la970413m
Semenov AP, Mendgaziev RI, Stoporev AS et al (2020) Gas hydrate nucleation and growth in the presence of water-soluble polymer, nonionic surfactants, and their mixtures. J Nat Gas Sci Eng 82:103491. https://doi.org/10.1016/j.jngse.2020.103491
Keagy JA, Zhang X, Johnston KP et al (2006) Cleaning of patterned porous low-k dielectrics with water, carbon dioxide and ambidextrous surfactants. J Supercrit Fluids 39:277–285. https://doi.org/10.1016/j.supflu.2006.04.009
Dey J, Shrivastava S (2012) Can molecules with an anionic head and a poly(ethylene glycol) methyl ether tail self-assemble in water? A surface tension, fluorescence probe, light scattering, and transmission electron microscopic investigation. Soft Matter 8:1305–1308. https://doi.org/10.1039/C2SM06931H
Shrivastava S, Matsuoka H (2014) Photoresponsive block copolymer: synthesis, characterization, and surface activity control. Langmuir 30:3957–3966. https://doi.org/10.1021/la4049677
Shrivastava S, Dey J (2010) Interaction of anionic surfactant with polymeric nanoparticles of similar charge. J Colloid Interface Sci 350:220–228. https://doi.org/10.1016/j.jcis.2010.06.055
Dey J, Shrivastava S (2012) Physicochemical characterization and self-assembly studies on cationic surfactants bearing mPEG tail. Langmuir 28:17247–17255. https://doi.org/10.1021/la303210f
Pal N, Samanta K, Mandal A (2019) A novel family of non-ionic gemini surfactants derived from sunflower oil: synthesis, characterization and physicochemical evaluation. J Mol Liq 275:638–653. https://doi.org/10.1016/J.MOLLIQ.2018.11.111
Li R, Manica R, Lu Y, Xu Z (2020) Role of surfactants in spontaneous displacement of high viscosity oil droplets from solid surfaces in aqueous solutions. J Colloid Interface Sci 579:898–908. https://doi.org/10.1016/j.jcis.2020.06.069
Kim D, Lee M, Kim JH, Lee J (2020) Dynamic contact angle measurements on lubricant infused surfaces. J Colloid Interface Sci 586:647–654. https://doi.org/10.1016/j.jcis.2020.10.134
Shah V, Bharatiya B, Shah DO (2018) Effect of molecular weight and diffusivity on the adsorption of PEO-PPO-PEO block copolymers at PTFE-water and oil-water interfaces. Colloid Polym Sci 296:1333–1340. https://doi.org/10.1007/s00396-018-4346-3
Zhang J, Kwok DY (2002) Calculation of solid−liquid work of adhesion patterns from combining rules for intermolecular potentials. J Phys Chem B 106:12594–12599. https://doi.org/10.1021/jp026676t
Giovambattista N, Debenedetti PG, Rossky PJ (2007) Effect of surface polarity on water contact angle and interfacial hydration structure. J Phys Chem B 111:9581–9587. https://doi.org/10.1021/jp071957s
Owens DK, Wendt RC (1969) Estimation of the surface free energy of polymers. J Appl Polym Sci 13:1741–1747. https://doi.org/10.1002/app.1969.070130815
Pal N, Saxena N, Mandal A (2018) Studies on the physicochemical properties of synthesized tailor-made gemini surfactants for application in enhanced oil recovery. J Mol Liq 258:211–224. https://doi.org/10.1016/J.MOLLIQ.2018.03.037
Pal N, Vajpayee M, Mandal A (2019) Cationic/ Nonionic mixed surfactants as enhanced oil recovery fluids: influence of mixed micellization and polymer association on interfacial, rheological, and rock-wetting characteristics. Energy Fuels 33:6048–6059. https://doi.org/10.1021/acs.energyfuels.9b00671
Pal N, Saxena N, Divya Laxmi KV, Mandal A (2018) Interfacial behaviour, wettability alteration and emulsification characteristics of a novel surfactant: implications for enhanced oil recovery. Chem Eng Sci 187:200–212. https://doi.org/10.1016/J.CES.2018.04.062
Pesonen-Leinonen E, Kuisma R, Redsven I et al (2006) Can contact angle measurements be used to predict soiling and cleaning of plastic flooring materials? In: Mittal KL (ed) Wettability and adhesion. CRC, Boston, pp 203–214
Schlisske S, Held M, Rödlmeier T et al (2018) Substrate-independent surface energy tuning via siloxane treatment for printed electronics. Langmuir 34:5964–5970. https://doi.org/10.1021/acs.langmuir.8b00304
Liu H, Xu W, Tan W et al (2016) Line printing solution-processable small molecules with uniform surface profile via ink-jet printer. J Colloid Interface Sci 465:106–111. https://doi.org/10.1016/j.jcis.2015.11.067
Vargas-Ruiz S, Lutzki J, von Klitzing R et al (2017) Wetting of planar solid surfaces by bicontinuous sugar surfactant-based microemulsions. Colloid Polym Sci 295. https://doi.org/10.1007/s00396-017-4188-4
Dolzhikova VD, Bogdanova YG (2019) A new technique for elucidation of the surfactants’ effect on the water uptake by Nafion membranes. Colloid Polym Sci 297:469–473. https://doi.org/10.1007/s00396-018-4446-0
Chotipong A, Scamehorn JF, Rirksomboon T et al (2006) Removal of solvent-based ink from printed surface of HDPE bottles by alkyltrimethylammonium bromides: effects of surfactant concentration and alkyl chain length. Colloid Polym Sci 284. https://doi.org/10.1007/s00396-005-1421-3
Ahner N, Zimmermann S, Schaller M, Schulz SE (2012) Determination of surface energy characteristics of plasma processed ultra low -K dielectrics for optimized wetting in wet chemical plasma etch residue removal. Solid State Phenom 195:110–113. https://doi.org/10.4028/www.scientific.net/SSP.195.110
Shrivastava S, Das A (2019) Interaction between ethoxylated emulsifiers and propylene glycol based solvents: gelation and rheology study. Colloids Surfaces A Physicochem Eng Asp 582:123905. https://doi.org/10.1016/j.colsurfa.2019.123905
Alshabib M, Onaizi SA (2019) Effects of surface active additives on the enzymatic treatment of phenol and its derivatives: a mini review. Curr Pollut Reports 5:52–65. https://doi.org/10.1007/s40726-019-00105-8
Rongsayamanont W, Tongcumpou C, Phasukarratchai N (2020) Diesel-contaminated soil washing by mixed nonionic surfactant emulsion and seed germination test. water, Air, Soil Pollut 231:267. https://doi.org/10.1007/s11270-020-04649-0
Saito S, Nakasato K, Kato Y, Nakata I (2009) Flux composition for solder, solder paste, and method of soldering
Yamamoto M, Shiomi T, Nakanishi K et al (2008) Soldering flux and solder paste composition
Parhar AK (2004) Water soluble fluxes and methods of using the same
Pal N, Saxena N, Mandal A (2017) Equilibrium and dynamic adsorption of gemini surfactants with different spacer lengths at oil/aqueous interfaces. Colloids Surfaces A Physicochem Eng Asp 533:20–32. https://doi.org/10.1016/J.COLSURFA.2017.08.020
Król P, Król B (2012) Surface free energy of polyurethane coatings with improved hydrophobicity. Colloid Polym Sci 290:879–893. https://doi.org/10.1007/s00396-012-2598-x
Turbini LJ (2006) Processing and material issues related to lead-free soldering. In: Subramanian KN (ed) Lead-free electronic solders. Springer US, Boston, MA
Timar-Balazsy A (2000) Wet cleaning of historical textiles: surfactants and other wash bath additives. Stud Conserv 45:46–64. https://doi.org/10.1179/sic.2000.45.1.46
Delcroix G, Bureau C (1990) A new detergent formulation. Text Museum J 29:58–64
Rakowska J, Radwan K, Porycka B, Prochaska K (2017) Experimental study on surface activity of surfactants on their ability to cleaning oil contaminations. J Clean Prod 144:437–447. https://doi.org/10.1016/j.jclepro.2016.12.158
Shrivastava S, Matsuoka H (2016) Photocleavable amphiphilic diblock copolymer micelles bearing a nitrobenzene block. Colloid Polym Sci 294:879–887. https://doi.org/10.1007/s00396-016-3839-1
Cox MF (1986) Surfactants for hard-surface cleaning: mechanisms of solid soil removal. J Am Oil Chem Soc 63:559–565. https://doi.org/10.1007/BF02645756
Jańczuk B, Białopiotrowicz T (1990) The total surface free energy and the contact angle in the case of low energetic solids. J Colloid Interface Sci 140:362–372. https://doi.org/10.1016/0021-9797(90)90356-S
Acknowledgements
Support and encouragement from management of MacDermid Alpha Electronics Solutions for this work is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shrivastava, S., Patra, M.R. & Das, A. A systematic approach for surfactant system selection and optimization for cleaning electronic assemblies’ residues. Colloid Polym Sci 299, 1979–1989 (2021). https://doi.org/10.1007/s00396-021-04914-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-021-04914-6