Abstract
Purpose
Whether youth who follow plant-based diets in Nordic countries meet their dietary needs for micronutrients remains unclear. This study aims to evaluate micronutrient intake and status in Norwegian youth following vegan, lacto-ovo-vegetarian, pescatarian, flexitarian and omnivore diets.
Methods
Cross-sectional design, with healthy 16-to-24-year-olds (n = 165). Participants were asked to complete a questionnaire and four 24-hour dietary recalls. Dried blood spots (DBS) and spot-urine samples were collected for analysis of methyl malonic acid (MMA) (n = 65), haemoglobin (Hb) (n = 164) and urinary iodine concentration (UIC) (n = 163).
Results
Vegans reported highest habitual supplement usage of multivitamin (58%), B12 (90%) and macroalgae consumption (32%), while flexitarians reported highest habitual usage of omega-3 supplements (56%). For daily supplement usage, vegans reported highest use of multivitamins (42%), B12 (79%), iodine (37%) and iron (63%). Increased risk of inadequate intake (energy-adjusted) were found for vitamin D (60% within lacto-ovo-vegetarians), selenium (70% within lacto-ovo-vegetarians, 65% within omnivores), and iodine (63% within vegans). Median MMA levels suggest low risk of insufficient B12 status across all groups (MMA 0.04‒0.37µmol/l) and 2% had MMA levels indicating possible B12 deficiency and 8% had elevated levels. Median Hb levels indicated low risk of anemia across all groups (≥ 12.0 females, ≥ 13.0 g/dl males), though 7% had Hb values indicating risk of mild anemia and 4% risk of moderate anemia. The median UIC indicates mild iodine deficiency in all groups (UIC < 100 µg/l), except vegans, who were moderately iodine deficient (UIC < 50 µg/l).
Conclusions
Our study indicated that the participating youth had low risk of inadequate intake of most micronutrients, partly due to high supplement usage. However, for iodine, vitamin D, and selenium higher risk of inadequate intake was found. UIC corroborated the low iodine intake among vegans. Thus, we suggest iodine status of youth in Norway should be monitored, especially among young fertile women who omits dietary iodine sources, until a mandatory iodine fortification program is implemented. Furthermore, we suggest that food education on how to secure sufficient nutrients from food in general should be provided to the Norwegian youth population, especially how to secure adequate intake of vitamin D, selenium and iodine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current evidence recommends limiting the consumption of processed and red meat for a reduced risk of noncommunicable diseases [1,2,3,4,5,6], and several reviews and studies have linked a diet with reduced meat consumption and vegetarian diets with a variety of health benefits [7,8,9,10]. By securing a well-planned plant-based diet containing a variety of fruits, vegetables, whole grains, legumes, nuts and seeds, the diet can be nutritionally adequate and promote health during all stages of the life course, although evidence of long-term effects is limited in critical stages of the life course, such as the youth period [11,12,13]. However, in addition to having a well-planned diet, supplementation of key nutrients is needed if following a vegan diet, if not obtaining these micronutrients through fortified foods. Particularly B12 is of concern for those who follow vegan diets, which is found mainly in foods such as meat, poultry, seafood, eggs, and dairy products [14], an essential vitamin that needs to be secured through the diet. Furthermore, the key nutrients that are of concern in plant-based diets are vitamin B12, vitamin D, calcium, zinc, selenium, iodine, vitamin A, riboflavin, and iron [15]. Although the nutrients of concern depend on the types of plant-based diets followed and the fortification strategy in that country. Thus, when omitting animal-source foods from the diet, it is necessary to substitute with food sources that are equivalent in most nutrients to avoid an increased risk of nutrient deficiencies over time. Ensuring adequate intake of the above mentioned key nutrients is also of utmost importance in the youth period due to increased nutritional requirements due to growth [16, 17].
Previously in Nordic countries, risk of inadequate intake of vitamin D, folate, calcium, and selenium have been reported in national dietary data among adults [18]. Furthermore, risk of inadequate iodine intake has also been documented in the general population across European countries [19], especially among fertile women, and is also widespread among the general population in Norway [20]. Thus, risk of inadequate intake of certain micronutrients are also present in the general population in Nordic countries. Currently, there is insufficient knowledge on micronutrient intake and status of youth who follows different plant-based diets in Nordic countries [11]. Thus, this study aims to evaluate the micronutrient intake and status of 16-to-24-year-olds in Norway following different plant-based diets compared to omnivores.
Methods
Study design and recruitment
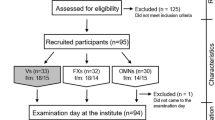
Between September 2021 and March 2022, 16-to-24-year-olds were recruited through convenience sampling and snowball sampling in the Agder area in southern Norway. Participants were recruited to participate in a cross-sectional project called VeggiSkills-Norway. The study eligibility criteria were: (1) able to read and understand Norwegian; (2) 16–24 years of age; (3) no acute or chronic illness; (4) currently not pregnant/lactating; (5) not having children; (6) having followed a vegan, lacto-ovo-vegetarian, pescatarian, flexitarian, or omnivore diet for a minimum of 6 months prior to participation, and no current plan to alter their dietary practice; (7) possibility of physical attendance at the University of Agder, Kristiansand, Norway.
After inclusion, participants completed an electronic questionnaire comprising 183 items (Supplemental Table 5) that evaluated the sociodemographic characteristics of the participants, health and lifestyle habits, sleeping habits and sedentary behavior and food literacy competencies. A dietary screener assessing consumption frequency of 33 selected food groups (“MyFoodMonth 1.1”) [21] was included in the questionnaire as well as questions concerning food habits in the previous six months (meal frequencies, supplements, dietary practice).
After filling out the electronic questionnaire onsite, participants were asked to complete the first out of four non-consecutive 24-hour dietary recalls (interview-administered). Subsequently, participants provided a non-fasted dried blood sample and a urinary sample for analysis of nutritional status biomarkers, and body composition measurements were also performed. After the study visit, participants completed the remaining three self-administered non-consecutive 24-hour dietary recalls. Participants were asked to report their intake of all foods, beverages, and supplements consumed the previous day in a Norwegian version of the web-based image-assistant dietary assessment tool, Measure Your Food on One Day (Myfood24) [22].
Sample size calculation
A priori, the sample size was determined based on a previously conducted study in young Swedish vegans and omnivores that detected group differences with 30 participants in each diet group [23]. Our final sample size was determined by the recruitment feasibility since the study took place during the COVID-19 pandemic.
Classification into dietary groups
Participants were classified into vegan, lacto-ovo-vegetarian, pescatarian, flexitarian and omnivore diet groups based on animal-sourced food groups included in their diet the previous six months, as previously described [24]. Out of the 165 participants, 19 were classified as vegans, with no inclusion of animal-source foods [1, 25], 20 participants were classified as lacto-ovo-vegetarians, including dairy / eggs to varying degree [1, 25] and 30 participants were classified as pescatarians, as they additionally reported eating seafood (regardless of the reported intake of dairy / eggs) [1]. Twenty-five participants were classified as flexitarians as they self-reported having a flexitarian diet in the electronic questionnaire and also reported consuming less than two servings per week of meat or poultry (any type) in the dietary screener [26]. Lastly, a total of 71 participants were classified as omnivores if they reported two or more servings of meat per week in the dietary screener.
24-hour dietary recalls
Participants were instructed to complete four 24-hour dietary recalls on random non-consecutive days, over a period of eight weeks using Myfood24 [22, 27]. Participants were asked to report their intake of all foods, beverages, and supplements consumed in the previous 24-hours based on the multiple pass method. The first 24-hour dietary recall was completed at the study site and carried out as an interview. To ensure 24-hour dietary recalls on weekends and weekdays, participants received an email with a link to complete recalls on different days. A maximum of two reminders were used to complete the dietary recalls, with one-week intervals. Participants who completed a minimum of one 24-hour dietary recall were included in the present study. In total, all the 165 participants completed day 1, 79% day 2, 60% day 3, and 44% day 4 of the 24-hour dietary recalls.
Myfood24 uses the Norwegian Food Composition Database [28] to calculate the energy and nutrient intake. If participants had consumed foods or beverages not listed in the database, they were instructed to choose an alternative but similar item (e.g., if consumed a plant-based substitute for yoghurt not present in the database, they were encouraged to choose another similar plant-based substitute available). It was also possible to report food items and amount consumed in an open text field at the end. All items reported in the open text option were processed manually. For recipes/foods not listed in the database (e.g., vegan mayonnaise), manual calculation was performed. In Norway, only plant-based substitutes to dairy products are allowed to be fortified, at present. If the participants reported consumption of plant-based substitutes to dairy products in the open text field, the researcher manually entered a plant-based substitute to dairy products available in the database with similar nutrient content. Furthermore, for foods reported with no similar food product available in the database, the product label was used to obtain a similar energy and macronutrient profile of the product. For example, if a participant reported consuming a vegan product mainly consisting of vegetable oil, water and sugar, the raw ingredients were entered to obtain the similar energy and macronutrient profile of the portion consumed.
Supplement use
Dietary supplements by 24-hour use was assessed using Myfood24 by collecting information on brand/type and amount/dose consumed the previous day in an open text question. Amount/dose of supplements reported in the 24-hour recalls are used when describing the total intake (including foods and supplements) in Tables 1, 2, 3, 5 and 4. The use of supplements reported in the 24-hour recalls is also reported as n (%) in Table 1.
Dietary supplements by habitual use the previous six months were assessed in the dietary screener (“MyFoodMonth 1.1”) [21], using a dichotomous variable, yes/no for the use of selected supplements including multivitamin, vitamin B12, iodine, omega-3 fatty acids, vitamin D, iron, folate, nutritional yeast and macroalgae. A string alternative ‘other supplements’ was also available. Habitual supplement use is presented in Table 1 as n (%).
Physical activity level
The physical activity level (PAL) of the participants was classified based on the reported frequency of moderate to vigorous-intensity activities in the previous six months using age specific PAL values [29, 30]. See Supplemental Table 5 for the items used to assess self-reported PAL. A low PAL value of 1.55 was assigned to participants who selected either of the options ‘never’ or ‘less than once a month’ or ‘once a week’ for the previous six-month period. Furthermore, a moderate PAL value of 1.7 was assigned if the option ‘two – three times a week’ was selected. A high and very high PAL value of 1.85-2.0 was assigned if options 4–5 times a week or ‘almost every day’ were selected.
Anthropometric measurements
Height measurement was carried out for all participants, barefoot, and standing to the nearest 0.1 cm using a portable stadiometer according to a measurement protocol (Sect. 7.2.2 in the protocol, page.72) [31]. Body weight was measured barefoot and in light clothing, in a non-fasted state, using a Tanita MC780 bioelectric impedance analyzer (Tokyo, Japan) and using the Tanita user manual. Body mass index (BMI) was calculated using height and weight (body weight (kg) /m2).
Blood sampling and analysis of methyl malonic acid (MMA) and haemoglobin (hb)
Whole blood was collected in non-fasted state by dried blood sampling (DBS), a biosampling method where capillary blood was obtained by a fingerprick using a disposable lancet. Complete DBS cards were allowed to dry overnight (maximum 12 h) in a room without light exposure, before being stored in a sealed aluminium bag with a desiccant pack at -20 degrees Celsius (°C) until analysis. Methylmalonic acid (MMA) was analyzed from the whole blood (VITAS™ Analytical Services, Norway). A sufficient volume of blood for analysis of MMA was available for 65 participants. MMA was extracted from 8 mm DBS punches using an isotope dilution GC-MS assay. Four punches were eluted in an aqueous alkaline solution containing stable isotope-labelled MMA prior to derivatization using propyl chloroformate. The liquid extraction of derivatized MMA was performed before injection into the GC-MS system. The GC-MS system utilized was an Agilent 6890 GC (Agilent Technologies, Palo Alto, CA, USA) equipped with an Agilent 5973 N mass-selective detector operated in EI-SIM mode. Separation was performed on a Zebron ZB-AAA GC column supplied by Phenomenex Inc (Torrance, CA, USA) and quantification was performed against a 5-point standard curve prepared from standards with known concentrations.
Haemoglobin (Hb) was measured from the same finger prick as the whole blood for DBS, using Hemocue® HB801 Analyzer and Microcuvettes (Sweden). In total 164 participants provided blood for analysis of Hb.
Collection of urinary samples and analysis of urinary iodine concentration (UIC)
Participants provided a non-fasted spot urine sample collected at random times throughout the day, using a 100 ml Vacuette® urine breaker (Greiner Bio-One, Kremsmünster, Austria). Thereafter, a subsample of urine was withdrawn from the urine breaker into a 9.5 ml Vacuette® urine tube (Greiner Bio-One, Kremsmünster, Austria). The urine samples were stored at -20 degrees Celsius (℃) until analysis at the Hormone Laboratory, Oslo University Hospital, Norway. In total, 163 participants provided enough urine volume to perform UIC analysis.
After thawing of the urine samples, 250 µl of each urine sample was diluted into 1 ml of ammonium persulfate for oxidation of interference substances. The UIC was determined using the Sandel-Kolthoff reaction method (colorimetric) based on the catalytic effect of iodine on the red/ox reaction between arsenic and cerium, after digestion of the samples. Samples were analyzed using microtiter plates (Delfia Microtitration Plate from Wallace Oy, Denmark) using 405 nm for Victor3 (Perkin Elmer, Finland). Method blank samples (H2O) were used, using reference material, KIO3, Sigma-Aldrich prepared in the same manner as the respective samples. The lower limit of detection was 0.01 µmol/l, and the lower limit of quantification was 0.2 µmol/l.
Evaluation of biomarkers
To indicate risk of insufficient B12 status, the functional marker MMA (µmol/l) was assessed. The reference interval for adequate MMA levels at 0.04‒0.37 µmol/l was used, for elevated MMA levels the cut-off at > 0.27 µmol/l was used and for risk of B12 deficiency the cut-off at > 0.37 µmol/l was used [32, 33].
To assess risk of anemia, WHO diagnostic criteria using Hb concentration was used [34]. Low risk of anemia was defined as Hb levels ≥ 12.0 g/dl for the women and ≥ 13.0 g/dl for men. Risk of mild anemia as Hb levels in the range 11.0–11.9 g/dl for women and 11.0–12.9 g/dl for men. Risk of moderate anemia as Hb levels in the range 8.0–10.9 g/dl and risk of severe anemia as Hb levels lower than 8.0 g/dl for both women and men.
To evaluate iodine status, WHO epidemiological criteria was used [35]. The iodine status was evaluated based on the median UIC, and the median UIC < 20 µg/l was considered as risk of severe iodine deficiency, median UIC at 20–49 µg/l as risk of moderate iodine deficiency, median UIC at 50–99 µg/l as risk of mild iodine deficiency, median UIC at 100–199 µg/l as adequate iodine status, median UIC at 200–299 µg/l as above the iodine requirements and median UIC ≥ 300 µg/l as risk of excessive iodine intake.
Statistical analysis
For data analysis the software used was IBM SPSS statistics version 29 (IBM Corp., Armonk, NY, United States) and Excel® (Microsoft 365). The calculation of the micronutrient intake of each supplement was performed manually in Excel®.
The data distribution was evaluated using tests for normality, and in addition visual inspection of the histogram and Q-Q plots were checked. Residual plots were also examined. To test for differences between groups, the ANOVA test (parametric), the Mann-Whitney U test (nonparametric, two groups), and the Kruskal-Wallis one-way ANOVA test (nonparametric, more than two groups) with correction for multiple comparisons were used. For categorical variables, cross-tabulation using the Pearson Chi-square or Fisher exact test was used to test for differences between groups. The adjusted p-value < 0.05 (two-sided) was used for the significance level for all tests.
Energy adjustment of micronutrient intake
Energy adjustment was carried out according to the principle of nutrient density [36] and individual energy requirements:
Subsequently, the micronutrient intake was adjusted for energy according to individual estimated basal metabolic rate (BMR) and individual estimated PAL value [37]. The BMR in MJ per day was estimated using Henry’s [38]. For the calculation of the total energy requirement, the estimated BMR was multiplied by individual PAL values for a low, moderate, and high activity level (see method section, physical activity).
We compared energy-adjusted intakes for each individual with the average requirements (AR) for females and males in the age group 16–17 years and 18–24 years with values in the Nordic Nutrition Recommendations 2023 (NNR2023) [39].
Multivariable adjustments
Quantile regression was performed to adjust the 25th, 50th and 75th percentiles of the micronutrient intake for potential influencing confounding factors [40]. The dietary groups were included as fixed factors using dummy variables. Further, age and gender were identified as potentially influencing confounding factors as these variables varied significantly between groups. Therefore, age and gender were included in the quantile regression model as covariates. Adjusted 95% confidence intervals (95%CI) for the medians were obtained from the quantile regression.
A previous publication found significant differences between diet groups in the general nutrition knowledge (GNKQ) score [24]. Since this variable also varied significantly between groups it was of relevance to try to adjust for it. However, due to the presence of groups with fewer than 30 participants within the plant-based diet groups it was not possible to adjust for the GNKQ score variable in a multivariable adjustment. Hence, we opted to adjust for age and gender. As we could not adjust for GNKQ as a covariate in the multivariable-adjustment model, a sensitivity analysis was carried out evaluating whether energy-adjusted total micronutrient intake (including food and supplements) varied according to poor, moderate and high GNKQ level (Table 4). Participants with a total sum score with less than 60% correct answers were considered poor, participants with 60–79% correct answers were considered moderate, and participants with 80–100% correct answers were considered high. Categorization into the different levels of GNKQ and calculation of the GNKQ score has previously been described elsewhere [24].
Results
Characteristics of study participants
The characteristics of the participants are presented in Table 1. The mean ± SD age of the participants was 21 ± 2 years. Omnivores had the lowest percentage of women versus the highest percentage among lacto-ovo-vegetarians (P = 0.005).
In total, 72% of all participants reported habitually consuming any type of supplement in the previous six months, in which pescatarians reported the lowest percentage (60%) and vegans the highest (95%) (P = 0.003). For habitual supplement usage within the dietary groups, vegans reported the highest habitual supplement use of multivitamins (58%) and of B12 (90%) and lowest usage was reported among omnivores (23% multivitamin and 9% B12, respectively) (P = 0.030 and P < 0.001). Furthermore, the highest habitual consumption of macroalgae (32%) was reported among vegans, compared to zero consumption in the other diet groups. Habitual usage of omega-3 supplements also varied between the groups, with the highest consumption among flexitarians (56%) and lowest among vegans (21%).
For supplement usage reported in the 24-hour dietary recalls, 58% of all participants reported consuming any type of dietary supplement. Reported 24-hour supplement use of multivitamin, iodine, B12 and iron varied between groups, in which vegans reported the highest usage of all the respective supplements (multivitamin 42%, iodine 37%, B12 79%, iron 63%) and lowest usage among omnivores (< 15% for each supplement).
Reported energy intake
Differences in reported energy intake was found between lacto-ovo-vegetarians (6 MJ/day) compared to omnivores (7 MJ/day, P = 0.011), and between lacto-ovo-vegetarians compared to pescatarians (8 MJ/day, P = 0.008) (Table 1).
Micronutrient intake
The energy-adjusted micronutrient intake solely from foods and the total intake from both foods and supplements are presented in Tables 2 and 3. For absolute micronutrient intake (not energy-adjusted) solely from foods and total absolute intake (not energy-adjusted) from both foods and supplements are presented in Supplemental Tables 1 and 2.
Multivariable adjustment of micronutrient intake
Multivariable adjustment (age and gender) of the energy-adjusted micronutrient intake is presented in Tables 2 and 3. Multivariable adjustment (age and gender) did not significantly affect the median intake of the energy-adjusted micronutrients solely from foods or total intake (including foods and supplements). Furthermore, after performing the multivariable adjustment, the 95%CI were found to be overlapping for nearly all the medians across the diet groups, except for total intake of iron (including foods and supplements), in which vegans still differed from the other diet groups.
Energy-adjusted total micronutrient intake (including foods and supplements) according to poor, moderate and high GNKQ level is presented in Table 4. Total energy-adjusted intakes (including foods and supplements) of vitamin A (P = 0.005), folate (P = 0.013), vitamin D (P = 0.006), vitamin C (P = 0.021) and iron (0.003) differed between the GNKQ levels, in which higher median intakes were found among those with high GNKQ level compared to those of poor GNKQ level.
Proportion of individuals below average requirements (AR)
The proportion of individuals with total energy-adjusted micronutrient intake (including foods and supplements) below AR is presented in Table 5. The proportions of individuals below AR using absolute micronutrient intake solely from foods and total intake (including foods and supplements) (not energy-adjusted) is presented in Supplemental Table 3.
Risk of inadequate micronutrient intake (including foods and supplements) were found for vitamin D (60% within lacto-ovo-vegetarians < AR), selenium (70% within lacto-ovo-vegetarians and 65% within omnivores < AR), and iodine (63% within vegans < AR) after energy-adjustment. Between the dietary groups, a significant difference in the percentage below AR was found for total intake of vitamin B12 (35% within lacto-ovo-vegetarians vs. 4% within flexitarians, P = 0.004) and for total intake of vitamin C (35% within omnivores vs. 5% within vegans, P = 0.031) after energy-adjustment.
The proportion of individuals with total energy-adjusted micronutrient intake (including foods and supplements) below AR according to GNKQ level is presented in Supplemental Table 4. Between the different GNKQ levels, energy-adjusted total micronutrient intakes (including foods and supplements) of vitamin A (P < 0.001), vitamin D (P = 0.012), vitamin C (P = 0.021) and zinc (P = 0.006) varied significantly, with highest proportion below AR among those with poor GNKQ level compared those with high GNKQ level. Risk of inadequate micronutrient intake based on GNKQ level was found for vitamin D in those with poor GNKQ level (73% < AR), and for selenium, for all GNKQ levels (64% within poor level, 57% within moderate level, 55% within high level).
Biomarkers of nutritional status
Methylmalonic acid (MMA) as a functional marker of B12 status
For the total group (n = 65), the median (25th, 75th ) level of MMA was 0.19 µmol/l (1.16, 0.23), and all the dietary groups had median MMA levels indicating lower risk of B12 deficiency, with no difference between the groups (Table 6). Indication of elevated MMA levels (> 0.27 µmol/l) were found in 8% of the participants, with no difference between the groups. Increased risk of B12 deficiency (MMA > 0.37 µmol/l) was found in one participant who adhered to a pescatarian diet.
Haemoglobin (hb) as a marker of anemia
The median Hb (n = 164) in the different dietary groups indicates low risk of anemia (Table 6). At the group level, males had higher median Hb levels (25th, 75th ) than females, 15 g/dl (15,16) vs. 14 g/dl (13,15) (P < 0.001), both indicating low risk of anemia. Lacto-ovo-vegetarians had the lowest median Hb (25th, 75th ) at 13 g/dl (12, 14) and omnivores the highest Hb level at 15 g/dl (13,15) (P = 0.007). None of the participants had Hb values that indicated risk of severe anemia (< 8 g/dl), 4% had Hb values that indicated risk of moderate anemia (< 11 g/dl) and 7% had Hb values that indicated risk of possible mild anemia (< 12 g/dl).
Urinary iodine concentration (UIC) as a marker of iodine status
For the total group (n = 163), the median UIC (25th, 75th ) was 75 µg/l (44, 114), indicating that 70% had risk of mild iodine deficiency (median UIC < 100 µg/l) and 31% had risk of moderate iodine deficiency (median UIC < 50 µg/l) Table 6. Vegans had the lowest median UIC at 20 µg/l (16, 53), compared to all other diet groups, flexitarians at 71 µg/l (39, 152) (P < 0.001), omnivores at 77 µg/l (56, 116) (P < 0.001), lacto-ovo-vegetarians at 82 µg/l (41, 110) (P = 0.018) and pescatarians at 83 µg/l (40, 133) (P = 0.001).
Discussion
In this study, micronutrient intake and status were evaluated in healthy 16-to-24-year-olds following vegan, lacto-ovo-vegetarian, pescatarian, flexitarian, and omnivore diets (n = 165). Most participants reported using a dietary supplement habitually (72%) the previous six months. The micronutrient intakes that persisted below AR when including foods and supplements (energy-adjusted) were vitamin D (lacto-ovo-vegetarians), selenium (lacto-ovo-vegetarians and omnivores), and iodine (vegans), indicating risk of inadequate intake of some micronutrients among youth with plant-based or omnivorous diets. The findings from the dietary intake data (including food and supplements), showed that intakes of B12 and iron were above AR for all groups, indicating low risk of inadequate intakes. This was supported by objective blood markers of MMA and Hb, which also indicated low risk of B12 deficiency and low risk of anemia across all dietary groups. The UIC indicated that risk of mild iodine deficiency was prevalent in all dietary groups indicated by a median UIC < 100 µg/l, except vegans who were found to be at risk of being moderately deficient in iodine, indicated by a median UIC < 50 µg/l.
Micronutrient adequacy of plant-based and omnivorous diets among youth
In our study, vegans did not meet AR solely through foods (energy-adjusted) for vitamin A, vitamin B12, vitamin D, selenium, and iodine, indicating an increased risk of inadequate intake of these nutrients without supplementation. Furthermore, none of the other diet groups had median energy-adjusted intakes solely through foods that met AR for vitamin D and selenium. Moreover, solely through foods, the lacto-ovo-vegetarians and flexitarians did not obtain AR for iodine.
From total intake including food and supplements (energy-adjusted), both youth with plant-based and omnivorous diets faced nutritional challenges in meeting AR, particularly selenium, vitamin D and iodine. The proportions of participants with total intake including food and supplements (energy-adjusted) below AR for vitamin D was 46%, with highest proportion among the lacto-ovo-vegetarians (60% < AR). Furthermore, 57% of the individuals had intakes below AR for selenium (70% within lacto-ovo-vegetarians and 65% within omnivores < AR). For iodine, 42% of the individuals had intakes below AR (63% within vegans < AR). Therefore, the risk of inadequate intake of these nutrients were present in these subgroups in our study. However, it needs to be notated that the AR values for selenium was revised and increased in the NNR2023, which might impact the percentage categorized with inadequate intake [39]. These findings highlight the importance of providing sufficient food education to youth on how to meet their dietary needs through foods. Also, the importance of consuming fortified foods or supplementing for certain micronutrients when following plant-based diets to compensate for the lower presence of some micronutrients in the diet. Our findings are consistent with previous research [11], including previous findings in young Swedish vegans [23]. Furthermore, our findings are also partly in coherence with a newly published study among German children and adolescents, in which the study found differences in biomarkers between the dietary groups who followed vegan, vegetarian and omnivore diets, but the study indicated no specific nutritional risk between the diet groups [41].
Since none of the dietary groups in this present study was able to obtain AR for vitamin D without supplementation, and only 22% of the young participants reported habitual consumption of vitamin D supplements, there is a risk of insufficient vitamin D status over time. Especially during the winter season without exposure to the sun in Norway, which necessitates the importance of adequate dietary intake of vitamin D. Our findings of inadequate vitamin D intake are consistent with national surveys among Norwegian young adults aged 18 to 29 years conducted a decade ago [42].
B12 intake and marker of status
In our study, the participating vegans did not obtain an adequate B12 intake solely through foods (energy-adjusted) which has also been shown in previous research [43]. Although in Norway most plant-based dairy substitutes are fortified with B12. However, total median B12 intake (energy-adjusted) including foods and supplements was well above AR in all dietary groups. The MMA level measured in DBS in our study also corroborated the lower risk of inadequate B12 intake, as 2% had possibly B12 deficiency with no significant difference between groups. Furthermore, 8% were found to have elevated MMA levels, but no significant differences between groups. Although MMA is the most sensitive single marker of B12, it is recommended to evaluate several markers of B12 for evaluating status [44]. Our finding of B12 intake above AR in the young vegans in our study, is explained by high usage of B12 supplements (90% habitually and 79% by 24-hour usage), which supports previous finding of the importance of supplementing with B12 in addition to using fortified foods when following plant-based diets, especially vegan diets [43, 45]. A concern is still expressed for young people following plant-based diets, especially young vegans who are not sufficiently educated on supplement usage or how to obtain nutrients from plant-based foods and fortified foods. Youth who are not sufficiently educated can be particularly vulnerable to inadequate intake of certain essential micronutrients as B12.
Our findings of adequate B12 intake are in coherence with previous studies, among young Germans who adhered to different plant-based diets and among Norwegian adults who adhered to vegan, vegetarian, and pescatarian diets [41, 45]. However, a previous review (including 40 studies with infants, children, adolescents, pregnant women, adults and elderly from several countries) has shown that vegans who did not supplement with vitamin B12 were at higher risk of B12 deficiency compared to vegetarians, based on serum B12 [43]. Due to our sample size, we were not able to perform analysis comparing B12 supplement users and non-supplement users within the dietary groups.
Iron intake and marker of status
In the present study, the energy-adjusted iron intake solely from foods and total iron intake (including foods and supplements) indicated low risk of inadequate intake. However, the findings did not account for the bioavailability of iron from plant sources, and a recently published study demonstrated that the uptake of iron and zinc from some plant-based meat substitutes could be poorer than anticipated [46]. On the other hand, higher intakes of vitamin C and organic acids in fruits and vegetables are known to improve iron absorption and could mitigate lower iron absorption from plant foods, if consumed together [47]. In our study, 7% had Hb values indicating mild anemia and 4% moderate anemia, although none of the dietary groups was found to differ in risk of anemia, which is consistent with previous studies investigating iron status in those following plant-based diets and omnivores [11, 41, 48]. On the contrary, a previous literature review found a higher prevalence of iron deficiency among adult vegans compared to vegetarians (from 10 different countries), in which 8 of 13 included studies used Hb as a marker of iron status. However, it should be noted that the included studies used different iron deficiency cutoff criteria [48].
Iodine intake and marker of status
The findings of median iodine intake including foods and supplements (energy-adjusted) below AR among the young vegans and lacto-ovo-vegetarians corresponds with the concentration of iodine measured in the urine, with UIC < 50 µg/l in the vegans and < 100 µg/l in the lacto-ovo-vegetarians. Our findings of UIC indicating mild to-moderate iodine deficiency are in line with previous findings in adult vegans and lacto-ovo-vegetarians in Norway [49, 50]. Furthermore, our findings are also in agreement with a newly published review reporting that vegans and vegetarians who did not consume iodine-containing supplements were at increased risk of having inadequate iodine intake [51], therefore also at increased risk of iodine deficiency. Furthermore, our findings that the pescatarians, flexitarians and omnivores, had a median UIC < 100 µg/l indicated inadequate intakes despite consumption of iodine-rich animal source foods, supports previous findings of mild iodine deficiency in young women in the general population of Norway [52]. Norway is still one of the countries that has voluntary salt iodization, which has been implemented in many countries to combat iodine deficiency globally [53]. Thus, the main sources of dietary iodine in Norway are lean fish, cow’s milk, dairy yoghurt, and eggs. Therefore, people who omit these food groups may be at particular risk of inadequate iodine intake. In the past decade, several population groups (women of fertile age, pregnant, breastfeeding women, infants who are exclusively breastfed, adult vegans, and immigrants) in Norway have been repeatedly found to have intakes below recommendations, showing that inadequate iodine intake is widespread [49, 52, 54]. For young women of reproductive age who exclude all animal dietary sources of iodine, it is particularly important that they obtain iodine from supplements or foods fortified with iodine [55]. If a pregnancy starts with low iodine stores, it can have consequences for the thyroid function in mothers, as well as neurodevelopment in their children [56].
Multivariable adjustment
Multivariable adjustment of the energy-adjusted micronutrient intakes using gender and age as covariates was performed in our study, in which minor changes were observed. Thus gender and age did not seem to significantly influence the reported micronutrient intake in our study sample. Although the findings from the multivariable adjustment needs to be interpreted with caution as all the 95%CI were overlapping between the groups, except for total intake of iron. Increased uncertainties in the results are probably caused by fewer than 30 participants in the plant-based diet groups. Furthermore, we did observe that the participants with higher level of GNKQ had significantly higher intakes of vitamin A, folate, vitamin D, vitamin C and iron compared to those with poor GNKQ level. This finding also emphasizes the importance of securing food education to youth for them to have a well-planned diet that meets their dietary needs. Furthermore, this finding indicates that future studies assessing dietary intake among youth following plant-based diets compared to omnivores should account for other factors that may impact micronutrient adequacy, aside from their dietary pattern.
Energy intake and misreporting
In our study, all dietary groups reported an energy intake below the lower energy requirements for females and males aged 18–24 years [39]. Thus, in this study, considerable underreporting is presumed, resulting in an underestimation of the micronutrient intake. Under-reporting is a well-known issue with self-reporting in dietary assessments [57]. In response to this, energy adjustments were performed for all micronutrients based on the nutrient density principle and according to individual energy requirements using the Henry equation to estimate the BMR and by using individual PAL values.
Intake estimates from 24-hour dietary recalls contain random within person error, particularly due to the day-to-day variation in the dietary intake. Thus, we need to acknowledge that there is a risk that participants might be wrongly categorized as having inadequate or adequate intakes when comparing individuals to AR. However, in this study, up to four non-consecutive 24-hour dietary recalls were evaluated, which can partially mitigate the concern about day-to-day variation. In addition, the 24-hour recalls were employed on both weekdays and weekends. We also assessed supplement use in both 24-hour dietary recalls and as habitual supplement use in the previous six months, strengthening the intake data on supplement use.
Strengths and limitations
Our study has strengths and limitations. To our knowledge, this is the first study to evaluate micronutrient intake and status of youth who follows different plant-based diets compared to omnivores in Norway. Therefore, providing up-to-date data in this population group are warranted. A major strength was the use of blood and urine biomarkers as objective markers, in addition to self-reported dietary data. However, it needs to be noted that MMA was measured using DBS and the Hb in capillary blood, which might be less precise compared to when measured in venous blood. Though, when avoiding venipuncture, we also reduce the participation burden. A previous validation study have shown that MMA measured using DBS can be used as a robust measurement with high precision and accuracy [58]. Another strength was the use of a previously validated tool for dietary assessment (Myfood24) that uses image-assisted portion sizes for a more accurate estimate of portion sizes [59]. However, a limitation of the Myfood24 database was the limited availability of vegan/vegetarian food products, which could potentially be one of the explanations for the low reported energy intake. However, an open text field was available, which allowed reporting of food items that were not present in the database.
A limitation of the study is the small sample size in the different diet groups; as a result, it was not possible to perform statistical analysis split by sex, age group, or supplement users within the different diet groups. In addition, we were not able to perform statistical analysis evaluating intrapersonal variability, due to the fit of the data. However, when using repeated 24-hour recalls these data can be used to evaluate the proportion at risk of inadequate intake, although there is a risk of wrongly categorizing participants with higher proportion of inadequate or adequate intake [60]. Due to the sample size, there is a risk of type II error, and therefore our results need to be interpreted with this kept in mind. Another limitation is the recruitment method used, which was based on convenience sampling, thus a risk is recruitment of a non-representative sample, which would impact the generalizability of our study findings. We also need to acknowledge that the PAL values used in the energy adjustment of the nutrient intakes is based on self-reported physical activity data, which can inaccurately result in higher or lower individual energy requirements, which needs to be taken into consideration when interpreting the data. Another limitation was the use of six months as diet duration as inclusion criteria, as it might be insufficient diet duration for a deficiency of anemia and B12 deficiency to manifest. Although our study will provide an insight into the current situation in a youth population, which is lacking. Furthermore, this study took place during the COVID-19 pandemic, which might have influenced recruitment and possibly their dietary intake. However, a report by the Norwegian Institute of Public Health [61], found that Norwegian adolescents did not change their diet to a considerable extent prior to, during, or after the COVID-19 pandemic, except those already consuming a higher sugar intake than recommended, as they reported an additional increase in sugary foods and sugary beverages.
Conclusion
Our findings show from the total intake including food and supplements (energy-adjusted) that both youth with plant-based and omnivorous diets faced nutritional challenges in meeting AR. Particularly for selenium, vitamin D and iodine. UIC supported increased risk of inadequate iodine intake, with mild iodine deficiency across all diet groups and moderate iodine deficiency in vegans. Due to the small sample size in our study, there is a risk of type II error, thus, our findings should be replicated in a larger sample examining dietary intake and status of youth, both those who follow plant-based and omnivore diets. Until then, we recommend that the iodine status of youth, especially young fertile women who omit all sources of dietary iodine, should be closely monitored until a mandatory salt iodization program is implemented in Norway. We also recommend youth who excludes most dietary iodine sources to obtain iodine from supplements or foods fortified with iodine as currently recommended from the Norwegian health authorities. Furthermore, we recommend that food education on how to secure sufficient nutrients from food in general and particularly adequate intake of vitamin D, selenium and iodine should be provided to the youth population in Norway.
References
World Health Organization (2021) Plant-based diets and their impact on health, sustainability and the environment: a review of the evidence. WHO European Office for the Prevention and Control of Noncommunicable Diseases
Willett W, Rockström J, Loken B et al (2019) Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 393:447–492
Oussalah A, Levy J, Berthezène C et al (2020) Health outcomes associated with vegetarian diets: an umbrella review of systematic reviews and meta-analyses. Clin Nutr 39:3283–3307
Papier K, Fensom GK, Knuppel A et al (2021) Meat consumption and risk of 25 common conditions: outcome-wide analyses in 475,000 men and women in the UK Biobank study. BMC Med 19:53
Knuppel A, Papier K, Fensom GK et al (2020) Meat intake and cancer risk: prospective analyses in UK Biobank. Int J Epidemiol 49:1540–1552
Dinu M, Abbate R, Gensini GF et al (2017) Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr 57:3640–3649
Crowe FL, Appleby PN, Travis RC et al (2013) Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC-Oxford cohort study. Am J Clin Nutr 97:597–603
Benatar JR, Stewart RAH (2018) Cardiometabolic risk factors in vegans; a meta-analysis of observational studies. PLoS ONE 13:e0209086
Dybvik JS, Svendsen M, Aune D (2023) Vegetarian and vegan diets and the risk of cardiovascular disease, ischemic heart disease and stroke: a systematic review and meta-analysis of prospective cohort studies. Eur J Nutr 62:51–69
Key TJ, Appleby PN, Crowe FL et al (2014) Cancer in British vegetarians: updated analyses of 4998 incident cancers in a cohort of 32,491 meat eaters, 8612 fish eaters, 18,298 vegetarians, and 2246 vegans. Am J Clin Nutr 100(Suppl 1):378s–385s
Bakaloudi DR, Halloran A, Rippin HL et al (2021) Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin Nutr 40:3503–3521
Global Burden of Disease Study (GBD) (2019) Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet 393:1958–1972
Sutter DO, Bender N (2021) Nutrient status and growth in vegan children. Nutr Res 91:13–25
Allen LH, Miller JW, de Groot L et al (2018) Biomarkers of Nutrition for Development (BOND): vitamin B-12 review. J Nutr 148, 1995S-2027S.
Neufingerl N, Eilander A (2021) Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 14
Rudloff S, Bührer C, Jochum F et al (2019) Vegetarian diets in childhood and adolescence. Mol Cell Pediatr 6:4
Schürmann S, Kersting M, Alexy U (2017) Vegetarian diets in children: a systematic review. Eur J Nutr 56:1797–1817
Lemming EW, Pitsi T (2022) The Nordic Nutrition recommendations 2022 - food consumption and nutrient intake in the adult population of the nordic and baltic countries. Food Nutr Res 66
Kayes L, Mullan KR, Woodside JV (2022) A review of current knowledge about the importance of iodine among women of child-bearing age and healthcare professionals. J Nutritional Sci 11:e56
Bath SC, Verkaik-Kloosterman J, Sabatier M et al (2022) A systematic review of iodine intake in children, adults, and pregnant women in Europe-comparison against dietary recommendations and evaluation of dietary iodine sources. Nutr Rev 80:2154–2177
Salvesen L, Wills AK, Øverby NC et al (2023) Relative validity of a non-quantitative 33-item dietary screener with a semi-quantitative food frequency questionnaire among young adults. J Nutritional Sci 12:e72
Carter MC, Albar SA, Morris MA et al (2015) Development of a UK Online 24-h Dietary Assessment Tool: myfood24. Nutrients 7, 4016–4032
Larsson CL, Johansson GK (2002) Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am J Clin Nutr 76:100–106
Groufh-Jacobsen S, Larsson C, Van Daele W et al (2023) Food literacy and diet quality in young vegans, lacto-ovo vegetarians, pescatarians, flexitarians and omnivores. Public Health Nutr, 1–26
Melina V, Craig W, Levin S (2016) Position of the Academy of Nutrition and Dietetics: vegetarian diets. J Acad Nutr Diet 116:1970–1980
Moreno LA, Meyer R, Donovan SM et al (2021) Perspective: striking a balance between Planetary and Human Health—Is there a path Forward? Adv Nutr 13:355–375
Salvesen L, Engeset D, Øverby NC et al (2021) Development and evaluation of image-series for portion size estimation in dietary assessment among adults. J Nutr Sci 10:e3
The Norwegian Health Authority (2022) The Norwegian food composition table, 2022
Black AE (2000) Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord 24:1119–1130
EFSA Panel on Dietetic Products N & Allergies (2013) Scientific opinion on dietary reference values for energy. EFSA J 11:3005
Viet L, Verschuren M (2008) Measurement protocols. The Feasibility of a European Health Examination Survey (FEHES) Recommendations.
Green R, Allen LH, Bjørke-Monsen A-L et al (2017) Vitamin B12 deficiency. Nat Reviews Disease Primers 3:17040
Fedosov SN (2010) Metabolic signs of vitamin B12 deficiency in humans: computational model and its implications for diagnostics. Metabolism 59:1124–1138
World Health Organization (2011) Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization
World Health Organization (2007) Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers, 3rd edn. World Health Organization, ed. Geneva
Dietary Assessment Primer Energy Adjustment. National Institutes of Health, National Cancer Institute. (https://dietassessmentprimer.cancer.gov/) [accessed 17.10.23]
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65:1220S–1228S
Henry CJK (2005) Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 8:1133–1152
Nutrition council of Ministers (2023) Nordic Nutrition Recommendations 2023 Integrating Environmental Aspects no. 2023:003
McGreevy KM, Lipsitz SR, Linder JA et al (2009) Using median regression to obtain adjusted estimates of Central Tendency for Skewed Laboratory and Epidemiologic Data. Clin Chem 55:165–169
Alexy U, Fischer M, Weder S et al (2021) Nutrient Intake and Status of German Children and Adolescents Consuming Vegetarian, Vegan or Omnivore Diets: Results of the VeChi Youth Study. Nutrients 13
Totland T, Melnæs B, Lundberg-Hallén N et al (2012) Norkost 3; En landsomfattende kostholdsundersøkelse blant menn og kvinner i Norge i alderen 18–70 år, 2010–11 (Norkost 3; a nationwide food consumption survey among men and women in Norway aged 18–70 years. Helsedirektoratet, Oslo, pp 2010–2011
Pawlak R, Lester SE, Babatunde T (2014) The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: a review of literature. Eur J Clin Nutr 68:541–548
Fedosov SN, Brito A, Miller JW et al (2015) Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points. 53:1215–1225
Henjum S, Groufh-Jacobsen S, Lindsay A et al (2023) Adequate vitamin B12 and folate status of Norwegian vegans and vegetarians. Br J Nutr 129:2076–2083
Mayer Labba I-C, Steinhausen H, Almius L et al (2022) Nutritional Composition and Estimated Iron and Zinc Bioavailability of Meat Substitutes Available on the Swedish Market. Nutrients 14, 3903
Siegenberg D, Baynes RD, Bothwell TH et al (1991) Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am J Clin Nutr 53:537–541
Pawlak R, Berger J, Hines I (2018) Iron Status of vegetarian adults: a review of literature. Am J Lifestyle Med 12:486–498
Groufh-Jacobsen S, Hess SY, Aakre I et al (2020) Vegans, Vegetarians and Pescatarians Are at Risk of Iodine Deficiency in Norway. Nutrients 12
Brantsæter AL, Knutsen HK, Johansen NC et al (2018) Inadequate Iodine Intake in Population Groups Defined by Age, Life Stage and Vegetarian Dietary Practice in a Norwegian Convenience Sample. Nutrients 10, 230
Eveleigh ER, Coneyworth L, Welham SJM (2023) Systematic review and meta-analysis of iodine nutrition in modern vegan and vegetarian diets. Br J Nutr, 1–15
Henjum S, Abel MH, Meltzer HM et al (2019) [Is iodine intake adequate in Norway?]. Tidsskr nor Laegeforen 139
Zimmermann MB, Andersson M (2021) GLOBAL ENDOCRINOLOGY: global perspectives in endocrinology: coverage of iodized salt programs and iodine status in 2020. Eur J Endocrinol 185:R13–r21
Medin AC, Carlsen MH, Andersen LF (2020) Iodine intake among children and adolescents in Norway: estimates from the national dietary survey Ungkost 3 (2015–2016). J Trace Elem Med Biol 58:126427
Nicol K, Nugent AP, Woodside JV et al (2024) Iodine and plant-based diets: a narrative review and calculation of iodine content. Br J Nutr 131:265–275
Zimmermann MB (2009) Iodine Deficiency. Endocr Rev 30:376–408
National Institutes of Health NCI Dietary Assesment Primer Describing Dietary Intake. https://dietassessmentprimer.cancer.gov/ (accessed 19.02 2024)
Schroder TH, Quay TAW, Lamers Y (2014) Methylmalonic acid quantified in dried blood spots provides a precise, valid, and stable measure of functional vitamin B-12 status in healthy women. J Nutr 144:1658–1663
Albar SA, Alwan NA, Evans CEL et al (2016) Agreement between an online dietary assessment tool (myfood24) and an interviewer-administered 24-h dietary recall in British adolescents aged 11–18 years. Br J Nutr 115:1678–1686
Willett W (2017) Nutritional epidemiology, 3rd ed. Oxford
Brantsæter ALPT, Biele G, Torheim LE, Abel MH (2023) Have restrictive measures throughout the pandemic had consequences for young people’s diet and eating habits? https://www.fhi.no/publ/2023/har-restriktive-tiltak-gjennom-pandemien-hatt-konsekvenser-for-unges-kostho/
Acknowledgements
The authors thank the bachelor students Stine Rambekk Henriksen and Silje Slettemoen, and the master students Marte Haugen and Lale Marie Aasland for being involved in the recruitment and data collection. We also thank the research assistants Camilla Bjornes and Christian Asbjørn Kvamsdal for being involved in the recruitment of participants.
Funding
The study was financed by the University of Agder.
Open access funding provided by University of Agder
Author information
Authors and Affiliations
Contributions
Conceptualization: AM, CL, and SG-J; Investigation: SG-J; Formal analysis: SG-J; Writing-original draft: SG-J; Writing-review & editing: SG-J, AM, CL, CM, IM, DA; Project administration: SG-J; Supervision: AM; Funding acquisition; AM. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Norwegian Regional Committees for Medical and Health Research Ethics (REK/217742), the Norwegian Centre for Research Data (NSD/168890). Written informed consent was obtained from all subjects prior to participation.
Standards of reporting
A STROBE checklist for reporting observational studies has been provided with this submission.
Conflict of interest
All authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Groufh-Jacobsen, S., Larsson, C., Margerison, C. et al. Micronutrient intake and status in young vegans, lacto-ovo-vegetarians, pescatarians, flexitarians, and omnivores. Eur J Nutr (2024). https://doi.org/10.1007/s00394-024-03453-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00394-024-03453-4