Abstract
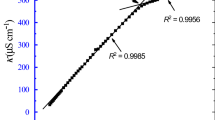
The specific conductivity of dodecyldimethylbenzylammonium bromide (C12BBr) in aqueous solutions, in the temperature range of 15 to 40 °C, has been measured as a function of molality. The two breaks which were found on the conductivity against molality plots were attributed to the critical micelle concentration, cmc, and second critical micelle concentration, 2nd cmc, respectively. The ratio of the slopes, S, of the three linear fragments on the plots, S2/S1 and S3/S1, was attributed to the degree of ionization of the micelles at cmc and 2nd cmc respectively. It was shown that the values of the 2nd cmc estimated above 27 °C are only apparent due to thermal disintegration of the micelles. In the temperature range of 15 to 27 °C, the values of the 2nd cmc increase gradually and the plot of the 2nd cmc against temperature is concave. The ratio of 2nd cmc/cmc for C12BBr at 25 °C amounts to 15 and appears to be high compared to the literature values for other surfactants. For comparative purposes the cmc and 2nd cmc values were also estimated conductometrically for decyldimethylbenzylammonium bromide (C10BBr) at 25 °C. The 2nd cmc value for this surfactant is higher compared to the value for the C12 homologue by a factor of 2.6.The standard Gibbs free energies of micellization at cmc and at the 2nd cmc were estimated from the experimental data for both surfactants at 25 °C.




Similar content being viewed by others
References
McBain JW (1913) Trans Faraday Soc 9:99
Rosen MJ (1989) Surfactants and Interfacial Phenomena, 2nd edition, Willey Publishers, New York
Moroi Y (1992) Micelles: Theoretical an Applied Aspects, Plenum Press, New York
Evans DF, Wennerström H (1999) The Colloidal Domain: Where Physics, Chemistry, Biology and Technology meet, 2nd edition, Wiley Publishers, New York
Holmberg K, Jönsson B, Kronberg B, Lindman B (2002) Surfactants and polymers in aqueous solution 2nd edition, Wiley Publishers, New York
Mc Bain JW, Laing ME, Titley AJ (1919) J Chem Soc 151:1279
Hess K, Philippoff W, Kiessing H (1939) Kolloid-Z 88:1939
Stauff J (1939) Kolloid-Z 89:224
Ekwall P, Eikren H, Mandell L (1963) Acta Chem Scand 17:111
Ekwall P, Holmberg P (1965) Acta Chem Scand 19:455
Ekwall P, Lemström KE, Eikrem H, Holmbreg P (1967) Acta Chem Scand 21:1401
Miura M, Kodama M (1972) Bull Chem Soc Jpn 45:428
Kodama M, Miura M (1972) Bull Chem Soc Jpn 45:2265
Kodama M, Kubota Y, Miura M (1972) Bull Chem Soc Jpn 45:2953
Sata N, Tyuzyo K (1953) Bull Chem Soc Jpn 26:177
Okuyama H, Tyuzyo K (1954) Bull Chem Soc Jpn 27:259
De Lisi R, Fisicaro E, Milioto S (1988) J Sol Chem 17:398
González-Pérez A, Czapkiewicz J, Prieto G, Rodríguez JR (2003) Colloid Polym Sci 281:1191
Treiner C, Makayssi JE (1992) Langmuir 8:794
González-Pérez A, Czapkiewicz, Del Castillo JL, Rodríguez JR (2001) Colloids Surf A 193:129
Zhao J, Fung BM (1993) Langmuir 9:1228
Lee YS, Woo KW (1995) J Colloid Interface Sci 169:34
La Mesa C (1990) J Phys Chem 94:323
Muller N (1993) Langmuir 9:96
Chen LJ, Lin SY, Huang CC (1998) J Phys Chem B 102:4350
Rodríguez JR, González-Pérez A, Del Castillo JL, Czapkiewicz J (2002) J Colloid Interface Sci 250:438
Zielinski R, Ikeda S, Nomura H, Kato S (1989) J Colloid Interface Sci 129:175
González-Pérez A, Del Castillo JL, Czapkiewicz J, Rodríguez JR (2002) Colloid Polym Sci 280:503
Monk CB (1961) Electrolytic dissociation. Academic, London
Hoffmann H, Ulbright W (1977) Z Phys Chem NF 106:167
González-Pérez A, Czapkiewicz J, Del Castillo JL, Rodríguez JR (2003) Colloid Polym Sci 280:503
Acknowledgement
This work was supported by the Xunta de Galicia, (Project PGIDIT03PXIB20601PR). A. González-Pérez is grateful to the University of Santiago de Compostela for his postdoctoral grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Pérez, A., Czapkiewicz, J., Ruso, J.M. et al. Temperature dependence of second critical micelle concentration of dodecyldimethylbenzylammonium bromide in aqueous solution . Colloid Polym Sci 282, 1169–1173 (2004). https://doi.org/10.1007/s00396-004-1053-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1053-z