Abstract
Late, repetitive or chronic remote ischaemic conditioning (CRIC) is a potential cardioprotective strategy against adverse remodelling following ST-segment elevation myocardial infarction (STEMI). In the randomised Daily Remote Ischaemic Conditioning Following Acute Myocardial Infarction (DREAM) trial, CRIC following primary percutaneous coronary intervention (P-PCI) did not improve global left ventricular (LV) systolic function. A post-hoc analysis was performed to determine whether CRIC improved regional strain. All 73 patients completing the original trial were studied (38 receiving 4 weeks’ daily CRIC, 35 controls receiving sham conditioning). Patients underwent cardiovascular magnetic resonance at baseline (5–7 days post-STEMI) and after 4 months, with assessment of LV systolic function, infarct size and strain (longitudinal/circumferential, in infarct-related and remote territories). At both timepoints, there were no significant between-group differences in global indices (LV ejection fraction, infarct size, longitudinal/circumferential strain). However, regional analysis revealed a significant improvement in longitudinal strain in the infarcted segments of the CRIC group (from − 16.2 ± 5.2 at baseline to − 18.7 ± 6.3 at follow up, p = 0.0006) but not in corresponding segments of the control group (from − 15.5 ± 4.0 to − 15.2 ± 4.7, p = 0.81; for change: − 2.5 ± 3.6 versus + 0.3 ± 5.6, respectively, p = 0.027). In remote territories, there was a lower increment in subendocardial circumferential strain in the CRIC group than in controls (− 1.2 ± 4.4 versus − 2.5 ± 4.0, p = 0.038). In summary, CRIC following P-PCI for STEMI is associated with improved longitudinal strain in infarct-related segments, and an attenuated increase in circumferential strain in remote segments. Further work is needed to establish whether these changes may translate into a reduced incidence of adverse remodelling and clinical events. Clinical Trial Registration: http://clinicaltrials.gov/show/NCT01664611.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The decline in mortality following ST-segment elevation myocardial infarction (STEMI) is mirrored by an increasing number of survivors with residual heart failure, a condition whose prognosis has not improved significantly in the last two decades [43, 57]. Therefore, key goals in combating ischaemic heart failure include (1) the early identification of at-risk individuals and (2) the development of effective strategies to limit adverse left ventricular (LV) remodelling.
Remote ischaemic conditioning (RIC) is a non-invasive cardioprotective strategy delivered through serial, short-lived periods of ischaemia–reperfusion in a tissue bed remote from the heart [19, 22, 40]. In some previous studies undertaken during the acute phase of STEMI, adjunctive RIC (with primary percutaneous coronary intervention [P-PCI]) attenuated infarct size and/or reduced the incidence of LV systolic dysfunction and major adverse cardiac events, albeit not consistently [1, 5, 9, 14, 31, 32, 42, 56, 60]. However, in the largest-scale randomised trial (CONDI-2/ERIC-PPCI, n = 5401), RIC failed to reduce infarct size or improve 12 month clinical outcomes (cardiac mortality/heart failure hospitalisation) [13, 18].
It has been suggested that a multi-targeted approach combining RIC with postconditioning immediately after stenting may afford greater cardioprotection [6, 8, 50]. Animal studies have also shown that late, repetitive ‘chronic’ remote ischaemic conditioning (CRIC) may mitigate against adverse LV remodelling [55]. We carried out the first randomised clinical trial evaluating CRIC (commencing on the third day following successful P-PCI and administered daily for 4 weeks) [51]. However, cardiovascular magnetic resonance (CMR) demonstrated no effect of CRIC on infarct size or global volumetric indices. Although CRIC commenced late after infarction may not be expected to impact infarct size, it may influence cardiac remodelling: hence, in this post-hoc analysis, we evaluated regional cardiac function as determined by myocardial strain imaging.
Methods
Our study was based on a non-specified post-hoc analysis of 73 STEMI patients recruited in the multicentre, prospective randomised Daily Remote Ischaemic Conditioning Following Acute Myocardial Infarction (DREAM) trial, assessing the impact of CRIC on LV systolic function. Trial design and methodology are as previously reported [51]. All subjects studied in the main trial were included in this post-hoc analysis. In brief, the trial comprised patients presenting to 4 P-PCI centres with a first STEMI successfully treated with P-PCI and baseline LVEF < 45% (determined by echocardiography). Participants were randomised in a 1:1 ratio (stratified by age, gender and infarct location) to 4 weeks’ duration of CRIC or sham treatment, beginning on the third day post-MI and self-administered at participants’ homes using the autoRIC® Device (CellAegis Devices Inc, Toronto, Canada). The device was applied to the upper arm and for CRIC, the device delivered four 5 min cycles of inflation at 200 mmHg separated by 5 min of deflation between each cycle [total treatment time 35 min]; in the control group, the sham device employed the same cycle durations with inflation to 10 mmHg. In both groups during inflation, the device made identical vibrating noises, and participants were not told of the level of inflation required to deliver active treatment and whether they were in the control group. Participants were instructed to apply the device at the same time of day and on the same arm. Participants were asked to keep a diary of use of the device and were removed from the trial if they returned an incomplete diary sheet, missed more than three treatment sessions or missed more than two consecutive days of treatments. Written informed consent was obtained from each patient prior to enrolment. The study was approved by the regional ethics committee (12/EM/0304) and registered with clinicaltrials.gov (NCT01664611). It was conducted according to the principles of Good Clinical Practice and according to the Declaration of Helsinki, under the oversight of the University of Leicester.
CMR imaging
Following their index presentation with STEMI, participants underwent CMR imaging at 5–7 days (baseline) and at 4 months (follow up, Fig. 1). CMR imaging was carried out on a 1.5-Tesla scanner at each of the four participating centres with retrospective electrocardiographic gating and dedicated cardiac receiver coils. Following standard pilot/localiser images, functional cine images were acquired according to standard clinical protocols using a steady-state free precession (SSFP) pulse sequence, in the three long-axis views and in contiguous short-axis slices covering the left ventricle. Late gadolinium enhancement (LGE) imaging was performed in the same slice positions using a segmented inversion-recovery gradient echo sequence.
CMR analysis was undertaken blinded to all clinical details, randomisation and temporal order of scans, and data presented according to the 17-segment American Heart Association segmentation model [2]. For functional assessment, endocardial and epicardial borders were manually contoured on contiguous short-axis LV slices, excluding papillary muscles and trabeculae at end-diastole and at end-systole, using CVI42® (Circle Cardiovascular Imaging Inc. Calgary, Canada) [51]. For assessment of strain, SSFP cine images were analysed using feature tracking software (Medis Qstrain 2.0, Medis Medical Imaging Systems, Leiden, The Netherlands). Epicardial and endocardial borders were manually contoured in the three long-axis views and three selected short-axis views (basal, mid and apical). By automated propagation of contours through the cardiac cycle, peak circumferential and longitudinal strain were derived, globally and at the segmental level. The software automatically generated strain in endocardial, mid-myocardial and epicardial layers, which were averaged for transmural strain. Intraobserver and interobserver agreement for strain analysis in our centre are excellent as previously reported [28]. For LGE analysis, areas of hyperenhancement were quantified using the full-width half maximum method, expressed in grams and as percentage of LV mass (CVI42®, Circle Cardiovascular Imaging Inc. Calgary, Canada) [11]. Additionally, infarct transmurality was graded visually on a 4-point scale as described previously (0–no LGE, 1–1–25%, 2–26–50%, 3–51–75%, 4–75–100%), with an LGE score being calculated as the sum of scores from all segments divided by 17 (yielding a maximum score of 4) [29]. Segments with LGE on the baseline scan were classified as infarct-related, and those without were classified as remote.
Statistical analysis
The original sample size was calculated on the basis of an improvement in LVEF with CRIC. For the present analysis, a sample size of 35 patients per group was needed to afford 90% power with α level 5% to detect a 25% difference in global longitudinal strain at follow up. Normality was assessed using histograms and Q–Q plots. Continuous data are expressed as mean (± standard deviation) or median (interquartile range) and compared with Student t tests or Mann–Whitney tests as appropriate. Binary data are expressed as numbers (percentages), and comparisons were performed with the Chi-square or Fisher exact test. For comparison of strain at the patient level, strain values for each patient were averaged in infarcted and remote segments respectively and compared with Mann–Whitney or Student t test as appropriate. To investigate whether the group difference in infarcted and remote segments varied between layers, we used individual segment strain values and a fitted linear mixed model which accounted for the dependency of segments from the same patients. We modelled ‘change from baseline’ with adjustment for baseline strains. Separate models were developed for longitudinal and circumferential strain. Given the exploratory nature of this post-hoc analysis, we did not adjust for multiple testing. In addition, we calculated AUC (area under the ROC curve) to examine the efficacy of strain parameters to predict adverse remodelling at follow up (defined as end-diastolic volume increase ≥ 20% and/or end-systolic volume increase ≥ 15% with ejection fraction ≤ 40%) [3, 4]. All statistical analyses were conducted using Medcalc 9.5.2.0 and SAS 9.4. Statistical tests were 2-tailed, and p < 0.05 was considered significant.
Results
Study participants
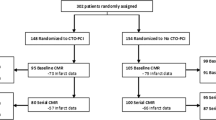
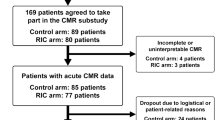
As previously described, patients in the two groups (control n = 35, treatment n = 38) were well matched with regards to baseline demographic and clinical characteristics (Table 1) [9]. The majority of infarcts involved the left anterior descending coronary artery. All participants received drug-eluting stents. Discharge medication was similar between the two groups, with high uptake of optimal medical therapy. The median time between hospital admission and baseline CMR assessment was 4.6 days (3.2–5.9) in the control group and 4.5 days (3.3–4.9) in the treatment group (p = 0.41). Follow up imaging was performed at a median of 122 days (120–126) after admission in the control group and at 123 days (122–125) in the treatment group (p = 0.50). All CMR images were of sufficient quality and no images were excluded from analysis (Fig. 1).
Global CMR parameters
At baseline, volumetric parameters were comparable in both groups (Table 1): there was no difference in LV ejection fraction at baseline or at follow up. Similarly, there were no differences in infarct size at either timepoint (at baseline, 33.5 ± 18.8 g in controls versus 32.6 ± 25.3 g in the treatment group, p = 0.86, and at follow up, 19.9 g (15.0–23.1) versus 15.2 g (7.8–29.9), respectively, p = 0.37). At baseline, 43.0 ± 15.2% of segments showed evidence of LGE in the control group and 41.0 ± 19.3% in the treatment group (p = 0.47), and at follow up, 38.7 ± 13.0% and 34.2 ± 19.3%, respectively (p = 0.10). At both timepoints, LGE score was comparable in the two groups (at baseline 1.12 ± 0.53 versus 1.03 ± 0.39, respectively, p = 0.52, and at follow up, 0.84 ± 0.39 versus 0.71 ± 0.27, respectively, p = 0.25). Microvascular obstruction was more prevalent in the sham group but this did not reach statistical significance.
Analysis of myocardial strain revealed no between-group differences at baseline in either global longitudinal strain or global circumferential strain (Fig. 2). Similarly, at follow up, there were no differences between control and treatment groups (change in global longitudinal strain −1.9±3.1 versus −2.4±3.0, respectively, p=0.39; change in global circumferential strain −2.9±3.4 versus −2.2±3.1, p=0.40).
Patient-level analysis myocardial strain in infarcted versus remote segments
Further patient-level analysis was carried out, evaluating infarcted (n = 73) and remote (n = 73) territories. At baseline, longitudinal strain was found to be lower in infarcted regions than in remote, with no between-group differences (Fig. 3A). At follow up, in the remote territories, there was a significant improvement in longitudinal strain relative to baseline in both arms (Fig. 3B, C). By contrast, in the infarct-related regions of the control arm, there was no improvement in longitudinal strain (− 15.5 ± 4.0 at baseline to − 15.2 ± 4.7 at follow up, p = 0.81). However, in the infarct-related regions of the treatment arm, there was a significant improvement in longitudinal strain (from − 16.2 ± 5.2 at baseline to − 18.7 ± 6.3 at follow up, p = 0.0006). Consequently, at follow up in the treatment arm, there was no significant disparity between infarcted and remote regions (− 18.7 ± 6.3 versus − 19.5 ± 5.2, respectively, p = 0.46), i.e. there was near normalisation of strain within infarcted territories. However, in the control arm, consistent with a lack of improvement in infarcted regions, there remained a significant disparity in longitudinal strain between these and remote regions at follow up (− 15.2 ± 4.7 versus − 20.5 ± 4.0, respectively, p < 0.0001). Examining the change in strain from baseline to follow up (Fig. 3C), there was a significant difference in the infarct-related regions of both groups (+ 0.3 ± 5.6 in the control group versus − 2.5 ± 3.6 in the treatment group, p = 0.027), with no difference in the remote regions of both groups (− 2.5 ± 3.5 versus − 2.4 ± 4.3, respectively, p = 0.90).
Longitudinal strain (transmurally) for control (n = 35) and treatment (n = 38) groups at A baseline (leftmost panel) and B follow up (middle panel), with C absolute change between timepoints (rightmost panel). Blue bars denote remote regions and orange bars denote infarcted regions. Error bars showing mean standard error
Circumferential strain at baseline was significantly lower in infarcted regions than in remote, with no between-group differences (Fig. 4A). At follow up, circumferential strain improved significantly from baseline in infarcted and remote myocardium in both arms (Fig. 4B). Although the magnitude of change was greater in the control group, this did not reach statistical significance in patient-level analysis (Fig. 4C).
Circumferential strain (transmurally) for control (n = 35) and treatment (n = 38) at A baseline (leftmost panel) and B follow up (middle panel), with C absolute change between timepoints (rightmost panel). Blue bars denote remote regions and orange bars denote infarcted regions. Error bars showing mean standard error
Segment-level analysis of myocardial strain in myocardial layers
A total of 1168 segments (475 infarcted and 693 remote) were available for segmental analysis with the mixed effects model. In remote segments, there was improvement in longitudinal strain in all three myocardial layers, with no between-group differences (Fig. 5, top left panel). By contrast, in segments with infarction, consistent with patient-level analysis, there were significant between-group differences in longitudinal strain, apparent in all three myocardial levels (Fig. 5, top right panel). Specifically, whereas longitudinal strain improved in all three layers of the CRIC arm, in the control arm, there was no significant change in any layer.
Mean longitudinal strain (upper two panels) and circumferential strain (lower two panels) at each timepoint depicted by myocardial layer -red denoting epicardial, yellow, mid-myocardial and blue, endocardial. Solid lines denote the treatment group and dashed lines, the control group. P values derived from mixed effects model accounting for differences in strain at baseline. Endo endocardial, Epi epicardial, Mid mid-myocardial
When circumferential strain was assessed, in contrast to longitudinal strain, in the infarcted segments, there was significant improvement in all three layers of both groups, with no between-group differences (Fig. 5, bottom right panel). However, in remote segments, there was a significant between-group difference: although circumferential strain improved from baseline to follow up, there was a higher increment in the endocardial layer of the control group than the corresponding layer in the treatment group (i.e. an attenuated increase in the latter, (− 1.2 ± 4.4 versus − 2.5 ± 4.0, p = 0.038, Fig. 5 bottom left panel). A trend towards higher increment in the mid-myocardial layer of the control group was also apparent (p = 0.09) with no difference in the epicardial layer (p = 0.32).
Strain parameters in relation to remodelling
Eight patients (11%) developed adverse remodelling at follow up (3 in the control group and 5 in the treatment group, p = 0.53). The ability of baseline infarct size to predict adverse remodelling at follow up did not reach statistical significance (Table 2). However, although baseline GLS was not of predictive value, baseline GCS was predictive of adverse remodelling (threshold < − 15.7, 88% sensitivity, 67% specificity, AUC 0.82 ± 0.06, p < 0.0001; p = 0.015 for difference with GLS). When examined separately in infarcted versus remote territories, circumferential strain in both remained predictive of adverse remodelling. By contrast, with longitudinal strain, diminished strain in infarcted regions predicted subsequent adverse remodelling but strain in remote territories was not of predictive value.
On evaluation of strain parameters on the follow up scan in relation to remodelling, all parameters (longitudinal and circumferential in remote and infarcted territories) were associated with adverse remodelling (Table 2). Circumferential strain remained most closely correlated with adverse remodelling (AUC 0.96 ± 0.02, p < 0.0001, with strain values < − 16.0 having 100% sensitivity and 91% specificity for identifying patients with adverse remodelling).
Discussion
This post-hoc analysis of the DREAM trial reveals that although CRIC does not alter infarct size or global LV indices (volumetry/strain), it is associated with altered regional strain in infarct-related and remote territories. The use of CRIC was associated with improvement in longitudinal strain in infarcted territories and an attenuated increase of circumferential strain in both infarcted and remote territories.
It remains unclear what underlies the disappointing failure to translate promising pre-clinical/early-phase clinical findings into hard clinical endpoints: potential reasons are extensively discussed in the literature.[20, 21, 23, 30] Interspecies differences mean that animal models may not fully replicate infarction in humans: whereas animal models utilise young, healthy animals, human disease is characterised by chronic atherosclerosis and the influence of risk factors such as diabetes, hypertension and hyperlipidaemia. Outcomes in clinical trials may be confounded by the influence of medications which can interfere with cardioprotection (e.g. P2Y purinoceptor 12 inhibitors or glyceryl trinitrate) or influence healing and remodelling independent of infarct size reduction (e.g. angiotensin-converting enzyme inhibitors and angiotensin II-receptor blockers).[17] Furthermore, improvements in reperfusion therapy (producing better and faster recanalisation) to attenuate infarct size and adjuvant pharmacotherapies to limit adverse remodelling may be so effective that no additional intervention with RIC may impact clinical outcome. Pre-infarct angina may also afford cardioprotection through the development of coronary collaterals or through a preconditioning-like effect. To date, most clinical studies have been small-scale and statistically underpowered, using surrogate measures rather than hard clinical endpoints. Even in the CONDI-2/ERIC-PPCI trial (n=5401), the utility of RIC may also have been limited by favourable patient factors (short symptom-to-PPCI time [median 3 hour] and spontaneous recanalisation at admission [TIMI 2-3 flow] in approximately 20% of participants). Greater benefit may be seen with RIC in higher risk patients, such as those with heart failure or large anterior infarcts [36].Another potential confounder is variation in the conditioned tissue mass: RIC administered to a leg may provide a greater stimulus than on a forearm: in a murine model, dual hindlimb RIC [with a greater mass of ischaemic/reperfused tissue] led to greater cardioprotection than single hindlimb RIC [33]. Similarly, a clinical trial utilising lower limb RIC demonstrated robust reduction in cardiac mortality and heart failure hospitalisation in contrast with many trials utilising forearm RIC which have failed to demonstrate clinical benefit [14]. However, another murine model found that one and two hindlimb preconditioning were equally protective, and a randomised clinical trial involving lower limb remote ischaemic per/postconditioning in 93 patients with anterior STEMI demonstrated no difference in myocardial salvage index or infarct size [26, 53].
In the CONDI-2/ERIC-PPCI trial, remote ischaemic perconditioning neither reduced infarct size (as assessed by 48-h troponin release or by CMR [n = 110]) nor improved the primary clinical endpoint (composite of cardiac mortality and heart failure hospitalisation at 12 months) [13, 18]. However, previous RIC studies have suggested the presence of discordant effects on infarct size and clinical outcomes. In the RIC-STEMI trial (n = 516), there was no reduction in infarct size with adjunctive RIC (based on 48 h troponin release) but the primary composite outcome of cardiac death and heart failure hospitalisation was significantly reduced (hazard ratio 0.35, 95% CI 0.15–0.78, median follow up 2.1 years) [14]. Other studies indicate the potential for additional cardioprotection by extending the period of conditioning beyond the time of ischaemia/reperfusion. A CMR study of postconditioning immediately after reperfusion in PPCI-treated STEMI patients (n = 122) showed no reduction in infarct size but at 1 year, adverse remodelling was reduced, especially in those with microvascular obstruction [50]. In the LIPSIA-CONDITIONING trial (n = 696), combined RIC and postconditioning resulted in greater myocardial salvage than conventional PPCI alone, and with extended follow up (median 3.6 years), a reduction in cardiac death, reinfarction and new congestive cardiac failure (10.2 versus 16.9%, p = 0.04) [8, 47]. However, in this trial, postconditioning alone did not reduce MACE (14.1 versus 16.9% in controls, p-0.41), and other postconditioning studies have also reported neutral outcomes [10, 16, 46]. Nonetheless, taken together, these results indicate that although infarct size may not be reduced with RIC, the subsequent remodelling process may be altered for therapeutic gain.
To our knowledge, ours is the only trial to evaluate CRIC post-STEMI: to date, no other clinical trial has evaluated late, repetitive RIC post-STEMI, though the CORIC-MI (n = 200) and i-RIC (n = 4700) trials will incorporate CRIC following STEMI (with additional per/postconditioning) [45, 63]. However, CRIC has been investigated in experimental animal studies. In an animal model of ischaemia/reperfusion injury, CRIC administered for 28 days resulted in improved LV remodelling and also survival (at 84 days), and, consistent with our findings, no change in infarct size [55]. Importantly, in our trial, CRIC was not commenced till day 3 post-MI. Given the critical 48 h timeframe ascribed for reperfusion injury when infarct size attenuation may be targeted therapeutically, any benefit from ‘late’ CRIC likely involves mechanisms distinct from infarct size reduction [34, 62]. Previous work has shown that cardioprotection may be mediated by dialysable humoral factors which can circulate for 6 days after a RIC stimulus [24]. Our data indicate that the benefits of CRIC are unlikely to involve haemodynamic parameters, which remained comparable in both groups at follow up (Table 1). Although microvascular obstruction, a known predictor of adverse remodelling, was more prevalent in the sham group, this did not reach statistical significance.
Nonetheless, if CRIC is beneficial, why was there no observed effect on LVEF or other volumetric indices of remodelling? Global volumetric indices integrate the function of infarcted and remote regions, and hence may be insensitive to regional dysfunction, particularly if compensatory mechanisms subsequently normalise global performance. Furthermore, gross volumetric change may only occur late in the remodelling process, in a maladaptive state beyond the point of no return. By contrast, strain imaging may prove more sensitive, identifying subtle, early and potentially reversible regional derangements in those at risk of at risk of adverse remodelling [37]. Impairment of longitudinal strain has been shown to occur early in many pathological disease states, preceding the onset of overt systolic dysfunction [7, 27].
Strain analysis may also provide pathophysiological insights into STEMI-induced remodelling. The myocardium comprises a complex spatial orientation of fibres, with subendocardial fibres orientated in a right-handed helix and subepicardial fibres, in a left-handed helix, with mid-myocardial fibres arranged circumferentially [15, 49]. This physiological arrangement is mechanically advantageous, providing energetic efficiency, with optimum redistribution of shear forces [52]. The subendocardial fibres are especially vulnerable to the effects of ischaemia, and post-MI, longitudinal function declines first [44, 54, 61]. This may be compensated by augmenting short-axis function, mediated by circumferential fibre shortening [54]. Clinical evidence suggests that whereas GLS is a better predictor of MACE (driven by ischaemic/scar-related events), GCS better predicts adverse remodelling [7, 25]. It is likely that circumferential function initially compensates for longitudinal dysfunction (and restrains ventricular dilatation), but subsequently may decline, with ensuing dilatation and adverse remodelling [48]. However, even prior to decompensation, augmented circumferential function may be maladaptive, increasing cardiac workload and shear stress in damaged regions. Myocardial stretching/thinning predisposes to the development of sphericity, which reduces mechanical advantage and is associated with adverse outcome [39, 41].
Our data show increased circumferential strain in remote as well as in infarcted segments. Studies using CMR diffusion tensor imaging have shown reorientation of fibres in remote myocardium post-MI [35, 58, 59]. Animal models show that local strain patterns may guide the alignment of collagen fibres during scar formation [12]. Hence, in the chronic phase post-STEMI, altered regional mechanics may influence the propensity to adverse remodelling. Intuitively, rather than augmenting a compensatory, potentially maladaptive mechanism, correction of the initial pathological derangement may be preferable. Our data indicate that CRIC may target the initial derangement in longitudinal function, minimising the disparity in longitudinal strain between infarcted segments and adjacent viable myocardium and lessening the requirement for circumferential compensation. This may prove mechanically and energetically advantageous and potentially mitigate against the development of heart failure [38].
Study limitations
This study has several limitations. As CRIC was self-administered in participants’ homes, it was not possible to objectively verify correct application of the device and achievement of a satisfactory postconditioning stimulus. The present work was a non-prespecified post-hoc analysis and a small number of participants were involved: hence, the results should be interpreted with caution. The study was not powered to determine potential improvements in adverse remodelling, as defined by global parameters. However, as a proof-of-concept study it serves the purpose of hypothesis generation. Whether the observed changes in regional strain translate into a reduction in adverse remodelling and altered clinical outcome warrants exploration in larger-scale prospective studies. Our data do not elucidate the mechanisms underlying observed changes in strain, albeit demonstrating that these do not involve infarct size reduction or changes in haemodynamic parameters.
Conclusions
Our analysis suggests that the beneficial effects of RIC may involve mechanisms distinct from infarct size limitation. This warrants further investigation in prospective studies as well as in analyses of imaging datasets acquired from previously studied RIC cohorts. Further study is required to determine whether a multi-target approach combining CRIC with ischaemic perconditioning interventions may afford a more effective, synergistic cardioprotective strategy.
Abbreviations
- CMR:
-
Cardiac Magnetic Resonance Imaging
- CRIC:
-
Chronic Remote Ischaemic Postconditioning
- DREAM:
-
Daily Remote Ischaemic Conditioning Following Acute Myocardial Infarction
- GCS:
-
Global Longitudinal Strain
- GLS:
-
Global Circumferential Strain
- LVEF:
-
Left Ventricular Ejection Fraction
- MI:
-
Myocardial Infarction
- P-PCI:
-
Primary Percutaneous Coronary Intervention
- STEMI:
-
ST-Elevation Myocardial Infarction
- RIC:
-
Remote Ischaemic Conditioning
- STEMI:
-
ST-Elevation Myocardial Infarction
References
Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT (2010) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet 375:727–734. https://doi.org/10.1016/S0140-6736(09)62001-8
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial S, Registration for Cardiac I (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation 105:539–542. https://doi.org/10.1161/hc0402.102975
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 35:569–582. https://doi.org/10.1016/s0735-1097(99)00630-0
Cong T, Sun Y, Shang Z, Wang K, Su D, Zhong L, Zhang S, Yang Y (2015) Prognostic value of speckle tracking echocardiography in patients with ST-elevation myocardial infarction treated with late percutaneous intervention. Echocardiography 32:1384–1391. https://doi.org/10.1111/echo.12864
Crimi G, Pica S, Raineri C, Bramucci E, De Ferrari GM, Klersy C, Ferlini M, Marinoni B, Repetto A, Romeo M, Rosti V, Massa M, Raisaro A, Leonardi S, Rubartelli P, Oltrona Visconti L, Ferrario M (2013) Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc Interv 6:1055–1063. https://doi.org/10.1016/j.jcin.2013.05.011
Davidson SM, Ferdinandy P, Andreadou I, Botker HE, Heusch G, Ibanez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D, Action CC (2019) Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol 73:89–99. https://doi.org/10.1016/j.jacc.2018.09.086
Eitel I, Stiermaier T, Lange T, Rommel KP, Koschalka A, Kowallick JT, Lotz J, Kutty S, Gutberlet M, Hasenfuss G, Thiele H, Schuster A (2018) Cardiac magnetic resonance myocardial feature tracking for optimized prediction of cardiovascular events following myocardial infarction. JACC Cardiovasc Imaging 11:1433–1444. https://doi.org/10.1016/j.jcmg.2017.11.034
Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H (2015) Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J 36:3049–3057. https://doi.org/10.1093/eurheartj/ehv463
Elbadawi A, Ha LD, Abuzaid AS, Crimi G, Azzouz MS (2017) Meta-analysis of randomized trials on remote ischemic conditioning during primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol 119:832–838. https://doi.org/10.1016/j.amjcard.2016.11.036
Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Clemmensen P, Holmvang L, Jorgensen E, Pedersen F, Saunamaki K, Ravkilde J, Tilsted HH, Villadsen A, Aaroe J, Jensen SE, Raungaard B, Botker HE, Terkelsen CJ, Maeng M, Kaltoft A, Krusell LR, Jensen LO, Veien KT, Kofoed KF, Torp-Pedersen C, Kyhl K, Nepper-Christensen L, Treiman M, Vejlstrup N, Ahtarovski K, Lonborg J, Kober L, Third Danish Study of Optimal Acute Treatment of Patients With STEMI-IPI (2017) Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol 2:490–497. https://doi.org/10.1001/jamacardio.2017.0022
Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC (2011) Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging 4:150–156. https://doi.org/10.1016/j.jcmg.2010.11.015
Fomovsky GM, Rouillard AD, Holmes JW (2012) Regional mechanics determine collagen fiber structure in healing myocardial infarcts. J Mol Cell Cardiol 52:1083–1090. https://doi.org/10.1016/j.yjmcc.2012.02.012
Francis R, Chong J, Ramlall M, Bucciarelli-Ducci C, Clayton T, Dodd M, Engstrom T, Evans R, Ferreira VM, Fontana M, Greenwood JP, Kharbanda RK, Kim WY, Kotecha T, Lonborg JT, Mathur A, Moller UK, Moon J, Perkins A, Rakhit RD, Yellon DM, Botker HE, Bulluck H, Hausenloy DJ (2021) Effect of remote ischaemic conditioning on infarct size and remodelling in ST-segment elevation myocardial infarction patients: The CONDI-2/ERIC-PPCI CMR substudy. Basic Res Cardiol 116:59. https://doi.org/10.1007/s00395-021-00896-2
Gaspar A, Lourenco AP, Pereira MA, Azevedo P, Roncon-Albuquerque R Jr, Marques J, Leite-Moreira AF (2018) Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol 113:14. https://doi.org/10.1007/s00395-018-0672-3
Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH (1981) Left ventricular fibre architecture in man. Br Heart J 45:248–263. https://doi.org/10.1136/hrt.45.3.248
Hahn JY, Song YB, Kim EK, Yu CW, Bae JW, Chung WY, Choi SH, Choi JH, Bae JH, An KJ, Park JS, Oh JH, Kim SW, Hwang JY, Ryu JK, Park HS, Lim DS, Gwon HC (2013) Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation 128:1889–1896. https://doi.org/10.1161/CIRCULATIONAHA.113.001690
Hauerslev M, Mork SR, Pryds K, Contractor H, Hansen J, Jespersen NR, Johnsen J, Heusch G, Kleinbongard P, Kharbanda R, Botker HE, Schmidt MR (2018) Influence of long-term treatment with glyceryl trinitrate on remote ischemic conditioning. Am J Physiol Heart Circ Physiol 315:H150–H158. https://doi.org/10.1152/ajpheart.00114.2018
Hausenloy DJ, Kharbanda RK, Moller UK, Ramlall M, Aaroe J, Butler R, Bulluck H, Clayton T, Dana A, Dodd M, Engstrom T, Evans R, Lassen JF, Christensen EF, Garcia-Ruiz JM, Gorog DA, Hjort J, Houghton RF, Ibanez B, Knight R, Lippert FK, Lonborg JT, Maeng M, Milasinovic D, More R, Nicholas JM, Jensen LO, Perkins A, Radovanovic N, Rakhit RD, Ravkilde J, Ryding AD, Schmidt MR, Riddervold IS, Sorensen HT, Stankovic G, Varma M, Webb I, Terkelsen CJ, Greenwood JP, Yellon DM, Botker HE (2019) Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet 394:1415–1424. https://doi.org/10.1016/S0140-6736(19)32039-2 (CONDI-2/ERIC-PPCI Investigators)
Heusch G (2018) 25 Years of remote ischemic conditioning: From laboratory curiosity to clinical outcome. Basic Res Cardiol 113:15. https://doi.org/10.1007/s00395-018-0673-2
Heusch G (2017) Critical issues for the translation of cardioprotection. Circ Res 120:1477–1486. https://doi.org/10.1161/CIRCRESAHA.117.310820
Heusch G (2020) Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17:773–789. https://doi.org/10.1038/s41569-020-0403-y
Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D (2015) Remote ischemic conditioning. J Am Coll Cardiol 65:177–195. https://doi.org/10.1016/j.jacc.2014.10.031
Heusch G, Gersh BJ (2020) Is cardioprotection salvageable? Circulation 141:415–417. https://doi.org/10.1161/CIRCULATIONAHA.119.044176
Hildebrandt HA, Kreienkamp V, Gent S, Kahlert P, Heusch G, Kleinbongard P (2016) Kinetics and signal activation properties of circulating factor(s) from healthy volunteers undergoing remote ischemic pre-conditioning. JACC Basic Transl Sci 1:3–13. https://doi.org/10.1016/j.jacbts.2016.01.007
Hung CL, Verma A, Uno H, Shin SH, Bourgoun M, Hassanein AH, McMurray JJ, Velazquez EJ, Kober L, Pfeffer MA, Solomon SD, Investigators V (2010) Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol 56:1812–1822. https://doi.org/10.1016/j.jacc.2010.06.044
Johnsen J, Pryds K, Salman R, Lofgren B, Kristiansen SB, Botker HE (2016) The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res Cardiol 111:10. https://doi.org/10.1007/s00395-016-0529-6
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100:1673–1680. https://doi.org/10.1136/heartjnl-2014-305538
Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP (2015) Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol 84:840–848. https://doi.org/10.1016/j.ejrad.2015.02.002
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343:1445–1453. https://doi.org/10.1056/NEJM200011163432003
Kleinbongard P, Botker HE, Ovize M, Hausenloy DJ, Heusch G (2020) Co-morbidities and co-medications as confounders of cardioprotection-does it matter in the clinical setting? Br J Pharmacol 177:5252–5269. https://doi.org/10.1111/bph.14839
Kleinbongard P, Peters J, Jakob H, Heusch G, Thielmann M (2018) Persistent survival benefit from remote ischemic pre-conditioning in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol 71:252–254. https://doi.org/10.1016/j.jacc.2017.10.083
Le Page S, Bejan-Angoulvant T, Angoulvant D, Prunier F (2015) Remote ischemic conditioning and cardioprotection: a systematic review and meta-analysis of randomized clinical trials. Basic Res Cardiol 110:11. https://doi.org/10.1007/s00395-015-0467-8
Lieder HR, Irmert A, Kamler M, Heusch G, Kleinbongard P (2019) Sex is no determinant of cardioprotection by ischemic preconditioning in rats, but ischemic/reperfused tissue mass is for remote ischemic preconditioning. Physiol Rep 7:e14146. https://doi.org/10.14814/phy2.14146
Matsumura K, Jeremy RW, Schaper J, Becker LC (1998) Progression of myocardial necrosis during reperfusion of ischemic myocardium. Circulation 97:795–804. https://doi.org/10.1161/01.cir.97.8.795
Mekkaoui C, Huang S, Chen HH, Dai G, Reese TG, Kostis WJ, Thiagalingam A, Maurovich-Horvat P, Ruskin JN, Hoffmann U, Jackowski MP, Sosnovik DE (2012) Fiber architecture in remodeled myocardium revealed with a quantitative diffusion CMR tractography framework and histological validation. J Cardiovasc Magn Reson 14:70. https://doi.org/10.1186/1532-429X-14-70
Munk K, Andersen NH, Schmidt MR, Nielsen SS, Terkelsen CJ, Sloth E, Botker HE, Nielsen TT, Poulsen SH (2010) Remote ischemic conditioning in patients with myocardial infarction treated with primary angioplasty: impact on left ventricular function assessed by comprehensive echocardiography and gated single-photon emission CT. Circ Cardiovasc Imaging 3:656–662. https://doi.org/10.1161/CIRCIMAGING.110.957340
Munk K, Andersen NH, Terkelsen CJ, Bibby BM, Johnsen SP, Botker HE, Nielsen TT, Poulsen SH (2012) Global left ventricular longitudinal systolic strain for early risk assessment in patients with acute myocardial infarction treated with primary percutaneous intervention. J Am Soc Echocardiogr 25:644–651. https://doi.org/10.1016/j.echo.2012.02.003
Neubauer S (2007) The failing heart–an engine out of fuel. N Engl J Med 356:1140–1151. https://doi.org/10.1056/NEJMra063052
Pfeffer MA, Braunwald E (1990) Ventricular remodeling after myocardial infarction. Exp Observations Clin Implic Circ 81:1161–1172. https://doi.org/10.1161/01.cir.81.4.1161
Pickard JM, Botker HE, Crimi G, Davidson B, Davidson SM, Dutka D, Ferdinandy P, Ganske R, Garcia-Dorado D, Giricz Z, Gourine AV, Heusch G, Kharbanda R, Kleinbongard P, MacAllister R, McIntyre C, Meybohm P, Prunier F, Redington A, Robertson NJ, Suleiman MS, Vanezis A, Walsh S, Yellon DM, Hausenloy DJ (2015) Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th biennial hatter cardiovascular institute workshop. Basic Res Cardiol 110:453. https://doi.org/10.1007/s00395-014-0453-6
Popescu BA, Beladan CC, Calin A, Muraru D, Deleanu D, Rosca M, Ginghina C (2009) Left ventricular remodelling and torsional dynamics in dilated cardiomyopathy: Reversed apical rotation as a marker of disease severity. Eur J Heart Fail 11:945–951. https://doi.org/10.1093/eurjhf/hfp124
Prunier F, Angoulvant D, Saint Etienne C, Vermes E, Gilard M, Piot C, Roubille F, Elbaz M, Ovize M, Biere L, Jeanneteau J, Delepine S, Benard T, Abi-Khalil W, Furber A (2014) The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res Cardiol 109:400. https://doi.org/10.1007/s00395-013-0400-y
Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, Chen AY, Klein LW, Masoudi FA, McKay C, Hewitt K, Brindis RG, Peterson ED, Rumsfeld JS (2010) Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol 56:254–263. https://doi.org/10.1016/j.jacc.2010.05.008
Sabbah HN, Marzilli M, Stein PD (1981) The relative role of subendocardium and subepicardium in left ventricular mechanics. Am J Physiol 240:H920-926. https://doi.org/10.1152/ajpheart.1981.240.6.H920
Song L, Yan H, Zhou P, Zhao H, Liu C, Sheng Z, Tan Y, Yi C, Li J, Zhou J (2018) Effect of comprehensive remote ischemic conditioning in anterior ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: design and rationale of the coric-mi randomized trial. Clin Cardiol 41:997–1003. https://doi.org/10.1002/clc.22973
Sorensson P, Ryden L, Saleh N, Tornvall P, Arheden H, Pernow J (2013) Long-term impact of postconditioning on infarct size and left ventricular ejection fraction in patients with ST-elevation myocardial infarction. BMC Cardiovasc Disord 13:22. https://doi.org/10.1186/1471-2261-13-22
Stiermaier T, Jensen JO, Rommel KP, de Waha-Thiele S, Fuernau G, Desch S, Thiele H, Eitel I (2019) Combined intrahospital remote ischemic perconditioning and postconditioning improves clinical outcome in ST-elevation myocardial infarction. Circ Res 124:1482–1491. https://doi.org/10.1161/CIRCRESAHA.118.314500
Takeuchi M, Nishikage T, Nakai H, Kokumai M, Otani S, Lang RM (2007) The assessment of left ventricular twist in anterior wall myocardial infarction using two-dimensional speckle tracking imaging. J Am Soc Echocardiogr 20:36–44. https://doi.org/10.1016/j.echo.2006.06.019
Torrent-Guasp F, Kocica MJ, Corno A, Komeda M, Cox J, Flotats A, Ballester-Rodes M, Carreras-Costa F (2004) Systolic ventricular filling. Eur J Cardiothorac Surg 25:376–386. https://doi.org/10.1016/j.ejcts.2003.12.020
Traverse JH, Swingen CM, Henry TD, Fox J, Wang YL, Chavez IJ, Lips DL, Lesser JR, Pedersen WR, Burke NM, Pai A, Lindberg JL, Garberich RF (2019) NHLBI-sponsored randomized trial of postconditioning during primary percutaneous coronary intervention for ST-elevation myocardial infarction. Circ Res 124:769–778. https://doi.org/10.1161/CIRCRESAHA.118.314060
Vanezis AP, Arnold JR, Rodrigo G, Lai FY, Debiec R, Nazir S, Khan JN, Ng LL, Chitkara K, Coghlan JG, Hetherington SL, McCann GP, Samani NJ (2018) Daily remote ischaemic conditioning following acute myocardial infarction: a randomised controlled trial. Heart 104:1955–1962. https://doi.org/10.1136/heartjnl-2018-313091
Vendelin M, Bovendeerd PH, Engelbrecht J, Arts T (2002) Optimizing ventricular fibers: uniform strain or stress, but not ATP consumption, leads to high efficiency. Am J Physiol Heart Circ Physiol 283:H1072-1081. https://doi.org/10.1152/ajpheart.00874.2001
Verouhis D, Sorensson P, Gourine A, Henareh L, Persson J, Saleh N, Settergren M, Sundqvist M, Tornvall P, Witt N, Bohm F, Pernow J (2016) Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J 181:66–73. https://doi.org/10.1016/j.ahj.2016.08.004
Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF (2008) Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J 29:1283–1289. https://doi.org/10.1093/eurheartj/ehn141
Wei M, Xin P, Li S, Tao J, Li Y, Li J, Liu M, Li J, Zhu W, Redington AN (2011) Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res 108:1220–1225. https://doi.org/10.1161/CIRCRESAHA.110.236190
White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, Tehrani S, Flett AS, Meier P, Ariti C, Davies JR, Moon JC, Yellon DM, Hausenloy DJ (2015) Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 8:178–188. https://doi.org/10.1016/j.jcin.2014.05.015
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB (2016) Executive summary: heart disease and stroke statistics–2016 update: a report from the American heart association. Circulation 133:447–454. https://doi.org/10.1161/CIR.0000000000000366
Wu MT, Su MY, Huang YL, Chiou KR, Yang P, Pan HB, Reese TG, Wedeen VJ, Tseng WY (2009) Sequential changes of myocardial microstructure in patients postmyocardial infarction by diffusion-tensor cardiac MR: correlation with left ventricular structure and function. Circ Cardiovasc Imaging 2:32–40. https://doi.org/10.1161/CIRCIMAGING.108.778902 (36 p following 40)
Wu MT, Tseng WY, Su MY, Liu CP, Chiou KR, Wedeen VJ, Reese TG, Yang CF (2006) Diffusion tensor magnetic resonance imaging mapping the fiber architecture remodeling in human myocardium after infarction: correlation with viability and wall motion. Circulation 114:1036–1045. https://doi.org/10.1161/CIRCULATIONAHA.105.545863
Yellon DM, Ackbarkhan AK, Balgobin V, Bulluck H, Deelchand A, Dhuny MR, Domah N, Gaoneadry D, Jagessur RK, Joonas N, Kowlessur S, Lutchoo J, Nicholas JM, Pauvaday K, Shamloll O, Walker JM, Hausenloy DJ (2015) Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J Am Coll Cardiol 65:2764–2765. https://doi.org/10.1016/j.jacc.2015.02.082
Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW (2002) Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation 105:1195–1201. https://doi.org/10.1161/hc1002.105185
Zhao ZQ, Nakamura M, Wang NP, Velez DA, Hewan-Lowe KO, Guyton RA, Vinten-Johansen J (2000) Dynamic progression of contractile and endothelial dysfunction and infarct extension in the late phase of reperfusion. J Surg Res 94:133–144. https://doi.org/10.1006/jsre.2000.6029
Zheng Y, Reinhardt JD, Li J, Hu D, Lin S, Wang L, Dai R, Fan Z, Ding R, Chen L, Yuan L, Xu Z, Cheng Y, Yan C, Zhang X, Wang L, Zhang X, Teng M, Yu Q, Yin A, Lu X, i RICTCG (2020) Can clinical and functional outcomes be improved with an intelligent “internet plus”-based full disease cycle remote ischemic conditioning program in acute ST-elevation myocardial infarction patients undergoing percutaneous coronary intervention? Rationale and design of the i-RIC trial. Cardiovasc Drugs Ther https://doi.org/10.1007/s10557-020-07022-9
Acknowledgements
The authors are grateful to the staff of Glenfield Hospital, Royal Derby Hospital, Royal Free Hospital and Kettering General Hospital who helped in patient recruitment and data collection. GPM and JRA are supported by the NIHR research professorship (RP-2017-08-ST2-007) and Clinician Scientist Award (CS-2018-18-ST2-007), respectively, and receive support from the NIHR Leicester Biomedical Research Centre.
Funding
The authors acknowledge the financial support from the NIHR Leicester Cardiovascular Biomedical Research Unit and the Masonic Charitable Foundation. The authors also thank CellAegis Devices Inc for providing the autoRIC™ devices free of charge for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arnold, J.R., P.Vanezis, A., Rodrigo, G.C. et al. Effects of late, repetitive remote ischaemic conditioning on myocardial strain in patients with acute myocardial infarction. Basic Res Cardiol 117, 23 (2022). https://doi.org/10.1007/s00395-022-00926-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-022-00926-7