Abstract
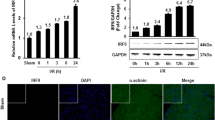
N-myc downstream-regulated gene 4 (NDRG4) is expressed weakly in heart and has been reported to modulate cardiac development and QT interval duration, but the role of NDRG4 in myocardial ischemia/reperfusion (I/R) injury remains unknown. In the present study, we analyzed the expression as well as potential function of cardiac NDRG4 and investigated how NDRG4 expression is regulated by inflammation. We found that NDRG4 was weakly expressed in cardiomyocytes and that its expression increased significantly both in I/R injured heart and in hypoxia-reoxygenation (H/R) injured neonatal rat ventricular myocytes (NRVMs). The increased NDRG4 expression aggravated myocardial I/R injury by inhibiting the activation of the reperfusion injury salvage kinase (RISK) pathway. Forced over-expression of NDRG4 inhibited RISK activation and exacerbated injury not only in I/R injured heart, but also in H/R treated NRVMs, whereas short hairpin RNA (shRNA)-mediated knock-down of NDRG4 enhanced RISK activation and attenuated injury. Upon injury, myocardial NDRG4 expression was induced by tumor necrosis factor-α (TNF-α) through nuclear factor kappa B (NF-κB), and we found that pre-treatment with inhibitors of either TNF-α or NF-κB blocked NDRG4 expression as well as I/R injury in vivo and H/R injury in vitro. Our study indicates that up-regulation of NDRG4 aggravates myocardial I/R injury by inhibiting activation of the RISK pathway, thereby identifying NDRG4 as a potential therapeutic target in I/R injury.








Similar content being viewed by others
References
Bozkurt B, Kribbs SB, Clubb FJ Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL (1998) Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation 97:1382–1391. doi:10.1161/01.CIR.97.14.1382
Cheshire JL, Baldwin AS Jr (1997) Synergistic activation of NF-kappaB by tumor necrosis factor alpha and gamma interferon via enhanced I kappaB alpha degradation and de novo I kappaBbeta degradation. Mol Cell Biol 17:6746–6754. doi:10.1128/MCB.17.11.6746
Chu D, Zhang Z, Zhou Y, Li Y, Zhu S, Zhang J, Zhao Q, Ji G, Wang W, Zheng J (2015) NDRG4, a novel candidate tumor suppressor, is a predictor of overall survival of colorectal cancer patients. Oncotarget 6:7584–7596. doi:10.18632/oncotarget.3170
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241. doi:10.1016/S0092-8674(00)80405-5
Ding W, Zhang J, Yoon JG, Shi D, Foltz G, Lin B (2012) NDRG4 is downregulated in glioblastoma and inhibits cell proliferation. OMICS 16:263–267. doi:10.1089/omi.2011.0146
Dupays L, Kotecha S, Angst B, Mohun TJ (2009) Tbx2 misexpression impairs deployment of second heart field derived progenitor cells to the arterial pole of the embryonic heart. Dev Biol 333:121–131. doi:10.1016/j.ydbio.2009.06.025
Eisenhardt SU, Weiss JB, Smolka C, Maxeiner J, Pankratz F, Bemtgen X, Kustermann M, Thiele JR, Schmidt Y, Bjoern Stark G, Moser M, Bode C, Grundmann S (2015) MicroRNA-155 aggravates ischemia-reperfusion injury by modulation of inflammatory cell recruitment and the respiratory oxidative burst. Basic Res Cardiol 110:32. doi:10.1007/s00395-015-0490-9
Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ (2010) A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res 107:1445–1453. doi:10.1161/CIRCRESAHA.110.223925
Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, Chilian WM, Zhang C (2007) TNF-alpha contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 27:1269–1275. doi:10.1161/ATVBAHA.107.142521
Gu C, Xing Y, Jiang L, Chen M, Xu M, Yin Y, Li C, Yang Z, Yu L, Ma H (2013) Impaired cardiac SIRT1 activity by carbonyl stress contributes to aging-related ischemic intolerance. PLoS ONE 8:e74050. doi:10.1371/journal.pone.0074050
Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res 61:448–460. doi:10.1016/j.cardiores.2003.09.024
Hausenloy DJ, Yellon DM (2007) Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev 12:217–234. doi:10.1007/s10741-007-9026-1
Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M (2012) STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res 110:111–115. doi:10.1161/CIRCRESAHA.111.259556
Hou W, Hu J, Li Y, Zhao J, Li Z, Liu X, Yao L, Zhang Y (2010) Altered expression of NDRG2 in the testes of experimental rat model of cryptorchidism. Urology 75:985–991. doi:10.1016/j.urology.2009.05.032
Hou WG, Zhao Y, Shen L, Zhao J, Liu XW, Li Z, Liu XP, Yao LB, Zhang YQ (2009) Differential expression of N-Myc downstream regulated gene 2 (NDRG2) in the rat testis during postnatal development. Cell Tissue Res 337:257–267. doi:10.1007/s00441-009-0814-x
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. doi:10.1016/B978-0-12-394309-5.00006-7
Kevin LG, Novalija E, Stowe DF (2005) Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg 101:1275–1287. doi:10.1213/01.ANE.0000180999.81013.D0
Kitowska A, Pawelczyk T (2010) N-myc downstream regulated 1 gene and its place in the cellular machinery. Acta Biochim Pol 57(1):15–21.
Kleinbongard P, Schulz R, Heusch G (2011) TNFalpha in myocardial ischemia/reperfusion, remodeling and heart failure. Heart Fail Rev 16:49–69. doi:10.1007/s10741-010-9180-8
Kotipatruni RP, Ferraro DJ, Ren X, Vanderwaal RP, Thotala DK, Hallahan DE, Jaboin JJ (2012) NDRG4, the N-Myc downstream regulated gene, is important for cell survival, tumor invasion and angiogenesis in meningiomas. Integr Biol (Camb) 4:1185–1197. doi:10.1039/c2ib20168b
Kovacevic Z, Richardson DR (2006) The metastasis suppressor, Ndrg-1: a new ally in the fight against cancer. Carcinogenesis 27:2355–2366. doi:10.1093/carcin/bgl146
Li J, Zhang H, Zhang C (2012) Role of inflammation in the regulation of coronary blood flow in ischemia and reperfusion: mechanisms and therapeutic implications. J Mol Cell Cardiol 52:865–872. doi:10.1016/j.yjmcc.2011.08.027
Li T, Hu J, He GH, Li Y, Zhu CC, Hou WG, Zhang S, Li W, Zhang JS, Wang Z, Liu XP, Yao LB, Zhang YQ (2012) Up-regulation of NDRG2 through nuclear factor-kappa B is required for Leydig cell apoptosis in both human and murine infertile testes. Biochim Biophys Acta 1822:301–313. doi:10.1016/j.bbadis.2011.11.013
Maekawa N, Wada H, Kanda T, Niwa T, Yamada Y, Saito K, Fujiwara H, Sekikawa K, Seishima M (2002) Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. J Am Coll Cardiol 39:1229–1235. doi:10.1016/S0735-1097(02)01738-2
Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J (2010) NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res 85:473–483. doi:10.1093/cvr/cvp305
Melotte V, Lentjes MH, van den Bosch SM, Hellebrekers DM, de Hoon JP, Wouters KA, Daenen KL, Partouns-Hendriks IE, Stessels F, Louwagie J, Smits KM, Weijenberg MP, Sanduleanu S, Khalid-de Bakker CA, Oort FA, Meijer GA, Jonkers DM, Herman JG, de Bruine AP, van Engeland M (2009) N-Myc downstream-regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancer. J Natl Cancer Inst 101:916–927. doi:10.1093/jnci/djp131
Newton-Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, Bis JC, Marciante K, Rivadeneira F, Noseworthy PA, Sotoodehnia N, Smith NL, Rotter JI, Kors JA, Witteman JC, Hofman A, Heckbert SR, O’Donnell CJ, Uitterlinden AG, Psaty BM, Lumley T, Larson MG, Stricker BH (2009) Common variants at ten loci influence QT interval duration in the QTGEN study. Nat Genet 41:399–406. doi:10.1038/ng.364
Qu X, Jia H, Garrity DM, Tompkins K, Batts L, Appel B, Zhong TP, Baldwin HS (2008) Ndrg4 is required for normal myocyte proliferation during early cardiac development in zebrafish. Dev Biol 317:486–496. doi:10.1016/j.ydbio.2008.02.044
Quam L, Smith R, Yach D (2006) Rising to the global challenge of the chronic disease epidemic. Lancet 368:1221–1223. doi:10.1016/S0140-6736(06)69422-1 (S0140-6736(06)69422-1[pii])
Schilling SH, Hjelmeland AB, Radiloff DR, Liu IM, Wakeman TP, Fielhauer JR, Foster EH, Lathia JD, Rich JN, Wang XF, Datto MB (2009) NDRG4 is required for cell cycle progression and survival in glioblastoma cells. J Biol Chem 284:25160–25169. doi:10.1074/jbc.M109.012484
Shimizu H, Mitomo K, Watanabe T, Okamoto S, Yamamoto K (1990) Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol 10:561–568. doi:10.1128/MCB.10.2.561
Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G (2015) Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res 117:279–288. doi:10.1161/CIRCRESAHA.117.306878
Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G (2007) Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res 100:140–146. doi:10.1161/01.RES.0000255031.15793.86
Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G (2009) Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 104:15–18. doi:10.1161/CIRCRESAHA.108.186429
Su H, Yuan Y, Wang XM, Lau WB, Wang Y, Wang X, Gao E, Koch WJ, Ma XL (2013) Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res Cardiol 108:315. doi:10.1007/s00395-012-0315-z
Sun Z, Tong G, Ma N, Li J, Li X, Li S, Zhou J, Xiong L, Cao F, Yao L, Wang H, Shen L (2013) NDRG2: a newly identified mediator of insulin cardioprotection against myocardial ischemia-reperfusion injury. Basic Res Cardiol 108:341. doi:10.1007/s00395-013-0341-5
Wang F, Li H, Shi H, Sun B (2012) Pro-apoptotic role of nuclear factor-kappaB in adriamycin-induced acute myocardial injury in rats. Mol Med Rep 5:400–404. doi:10.3892/mmr.2011.636
Wang W, Li Y, Li Y, Hong A, Wang J, Lin B, Li R (2009) NDRG3 is an androgen regulated and prostate enriched gene that promotes in vitro and in vivo prostate cancer cell growth. Int J Cancer 124:521–530. doi:10.1002/ijc.23961
Xie QW, Kashiwabara Y, Nathan C (1994) Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem 269(7):4705–4708.
Yamamoto H, Kokame K, Okuda T, Nakajo Y, Yanamoto H, Miyata T (2011) NDRG4 protein-deficient mice exhibit spatial learning deficits and vulnerabilities to cerebral ischemia. J Biol Chem 286:26158–26165. doi:10.1074/jbc.M111.256446
Yao L, Zhang J, Liu X (2008) NDRG2: a Myc-repressed gene involved in cancer and cell stress. Acta Biochim Biophys Sin (Shanghai) 40:625–635. doi:10.1111/j.1745-7270.2008.00434.x
Zeng M, Yan H, Chen Y, Zhao HJ, Lv Y, Liu C, Zhou P, Zhao B (2012) Suppression of NF-kappaB reduces myocardial no-reflow. PLoS ONE 7:e47306. doi:10.1371/journal.pone.0047306
Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ (1996) Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 87:619–628. doi:10.1016/S0092-8674(00)81382-3
Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM (2006) TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 26:475–480. doi:10.1161/01.ATV.0000201932.32678.7e
Zhang XQ, Tang R, Li L, Szucsik A, Javan H, Saegusa N, Spitzer KW, Selzman CH (2013) Cardiomyocyte-specific p65 NF-kappaB deletion protects the injured heart by preservation of calcium handling. Am J Physiol Heart Circ Physiol 305:H1089–H1097. doi:10.1152/ajpheart.00067.2013
Zhao W, Tang R, Huang Y, Wang W, Zhou Z, Gu S, Dai J, Ying K, Xie Y, Mao Y (2001) Cloning and expression pattern of the human NDRG3 gene. Biochim Biophys Acta 1519:134–138. doi:10.1016/S0167-4781(01)00210-X
Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T (2001) Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics 73:86–97. doi:10.1006/geno.2000.6496
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None declared.
Funding
This work was supported by the National Natural Science Foundation of China, NSFC (Nos. 31371216 and 31171154 to YQ Z), and by the Program of China Scholarship Council (No. (2014)3026 to CC Z).
Additional information
Y. Xing, B. Tang and C. Zhu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2015_519_MOESM1_ESM.tif
Figure S1 The effect of NDRG4 knockdown on I/R-induced myocardial injury. (A) Measurement of cardiac function using echocardiography was performed at 24 h following reperfusion to assess left ventricular ejection fraction (LVEF) in hearts with adenovirus-packaged shRNA-NC or shRNA-ND4. (*P < 0.05 vs. sham) (B) Serum was collected at 24-h reperfusion to analyze the total LDH level in each group. (n = 6 per group, *P < 0.05 vs. sham, # P < 0.05 vs. shRNA-NC + I/R). Values are presented as mean ± SEM. (C) Quantitation of caspase 3 activation results. (n = 5 per group, *P < 0.05 vs. Con + shRNA-NC group; # P < 0.05 vs. H/R + shRNA-NC group). (D, E) Flow cytometry assay results indicating the apoptosis rate of NRVMs with or without NDRG4 knockdown after H/R. The percentage of apoptotic cells in H/R-treated cardiomyocytes without NDRG4 knockdown is 13.9%, whereas the percentage of apoptotic cells in H/R-treated myocytes with NDRG4 knockdown is 6.3%. (n = 3 per group, *P < 0.05 vs. Con + shRNA-NC group; # P < 0.05 vs. H/R + shRNA-NC group). Data are presented as the mean ± SEM. (TIFF 5952 kb)
395_2015_519_MOESM2_ESM.tif
Figure S2 The effect of NDRG4 over-expression on I/R-induced myocardial injury. (A) Measurement of cardiac function using echocardiography was performed at 24 h following reperfusion in hearts with adenovirus-packaged Adv-NC or Adv-ND4. LVEF showed no significant difference. (B) Serum was collected at 24-h reperfusion to analyze the total LDH level in each group. (n = 6 per group, *P < 0.05 vs. Adv-NC + I/R). (C) Representative immunoblot image and quantitation of caspase 3 activation results. (n = 4 per group, *P < 0.05 vs. Con + Adv-NC group; # P < 0.05 vs. H/R + Adv-NC group). (D, E) Flow cytometry assay results showing the apoptosis rate of NRVMs with or without NDRG4 over-expression after H/R. The percentage of apoptotic cells in H/R-treated cardiomyocytes without NDRG4 over-expression is 13.3% whereas the percentage of apoptotic cells in H/R-treated cardiomyocytes with NDRG4 over-expression is 31.9%. (n = 3 per group, *P < 0.05 vs. Con + Adv-NC group; # P < 0.05 vs. H/R + Adv-NC group). Data are presented as the mean ± SEM. (TIFF 6319 kb)
395_2015_519_MOESM3_ESM.tif
Figure S3 NDRG4 regulated myocardial I/R injury through inhibiting RISK pathway. (A) Representative immunoblot image of pAkt and pERK levels in NDRG4 knockdown NRVMs subjected to H/R with vehicle, LY294002 (10 μM) and PD98059 (10 μM)), respectively. (B) Quantitation of Akt activation results. (C) Quantitation of ERK activation results. (n = 5 per group, *P < 0.05 vs. shRNA-NC group; # P < 0.05 vs. LY294002 and PD98059 + shRNA-NC group). (D) Representative immunoblot image of pAkt and pERK levels in inhibitors treated NRVMs subjected to H/R with NDRG4 over-expression. (E) Quantitation of Akt activation results. (F) Quantitation of ERK activation results. (n = 5 per group, *P < 0.05 vs. Adv-NC group; # P < 0.05 vs. LY294002 and PD98059 + Adv-NC group). Data are presented as the mean ± SEM. (TIFF 7102 kb)
395_2015_519_MOESM4_ESM.tif
Figure S4 Determination of TNF-α level in vivo and in vitro. (A) TNF-α expression at various time points of reperfusion in the heart. (B) Myocardial I/R-stimulated serum TNF-α levels in vivo. Blood samples were collected at the end of reperfusion and detected by ELISA test. (n = 5 per group, *P < 0.05, compared to that observed in the sham group). (C) TNF-α expression increased in NRVMs subjected to H/R. NRVMs were subjected to hypoxia for 4 h and reoxygenation at three different time points (2, 4 and 6 h). Western blot analysis of TNF-α expression in lysates of NRVMs. (D) TNF-α level in the supernatant of H/R-treated cardiomyocytes. (n = 5 per group, *P < 0.05, compared to control group). Data are presented as the mean ± SEM. (TIFF 7240 kb)
395_2015_519_MOESM5_ESM.tif
Figure S5 TNF-α inhibitor pre-treatment inhibited NF-κB expression in both I/R heart and H/R NRVMs. (A) Representative immunoblot images showing the expression of NF-κB in I/R-injured hearts with pre-treatment of Et (100 μM). (B) Quantitation of NF-κB expression in the I/R experiments. (n = 5 per group, *P < 0.05, compared to sham group, # P < 0.05 vs. I/R 2 h group). (C) Representative immunoblot image of NF-κB in H/R-treated NRVMs with or without Et pre-treatment. (D) Quantitation of NF-κB expression in the H/R experiments. (n = 5 per group, *P < 0.05, compared to control group, # P < 0.05 vs. H/R 2 h group). (TIFF 6716 kb)
395_2015_519_MOESM6_ESM.tif
Figure S6 NF-κB inhibitor pre-treatment inhibited apoptosis in I/R heart and H/R NRVMs. (A, B) The number of TUNEL-positive cells decreased in I/R-injured hearts with PDTC pre-treatment. (n = 5 per group, *P < 0.05, compared to I/R group). Data are presented as the mean ± SEM. (Scare bar 50 μm) (C) Representative immunoblot images and quantitation of caspase 3 activation in I/R-injured hearts with pre-treatment of PDTC (100 μM). (n = 4 per group, *P < 0.05 vs. sham group; # P < 0.05 vs. I/R 2 h group). Data are presented as the mean ± SEM (D) Representative immunoblot images and quantitation of caspase 3 activation in. H/R injured NRVMs with pre-treatment of PDTC (100 μM). (n = 4 per group, *P < 0.05 vs. control group; # P < 0.05 vs. H/R 2 h group). (TIFF 6391 kb)
395_2015_519_MOESM7_ESM.tif
Figure S7 NF-κB inhibitor pre-treatment inhibited TNF-α-induced RISK activation in NRVMs with H/R treatment. (A) Representative immunoblot image of RISK activation in H/R-treated NRVMs with or without PDTC pre-treatment. (B) Quantitation of Akt activation. (C) Quantitation of ERK activation. (n = 4 per group, *P < 0.05 vs. Con + vehicle group; # P < 0.05 vs. I/R + vehicle group). (D) Representative immunoblot image showing RISK activation in TNF-α-treated NRVMs with or without PDTC pre-treatment. (E) Quantitation of Akt activation. (F) Quantitation of ERK activation. (n = 4 per group, *P < 0.05 vs. Con group; # P < 0.05 vs. TNF-α 2 h group). (TIFF 10595 kb)
Rights and permissions
About this article
Cite this article
Xing, Y., Tang, B., Zhu, C. et al. N-myc downstream-regulated gene 4, up-regulated by tumor necrosis factor-α and nuclear factor kappa B, aggravates cardiac ischemia/reperfusion injury by inhibiting reperfusion injury salvage kinase pathway. Basic Res Cardiol 111, 11 (2016). https://doi.org/10.1007/s00395-015-0519-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-015-0519-0