Abstract
Purpose
Adherence to the Mediterranean diet is associated with beneficial health effects, including gastrointestinal disorders. Preclinical studies suggest that omega-3 polyunsaturated fatty acids (n-3 PUFAs), found in Mediterranean foods like nuts and fish, improve intestinal barrier integrity. Here, we assessed possible effects of n-3 PUFAs on barrier integrity in a randomized controlled trial.
Methods
We studied 68 women from the open-label LIBRE trial (clinicaltrials.gov: NCT02087592) who followed either a Mediterranean diet (intervention group, IG) or a standard diet (control group, CG). Study visits comprised baseline, month 3, and month 12. Barrier integrity was assessed by plasma lipopolysaccharide binding protein (LBP) and fecal zonulin; fatty acids by gas chromatography with mass spectrometry. Median and interquartile ranges are shown.
Results
Adherence to the Mediterranean diet increased the proportion of the n-3 docosahexaenoic acid (DHA) (IG + 1.5% [0.9;2.5, p < 0.001]/ + 0.3% [− 0.1;0.9, p < 0.050] after 3/12 months; CG + 0.9% [0.5;1.6, p < 0.001]/ ± 0%) and decreased plasma LBP (IG − 0.3 µg/ml [− 0.6;0.1, p < 0.010]/ − 0.3 µg/ml [− 1.1; − 0.1, p < 0.001]; CG − 0.2 µg/ml [− 0.8; − 0.1, p < 0.001]/ ± 0 µg/ml) and fecal zonulin levels (IG − 76 ng/mg [− 164; − 12, p < 0.010]/ − 74 ng/mg [− 197;15, p < 0.001]; CG − 59 ng/mg [− 186;15, p < 0.050]/ + 10 ng/mg [− 117;24, p > 0.050]). Plasma DHA and LBP (R2: 0.14–0.42; all p < 0.070), as well as plasma DHA and fecal zonulin (R2: 0.18–0.48; all p < 0.050) were found to be inversely associated in bi- and multivariate analyses. Further multivariate analyses showed that the effect of DHA on barrier integrity was less pronounced than the effect of fecal short-chain fatty acids on barrier integrity.
Conclusions
Our data show that n-3 PUFAs can improve intestinal barrier integrity.
Trial registration number: The trial was registered prospectively at ClinicalTrials.gov (reference: NCT02087592).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The intestinal barrier is a complex anatomical structure, protecting the host against gut microbes, food antigens, and toxins present in the gastrointestinal tract. A functioning intestinal barrier is required for gut health, whereas intestinal barrier impairment has been associated with numerous diseases such as cardiovascular disease, cancer, type 2 diabetes, and inflammatory bowel disease [1, 2].
Intestinal barrier function is affected by several endogenous and exogenous factors including diet, stress, excessive body weight, and low or extreme physical activity [1, 3, 4]. Previous findings have determined that the intestinal barrier plays a central role in disease occurrence, yet the mechanisms by which the barrier function is regulated are not well known. Dietary factors might play an important role here. We and others have shown that short-chain fatty acids, derived from bacterial fermentation of dietary fibers, improve intestinal barrier function [5,6,7]. Also, vitamins, minerals, amino acids and polyphenols might have an effect [8, 9]. Furthermore, several preclinical studies suggest that diet-derived omega-3 polyunsaturated fatty acids (n-3 PUFAs) improve intestinal barrier integrity [10, 11] yet to date there is no data including human subjects regarding this.
The Mediterranean diet describes the traditional dietary pattern in south Italy and Greece in the mid 1950s, which was characterized by a high intake of n-3 PUFAs like alpha-linolenic acid and docosahexaenoic acid (DHA) due to a regular consumption of seafood and nuts [12]. Such n-3 PUFAs have been shown to be cardioprotective mainly due to beneficial effects on atherosclerosis, arrhythmias, inflammation, and thrombosis [13]. Furthermore, there is evidence that they improve endothelial function, lower blood pressure, and significantly lower triglycerides [13]. Adherence to the Mediterranean diet has been associated with primary and secondary prevention of diseases, which are also linked to intestinal barrier impairment, including cardiovascular disease [14, 15], cancer [16,17,18], and type 2 diabetes [19]. Also, this diet has been shown to be effective in the prevention of and improvement of gastrointestinal disorders, including intestinal barrier impairment [5, 20].
In the present study, we aimed to assess possible associations between the Mediterranean diet, plasma fatty acid composition, and intestinal barrier integrity for the first time in a human study. We hypothesized that n-3 PUFAs improve intestinal barrier function, assessed by the two validated biomarkers plasma lipopolysaccharide binding protein (LBP) and fecal zonulin [21].
Materials and methods
Study design
Data for the present exploratory study derived from the randomized controlled LIBRE (Lifestyle Intervention Study in Women with Hereditary Breast and Ovarian Cancer) trial. The LIBRE study was a randomized (1:1 ratio), prospective, open-label, two-armed controlled multicenter trial, conducted in Germany. It aimed to test the effect of a structured lifestyle intervention program focussing on the Mediterranean diet and increased physical activity on cancer-relevant outcomes. The study included women at high risk for breast and ovarian cancer due to a pathogenic germline mutation in the BRCA1 and/or BRCA2 genes. BRCA mutations have been shown to be associated with an altered intestinal barrier function [5, 20] and it is suggested that intestinal barrier impairment is linked to breast cancer initiation and progression [22].
In the present explorative analyses we included all 68 participants from the completed LIBRE-1 study [23], which started in 2014. The LIBRE-1 study was a feasibility study with the number of participants who successfully completed the first 3 months of lifestyle intervention used as primary endpoint. A rate of 70% adherence or more was considered as success. Secondary endpoints comprised body mass index (BMI), physical activity, which is measured objectively by spiroergometry and is expressed as the ventilatory threshold (VT1), the analyses of omega fatty acids, and fecal metabolites. VT1 is an objective marker of physical fitness and represents the level of physical activity at which blood lactate accumulates faster than it can be cleared in spiroergometry. The sample size in LIBRE-1 was adjusted to this goal but was not calculated based on statistical assumptions and tests [23]. The main focus of the present analysis was to assess possible changes in the plasma fatty acid composition, especially in the proportion of n-3 PUFAs upon intervention. Considering the mean increase in the proportion of n-3 PUFAs in the plasma fatty acids between baseline and month 3, a post-hoc power calculation showed a power of 77% given an alpha error of 5% (intervention group 1.8% ± 2.3% [mean ± SD]; control group 0.3% ± 2.3%). The LIBRE-2 confirmatory study, which aims to include 600 women, started 2015 and recruitment is ongoing [24].
In LIBRE, women with a history of breast cancer prior to study start as well as women without previous breast cancer were included. Inclusion criteria were female sex, age between 18 and 69 years, a pathogenic BRCA1/2 mutation and written informed consent. Exclusion criteria comprised, among others, a BMI below 15 kg/m2, neoplastic diseases currently in treatment, as well as food allergies and/or dietary patterns which prevent the implementation of the Mediterranean diet, like veganism [23].
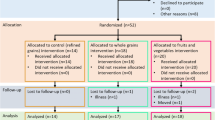
Individuals from the intervention group (n = 33) received a structured lifestyle-intervention program, consisting of a three-month intensive phase with bi-weekly group classes on the Mediterranean diet as well as professionally guided sport training, focussed on endurance-oriented exercises. The intensive phase was followed by a nine-month less intensive phase with monthly meetings. The control group (n = 35) were lectured once on the dietary recommendations of the German Nutrition Society (DGE) and once on the beneficial effects of regular physical activity on breast cancer incidence, prognosis, and recurrence at the beginning of the study. Study visits were at baseline, as well as 3 months (time point V1) and 12 months (time point V2) after baseline. Details on the enrolment, randomization, drop-outs, and available data for each time point are shown in the CONSORT flow chart in Supplementary Fig. 1.
The ethics review board of the Klinikum Rechts der Isar of the Technical University of Munich approved the study protocol (reference 5686/13) which was in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The trial was registered at ClinicalTrials.gov (reference: NCT02087592).
Dietary measurements
Two validated questionnaires were used to assess dietary habits. The Mediterranean Diet Adherence Screener (MEDAS), developed in the Prevención con Dieta Mediterránea (PREDIMED) studies [25], is a validated tool to measure adherence to the Mediterranean diet. As part of the LIBRE-1 study, we translated the original version into German and validated the German version again [26]. The MEDAS consists of 14 dichotomous questions, focussing on food consumption and typically Mediterranean dietary habits. Each question is scored with either 0 or 1, with 1 representing the answers that are related to the Mediterranean diet. Therefore, the MEDAS-Score ranges from 0 to 14, with 14 points representing the highest adherence to the Mediterranean diet. Two MEDAS questions imply meat consumption (#5 & #13, [26]) and were occasionally left out by vegetarians. Therefore, we calculated the MEDAS-Score as the percentage of the achieved score related to the achievable score (e.g. 7/14 = 50%; 7/13 = 54%).
In addition to the MEDAS, participants were asked to complete a 33-page long semi-quantitative Food Frequency Questionnaire (FFQ) established and validated by the European Prospective Investigation into Cancer and Nutrition (EPIC) consortium [27]. The EPIC-FFQ contains various questions asking qualitatively and quantitatively about food and beverage consumption, covering the previous 12 months (baseline), the previous 3-month-intervention phase (V1), or the previous 9-month-intervention phase (V2). Data input and data evaluation were performed using the study management system for health research, which has been developed by the Department of Epidemiology of the German Institute of Human Nutrition. The EPIC-FFQ provides the daily intakes of food groups (e.g. fruits, vegetables, nuts) and nutrients (e.g. fats, carbohydrates, protein). In the EPIC-FFQ results, vegetable oil refers to the sum of all vegetable oils consumed; processed meat refers to meat and meat products which have been processed by e.g. salting, curing, fermentation, smoking, and/or the addition of chemical preservatives (including e.g. bacon, ham, and sausages); red meat refers to unprocessed meat of red color, e.g. beef and pork.
Data from the EPIC-FFQ were adjusted for energy intake in accordance to Willett et al. [28]. Since the EPIC-FFQ does not per se measure adherence to the Mediterranean diet, we calculated the Mediterranean Diet Score (MedD-Score), a commonly used score established by Trichopoulou et al. [29]. Thus, in the present analyses, we included two independent scores which determine adherence to the Mediterranean diet, i.e. the MEDAS-Score and the MedD-Score. Dietary data for all variables and all time points is shown in Supplementary Table 1.
Blood and fecal sample collection
Blood and fecal samples were collected in 2014 and 2015 at the participating study centers and sent to the University of Hohenheim overnight. All samples were stored at −80 °C and were analyzed in 2017 and 2018.
Plasma fatty acid composition
Plasma fatty acids were transferred into fatty acid methyl esters (FAME) and were determined by gas chromatography with mass spectrometry (GC/MS). Afterwards, the proportion (%) of each fatty acid in the total fatty acid composition (= 100%) was determined. Therefore, each fatty acid is shown as the proportion of the respective fatty acid (%), as shown in Supplementary Table 2.
In detail, blood samples were collected in ethylenediaminetetraacetic acid (EDTA)-coated tubes. To separate the plasma, the samples were centrifuged at 500g for 7.5 min at 15 °C. Plasma fatty acid composition was assessed similar to a method used for fatty acid analysis in erythrocyte membranes, which was described previously [30]. In brief, 0.05 ml of plasma was supplemented with a solution containing 2 µg the internal standard 10,11-dichloro-undecanoic acid (DC 11:0) which was synthesized according to Thurnhofer and Vetter [31]. Transesterification (60 min at 80 °C) was carried out by adding 2 ml methanol with 1% sulphuric acid according to Wendlinger et al. [32]. Finally, 2.5 μg of the second internal standard, myristic acid ethyl ester (14:0 EE), was added to the resulting solution with the fatty acid methyl esters. This internal standard, which does not interfere with the fatty acid methyl esters in the samples, was used to level off variations in the instrumental performance between the individual measurements [33]. The final sample solutions were analyzed by GC/MS on a 5890 series II/5972A system (Hewlett-Packard, Waldbronn, Germany) equipped with a 60 m × 0.25 mm i.d. capillary column coated with 0.1 µm film thickness 10% cyanopropylphenyl, 90% bis-cyanopropyl polysiloxane (Rtx 2330, Restek, Belafonte, PA, USA) operated in selected ion monitoring (SIM) mode according to Thurnhofer et al. [34].
Intestinal barrier biomarkers
All intestinal barrier biomarkers were analyzed using enzyme-linked immunosorbent assays following the manufacturer’s protocols. Zonulin was measured in fecal samples (K5600; Immundiagnostik AG, Bensheim, Germany). LBP was measured in plasma (REFs: DY870-05 and DY008; Bio-Techne GmbH, Wiesbaden, Germany).
Short-chain fatty acids
To analyze fecal short-chain fatty acid (SCFA) levels, 400–500 mg feces were first diluted 1:4 in water. Then, 0.1 ml of 50% ortho-phosphoric acid (AppliChem GmbH, Darmstadt, Germany) was added before the samples were homogenized and filtered using polyester syringe filters (REF: 729033; Macherey–Nagel GmbH & Co. KG, Düren, Germany). Afterwards, 1 µl filtrate was analyzed using a capillary gas chromatograph (HP6890 Series; Hewlett Packard Corp., Paolo Alto, California, USA) with a flame ionization detector using the column OPTIMA-FFAP (REF: 726344.10; Macherey–Nagel GmbH & Co. KG, Düren, Germany) with standards for all SCFAs (Merck Schuchardt OHG, Hohenbrunn, Germany). Fecal dry mass was assessed by drying 300–500 mg feces overnight at 103 °C [35]. The SCFA data are expressed in relation to dry mass to overcome bias due to differing fecal water contents.
Statistical analyses
Prior to analyses, normal distribution was tested for all variables using Shapiro–Wilk tests, showing that 76% of the variables were not normally distributed. Hence, non-parametric tests were used for all uni- and bivariate analyses. Differences between the intervention and control groups were tested using Fisher’s exact test for categorical variables or Mann–Whitney U tests for quantitative data. Within-group differences over time were assessed using Wilcoxon matched-pairs signed rank tests. To assess changes over time we calculated the shift for each parameter (baseline [BL] values subtracted from the respective values at time point V1 and V2, shown as ∆V1-BL and ∆V2-BL). Correlations were determined using Spearman's rank coefficient. Multivariate analyses were performed using multiple regressions. Before performing multiple regressions, the variables were tested for intercorrelation (intercorrelations were defined as Spearman’s correlation coefficient > 0.8) showing no significant results. A p ≤ 0.07 was considered as a trend, a p < 0.05 was considered as statistically significant.
As these analyses are not confirmatory and rather to generate hypotheses to be further analysed in studies with larger populations, e.g. LIBRE-2, we did not perform post-hoc adjustment for multiple testing. Also, in order to try to compensate for random findings in the analyses, we only draw conclusions based on results, which were found (i) consistently for the intervention group and the control group and/or (ii) consistently for both shifts ∆V1-BL and ∆V2-BL. All statistical analyses were performed using GraphPad Prism version 9.1.0 (GraphPad Software, San Diego, CA, USA). Data are shown as medians with interquartile ranges (25th; 75th percentiles).
Results
Baseline characteristics
At baseline, the intervention and control groups had similar numbers of women with previously diagnosed breast cancer, vegetarians, and smokers, and did not differ in age, BMI (Table 1), and physical fitness (data not shown).
At baseline, both groups showed similar adherence to the Mediterranean diet according to the MedD-Score, yet the MEDAS-Score was slightly higher in the intervention group compared to the control group (50% [36%;59%] vs 42% [29%;50%], p = 0.045) (Supplementary Table 1). Besides the small difference in the MEDAS-Score, there was no baseline difference in diet. The baseline proportion of eicosanoid acid (20:0) in the plasma fatty acids was higher in the control group compared to the intervention group (0.2% [0.1%;0.2%] vs 0.1% [0.1%;0.2%], p = 0.043), while the proportion of linoleic acid (18:2, n-6) was higher in the intervention group compared to the control group (30% [26%;32%] vs 28% [26%;30%], p = 0.039). All other fatty acids as well as the levels of the intestinal barrier biomarkers LBP and zonulin did not differ between the groups at baseline (Supplementary Table 2).
The effect of the LIBRE intervention program on dietary and physical outcomes
As described elsewhere [30], adherence to the Mediterranean diet, assessed by both the MEDAS-Score and the MedD-Score, increased markedly in the intervention group for at least 1 year (all p < 0.01) (Supplementary Table 1). In the control group, there was a mild but significant increase in the MEDAS-Score and the MedD-Score between baseline and V1 (all p < 0.001), which was absent at month 12 (all p > 0.05).
Data derived from the EPIC-FFQ showed that participants from the intervention group increased the intake of the typically Mediterranean food groups nuts and seafood (Fig. 1a, b), vegetables, legumes, fruits, olives, and vegetable oil (Fig. 1c) (all p < 0.05) (Supplementary Table 1). At the same time, the intake of processed meat (p < 0.05 for ∆V1-BL and ∆V2-BL) and red meat (p = 0.070 for ∆V1-BL) decreased in the intervention group, but not in the control group (Fig. 1d,e). The participants from the control group decreased the intake of lignin and total diet-derived fat in the first three months and decreased the intake of animal- and plant-derived protein, total fibers, and total diet-derived fat over the 12 month-period (all p < 0.05). Dietary data for all time points is shown in Supplementary Table 1.
Effect of the intervention on dietary habits. a–e Shown are data for baseline (BL), as well as after month 3 (V1) and month 12 (V2) for the intake of typical Mediterranean foods. Tukey boxplots with median, whiskers (1.5 × interquartile ranges), and outliers are shown in green (intervention group; BL: n = 31, V1, V2: n = 26) and orange (control group; BL: n = 33, V1: n = 31, V2: n = 29). Within group difference to baseline is indicated by asterisks (*p < 0.05; **p < 0.1; ***p < 0.001; Wilcoxon signed-rank test). This figure summarizes data shown in Supplementary Table 1
Physical fitness, assessed objectively in spiroergometry as the ventilatory threshold 1 (VT1), did not change in the intervention group, and showed a slight but significant decrease in the control group between baseline and V1 (−12 W [−62 W;3 W], p = 0.020) but not between baseline and V2 (p > 0.05). The mean BMI did not change in the intervention group and increased mildly in the control group between baseline and V1 (+ 0.32 kg/m2 [− 0.1 kg/m2;0.8 kg/m2], p = 0.013) and increased by trend between baseline and V2 (+ 0.27 kg/m2 [− 0.5 kg/m2; 0.7 kg/m2] p = 0.062) (Supplementary Table 1).
Effect of the Mediterranean diet on plasma fatty acid patterns and intestinal barrier biomarkers
After the 3-month intensive intervention phase, we observed several changes in the plasma fatty acid composition in both study arms. For example, in the first 3 months the proportion of the n-3 PUFA DHA (22:6) increased in both groups, while the proportion of the n-6 PUFA arachidonic acid (20:4) decreased (all p < 0.001) (Fig. 2a, b). After 1 year, these changes were still present in the intervention group (all p < 0.05), but not in the control group. Accordingly, the total n-3/n-6 ratio increased in the intervention group (all p < 0.05) (Fig. 2c). Compared to baseline, the proportion of oleic acid (18:1, n-9) increased mildly in the first three months (+ 0.2% [−1.3%;1.2%], p = 0.062) and increased markedly after 1 year (+ 1.3% [0.2%;0.4%], p = 0.001) in the intervention group (Fig. 2d). There was no change in the proportion of plasma oleic acid in the control group. In both study arms there was a decrease in the proportion of total saturated fatty acids (SFAs) for at least 1 year (intervention group, all p < 0.001; control group, all p < 0.05) (Fig. 2e).
Effect of the intervention on plasma fatty acid composition and intestinal barrier biomarkers Shown are data for baseline (BL), as well as after month 3 (V1) and month 12 (V2) for the proportion (%) of docosahexaenoic acid (DHA), arachidonic acid (ARA), oleic acid (OA), and total saturated fatty acids (SFAs) in the total plasma fatty acid composition (plasma FAs) (a–e), and for the intestinal barrier biomarkers plasma lipopolysaccharide binding protein (LBP) and fecal zonulin (f, g). Tukey boxplots with median, whiskers (1.5 × interquartile ranges), and outliers are shown in green (intervention group; BL: n = 33, V1: n = 26–33, V2: n = 23–29) and orange (control group; BL: n = 35, V1: n = 29–35, V2: n = 28–32). Within group difference to baseline is indicated by asterisks (*p < 0.05; **p < 0.1; ***p < 0.001; Wilcoxon signed-rank test). This figure summarizes data shown in Supplementary Table 2
Both biomarkers of intestinal barrier function decreased in the intervention group in the first 3 months (LBP −0.3 µg/ml [−0.6 µg/ml; 0.1 µg/ml], p = 0.007; zonulin −76 ng/mg [−164 ng/mg; −12 ng/mg], p = 0.006). In the control group, both biomarkers decreased in the first three months (LBP −0.2 µg/ml [−0.8 µg/ml; −0.1 µg/ml], p < 0.001; zonulin −59 ng/mg [−186 ng/mg; 15 ng/mg], p = 0.023) but returned to baseline levels after 1 year (Fig. 2f,g). All data on plasma fatty acid composition and the gut barrier biomarkers is shown in detail in Supplementary Tables 1 and 2.
Omega-3 polyunsaturated fatty acids and intestinal barrier integrity
As a first step, we analyzed correlations between diet, plasma fatty acids, and the intestinal barrier biomarkers. Here, we first calculated the shift for each parameter that was significantly altered during the study (see Supplementary Tables 1 and 2).
As shown in Table 2, the increase in adhering to the Mediterranean diet correlated with the increase in the proportion of DHA and the Omega-3-Index. The increase in the adherence to the Mediterranean diet was furthermore inversely correlated with the proportion of n-6 PUFAs. These associations were found consistently in the intervention and control groups. In the intervention group, the MedD-Score correlated with the proportion of the n-9 oleic acid at ∆V1-BL.
The intake of nuts was associated with the proportion of DHA, and the intake of processed meat was associated with the proportion of SFAs. These associations were found consistently for both ∆V1-BL and ∆V2-BL in the intervention group. The intake of vegetable oils correlated strongly with the proportion of oleic acid in the intervention group for ∆V2-BL but not the other time points. As shown in Fig. 3a, the decrease in the proportion of SFAs correlated with the decrease in the plasma levels of the intestinal barrier biomarker LBP for both ∆V1-BL and ∆V2-BL in the intervention group as well as for ∆V1-BL in the control group. Also, the increase in the proportion of DHA correlated with the decrease in plasma LBP (Fig. 3b) and fecal zonulin (Fig. 3c). All results from the correlation analyses are shown in Table 2.
Omega-3 polyunsaturated fatty acids improve intestinal barrier integrity. Shown are the correlations between the proportion (%) of saturated fatty acids (SFAs) in the total plasma fatty acid composition (plasma FAs) and plasma levels of lipopolysaccharide binding protein (LBP) (a); the proportion of the omega-3 polyunsaturated docosahexaenoic acid (DHA) and plasma levels of LBP (b); and the proportion of DHA and fecal levels of zonulin (c). Spearman correlations were conducted for the intervention and the control groups (n = 33/35) using shift values (baseline [BL] values subtracted from the respective values after month 3 [V1] and month 12 [V2]. This figure summarizes the main findings shown in detail in Table 2
To further investigate the observed associations between the plasma fatty acid composition and the intestinal permeability biomarkers, we ran multiple regression models, including BMI, physical fitness, and previous cancer disease state as possible confounders. As shown in Supplementary Table 3, these multivariate analyses confirmed the initial correlation analyses to a large degree. In detail, the association between the proportion of SFAs and plasma LBP was also significant in the intervention group for ∆ V1-SE (p = 0.048, R2 = 0.45), but was not significant at the other time points and not in the control group. Furthermore, the multivariate analyses confirmed the inverse association between the proportion of DHA and plasma LBP for ∆ V1-SE both in the intervention and the control groups (intervention group: p = 0.070, R2 = 0.42; control group: p = 0.023, R2 = 0.37). Also, the regression models confirmed the inverse association between the proportion of DHA and fecal zonulin in the intervention group (∆ V1-BL: p = 0.049, R2 = 0.40; ∆ V2-BL: p = 0.040, R2 = 0.42).
Taken together, the initial correlation analyses as well as the subsequent multivariate analyses showed an inverse association between the proportion of DHA in the plasma fatty acids and the two intestinal barrier biomarkers LBP and zonulin. Furthermore, the results showed an association between the proportion of SFAs and LBP.
Intestinal short-chain fatty acids have a greater effect on intestinal barrier integrity than plasma omega-3 polyunsaturated fatty acids
We have previously shown that fecal short-chain fatty acids (SCFAs) are key mediators for the favorable effects of the Mediterranean diet on intestinal barrier integrity [5]. In this previous article we showed strong inverse associations between fecal levels of the SCFAs propionate and butyrate and the intestinal barrier biomarkers plasma LBP and fecal zonulin.
As a final step in the present analysis, we compared the effect size of the association between the proportion of DHA in the plasma fatty acids and the two intestinal barrier biomarkers (plasma LBP and fecal zonulin) with the effect size of the association between fecal SCFAs (propionate and butyrate) and the two intestinal barrier biomarkers.
First, we ran correlation analyses, including the fecal amounts of the SCFAs propionate and butyrate (mg SCFA/g fecal sample) and the levels of the intestinal barrier biomarkers plasma LBP and fecal zonulin. As shown in Table 2 and summarized in Fig. 4a, b, the effect size (R2) was markedly higher for the association between fecal SCFAs and the intestinal barrier biomarkers (R2 0.37–0.57; all p < 0.0001) compared to the effect size of the association between the proportion of DHA in the plasma fatty acids and the intestinal barrier biomarkers (R2 0.14–0.32; all p ≤ 0.070).
Omega-3 polyunsaturated fatty acids improve intestinal barrier integrity—albeit to a lesser degree than fecal short-chain fatty acids. Shown are the comparisons of the effect sizes (R2) of the correlation between the proportion (%) of the omega-3 polyunsaturated fatty acid (n-3 PUFA) docosahexaenoic acid (DHA) in the total plasma fatty acid composition and the fecal short-chain fatty acids (SCFAs) propionate and butyrate with plasma lipopolysaccharide binding protein (LBP) (a), and fecal zonulin (b). Panels c, d show the comparisons of the effect sizes of the multiple linear regressions (MLR) between the proportion of DHA and the fecal SCFAs propionate and butyrate with plasma LBP (c) and fecal zonulin (d). #p < 0.07; *p < 0.05; **p < 0.01; ***p < 0.001; n.s. not significant. This figure summarizes the main findings shown in detail in Table 2 and Supplementary Table 3
Subsequently, we ran multivariate regression analyses to verify our findings from the correlation analyses with BMI, physical fitness, and previous cancer disease state as possible confounders. As summarized in Fig. 4c, d, these multivariate analyses supported the results from the correlation analyses, showing that fecal SCFAs had a more pronounced effect on the intestinal barrier biomarkers than the proportion of DHA (SCFAs and barrier biomarkers: R2 0.60–0.71; DHA and barrier biomarkers: R2 0.37–0.42; all p ≤ 0.070). All results from the multiple regression models are shown Supplementary Table 3.
Discussion
Intestinal barrier dysfunction is a major cause that drives low-grade inflammation found in numerous chronic diseases, including cardiometabolic diseases like type 2 diabetes, and cancer [2, 36]. Adherence to the Mediterranean diet, on the other hand, shows beneficial effects on these chronic diseases [14,15,16,17,18]. In the present study, we show that an increase in the proportion of n-3 PUFAs in the plasma fatty acids, derived from adherence to the Mediterranean diet, improves intestinal barrier dysfunction.
In a randomized controlled trial, we assessed the effect of a 1-year Mediterranean diet on plasma fatty acid levels and intestinal barrier integrity in a cohort of women with mild barrier impairment due to BRCA germline mutations [5, 20]. Our data show that adherence to the Mediterranean diet changes the plasma fatty acid composition, increasing the proportion of anti-inflammatory n-3 PUFAs and n-9 MUFAs, while decreasing the proportion of SFAs and pro-inflammatory n-6 PUFAs.
Thus far, it has been largely unclear how adherence to the Mediterranean diet exerts its beneficial effects on chronic diseases. Preclinical studies suggested that diet-derived organic acids like ferulic acid found in grains [37], but especially n-3 PUFAs might be of major relevance here. For the first time in a clinical setting, we show that n-3 PUFAs improve intestinal barrier function, which has so far only been shown in cell lines and animal models. Using bi- and multivariate analyses we found that n-3 PUFAs, especially DHA found in fat fish like salmon, improved intestinal barrier function, while SFAs, found in confectionery and fast food, were associated with barrier dysfunction. Of note, we found no association between the proportion of n-6 PUFAs or n-9 MUFAs in the plasma fatty acids and the two barrier biomarkers, indicating that an effect of omega fatty acids on barrier integrity is exclusive for n-3 PUFAs.
Marine-derived n-3 PUFAs like eicosapentaenoic acid (EPA) and DHA are incorporated into cell membranes, including intestinal epithelial cell membranes, and exert several biological effects. The best-known mechanisms comprise the induction of anti-inflammatory eicosanoids derived from EPA and docosanoids derived from DHA [38], which are linked to lower cancer incidence [39]. Also, recent studies showed that marine-derived n-3 PUFAs improve intestinal barrier function, which has been shown in cell lines [40,41,42,43,44,45,46,47] and rodents [43, 48,49,50,51,52,53,54,55,56].
According to in vitro and in vivo studies, n-3 PUFAs affect tight junction proteins, including occludin and zonula occludens-1 (ZO-1), which are essential for effective cell–cell connections, which are necessary to prevent uncontrolled paracellular permeability. In detail, in vitro studies [44,45,46] and rodent models [50, 51, 57] showed that long-chain n-3 PUFAs (eicosapentaenoic acid and DHA) improved gut barrier stability via an increased expression of occludin and ZO-1 in cell membranes and decreased cellular degeneration. Furthermore, n-3 PUFAs induce the G-protein coupled receptor 120, which exerts anti-inflammatory effects and increases tight junction stability [10, 58, 59]. For the first time in a clinical setting, our data show a barrier-stabilizing effect of n-3 PUFAs in humans, using two validated barrier biomarkers LBP and zonulin.
Intestinal barrier function is affected by numerous exogenous and endogenous factors, including lifestyle factors like diet, physical activity, alcohol intake or smoking, but also gut microbiota composition and function [2, 3, 60]. As shown by our data, n-3 PUFAs have a significant influence on barrier integrity. However, barrier-stabilizing effects are not limited to n-3 PUFAs. Early and recent research showed that SCFAs, derived from bacterial fermentation of dietary fibers in the colon, improve intestinal barrier function [2, 60, 61], exert anti-inflammatory effects [62, 63], and might regulate cancer progression [64]. In the present study, we assessed fecal SCFA levels to compare the effect size of n-3 PUFAs on intestinal barrier integrity with the effect size of SCFAs on barrier integrity. To the best of our knowledge, this is the first clinical study to investigate this. Our data suggest that the effect of fecal SCFAs on barrier function is more pronounced than the effect of n-3 PUFAs on barrier function. Obviously, the LIBRE study design does not allow to draw conclusions regarding the underlying molecular mechanisms, as extensive phenotyping would be necessary to do so. We assume, however, that several aspects play a role here. Most importantly, SCFAs are produced in the colon and are metabolized to a large degree by enterocytes where SCFAs directly improve barrier integrity [2, 60]. On the other hand, n-3 PUFA intake is regulated in proximal parts of the small intestine. Therefore, only smaller amounts of n-3 PUFAs reach enterocytes via the bloodstream which might lessen the effect size of n-3 PUFAs. Future studies should assess whether n-3 PUFAs applicated rectally or via capsules which release n-3 PUFAs in more distal parts of the intestinal tract increase the effect of these PUFAs on intestinal barrier function. Furthermore, the effect of food components other than fatty acids, fibers, and SCFAs, e.g. vitamins, minerals, amino acids and polyphenols, which might also affect intestinal barrier integrity, should be evaluated [8, 9].
Limitations and strengths
Our study has both limitations and strengths. A limitation of our study is that we only included women with BRCA mutations, which implies an associated mild intestinal barrier dysfunction. To what extent our finding will also hold true for other populations with or without intestinal barrier dysfunction needs to be explored in future studies. A strength of our study is that our findings on the effects of omega-3 polyunsaturated fatty acids in the Mediterranean diet on intestinal barrier function is based on a rigorous approach in the statistical analyses. To omit reporting random findings, we only show results which were found consistently in the two study groups and/or found for more than one time point.
In conclusion, our data show that n-3 PUFAs, derived from typical Mediterranean foods like fatty fish, improve intestinal barrier function. However, the effect of gut bacteria-derived SCFAs on intestinal barrier function was more pronounced than the effect of diet-derived n-3 PUFAs. Our study offers new insights in the interplay between dietary components and intestinal health.
Data sharing plan
Data described in the manuscript will be made available upon request pending approval by the corresponding author SCB
References
Martel J, Chang S-H, Ko Y-F et al (2022) Gut barrier disruption and chronic disease. Trends Endocrinol Metab 33:247–265. https://doi.org/10.1016/j.tem.2022.01.002
Bischoff SC, Barbara G, Buurman W et al (2014) Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol 14:189. https://doi.org/10.1186/s12876-014-0189-7
Bischoff SC, Kaden-Volynets V, Filipe Rosa L et al (2021) Regulation of the gut barrier by carbohydrates from diet—underlying mechanisms and possible clinical implications. Int J Med Microbiol IJMM 311:151499. https://doi.org/10.1016/j.ijmm.2021.151499
Chantler S, Griffiths A, Matu J et al (2021) The effects of exercise on indirect markers of gut damage and permeability: a systematic review and meta-analysis. Sports Med Auckl NZ 51:113–124. https://doi.org/10.1007/s40279-020-01348-y
Seethaler B, Nguyen NK, Basrai M et al (2022) Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: data from the randomized controlled LIBRE trial. Am J Clin Nutr 116:928–942. https://doi.org/10.1093/ajcn/nqac175
Parada Venegas D, De la Fuente MK, Landskron G et al (2019) Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 10:277. https://doi.org/10.3389/fimmu.2019.00277
Liu P, Wang Y, Yang G et al (2021) The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res 165:105420. https://doi.org/10.1016/j.phrs.2021.105420
Bernardi S, Del Bo C, Marino M et al (2020) Polyphenols and intestinal permeability: rationale and future perspectives. J Agric Food Chem 68:1816–1829. https://doi.org/10.1021/acs.jafc.9b02283
Scott SA, Fu J, Chang PV (2020) Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 117:19376–19387. https://doi.org/10.1073/pnas.2000047117
Durkin LA, Childs CE, Calder PC (2021) Omega-3 polyunsaturated fatty acids and the intestinal epithelium—a review. Foods 10:199. https://doi.org/10.3390/foods10010199
Millman J, Okamoto S, Kimura A et al (2020) Metabolically and immunologically beneficial impact of extra virgin olive and flaxseed oils on composition of gut microbiota in mice. Eur J Nutr 59:2411–2425. https://doi.org/10.1007/s00394-019-02088-0
Davis C, Bryan J, Hodgson J, Murphy K (2015) Definition of the Mediterranean diet: a literature review. Nutrients 7:9139–9153. https://doi.org/10.3390/nu7115459
Schwalfenberg G (2006) Omega-3 fatty acids. Can Fam Physician 52:734–740
Esposito K, Maiorino MI, Ciotola M et al (2009) Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med 151:306–314. https://doi.org/10.7326/0003-4819-151-5-200909010-00004
Estruch R, Ros E, Salas-Salvadó J et al (2018) Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 378:e34. https://doi.org/10.1056/NEJMoa1800389
Toledo E, Salas-Salvadó J, Donat-Vargas C et al (2015) Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med 175:1752–1760. https://doi.org/10.1001/jamainternmed.2015.4838
Dieli-Conwright CM, Lee K, Kiwata JL (2016) Reducing the risk of breast cancer recurrence: an evaluation of the effects and mechanisms of diet and exercise. Curr Breast Cancer Rep 8:139–150. https://doi.org/10.1007/s12609-016-0218-3
Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G (2017) Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients 9:1063. https://doi.org/10.3390/nu9101063
de Lorgeril M (2011) PREDIMED trial: Mediterranean diet may reduce the risk of type 2 diabetes. BMJ Evid-Based Med 16:152–153. https://doi.org/10.1136/ebm1400
van Voss MRH, van Diest PJ, Smolders YHCM et al (2014) Distinct claudin expression characterizes BRCA1-related breast cancer. Histopathology 65:814–827. https://doi.org/10.1111/his.12490
Seethaler B, Basrai M, Neyrinck AM et al (2021) Biomarkers for assessment of intestinal permeability in clinical practice. Am J Physiol Gastrointest Liver Physiol 321:G11–G17. https://doi.org/10.1152/ajpgi.00113.2021
Brennan K, Offiah G, McSherry EA, Hopkins AM (2010) Tight junctions: a barrier to the initiation and progression of breast cancer? J Biomed Biotechnol 2010:460607. https://doi.org/10.1155/2010/460607
Kiechle M, Engel C, Berling A et al (2016) Lifestyle intervention in BRCA1/2 mutation carriers: study protocol for a prospective, randomized, controlled clinical feasibility trial (LIBRE-1 study). Pilot Feasibility Stud 2:74. https://doi.org/10.1186/s40814-016-0114-7
Kiechle M, Engel C, Berling A et al (2016) Effects of lifestyle intervention in BRCA1/2 mutation carriers on nutrition, BMI, and physical fitness (LIBRE study): study protocol for a randomized controlled trial. Trials 17:368. https://doi.org/10.1186/s13063-016-1504-0
Schröder H, Fitó M, Estruch R et al (2011) A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr 141:1140–1145. https://doi.org/10.3945/jn.110.135566
Hebestreit K, Yahiaoui-Doktor M, Engel C et al (2017) Validation of the German version of the Mediterranean Diet Adherence Screener (MEDAS) questionnaire. BMC Cancer 17:341. https://doi.org/10.1186/s12885-017-3337-y
Bohlscheid-Thomas S, Hoting I, Boeing H, Wahrendorf J (1997) Reproducibility and relative validity of energy and macronutrient intake of a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 26(Suppl 1):S71–S81. https://doi.org/10.1093/ije/26.suppl_1.s71
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65:1220S-1228S. https://doi.org/10.1093/ajcn/65.4.1220S
Trichopoulou A, Kouris-Blazos A, Wahlqvist ML et al (1995) Diet and overall survival in elderly people. BMJ 311:1457–1460. https://doi.org/10.1136/bmj.311.7018.1457
Seethaler B, Basrai M, Vetter W et al (2020) Fatty acid profiles in erythrocyte membranes following the Mediterranean diet—data from a multicenter lifestyle intervention study in women with hereditary breast cancer (LIBRE). Clin Nutr Edinb Scotl 39:2389–2398. https://doi.org/10.1016/j.clnu.2019.10.033
Thurnhofer S, Vetter W (2005) A gas chromatography/electron ionization-mass spectrometry-selected ion monitoring method for determining the fatty acid pattern in food after formation of fatty acid methyl esters. J Agric Food Chem 53:8896–8903. https://doi.org/10.1021/jf051468u
Wendlinger C, Hammann S, Vetter W (2014) Various concentrations of erucic acid in mustard oil and mustard. Food Chem 153:393–397. https://doi.org/10.1016/j.foodchem.2013.12.073
Thurnhofer S, Vetter W (2006) Application of ethyl esters and d3-methyl esters as internal standards for the gas chromatographic quantification of transesterified fatty acid methyl esters in food. J Agric Food Chem 54:3209–3214. https://doi.org/10.1021/jf053022j
Thurnhofer S, Lehnert K, Vetter W (2008) Exclusive quantification of methyl-branched fatty acids and minor 18:1-isomers in foodstuff by GC/MS in the SIM mode using 10,11-dichloroundecanoic acid and fatty acid ethyl esters as internal standards. Eur Food Res Technol 226:975–983. https://doi.org/10.1007/s00217-007-0619-7
Rodriguez J, Neyrinck AM, Zhang Z et al (2020) Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut Microbes 12(1810530):1810530. https://doi.org/10.1080/19490976.2020.1810530
Fukui H (2016) Increased intestinal permeability and decreased barrier function: does it really influence the risk of inflammation? Inflamm Intest Dis 1:135–145. https://doi.org/10.1159/000447252
Tian B, Geng Y, Wang P et al (2022) Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet-induced mice. Eur J Nutr. https://doi.org/10.1007/s00394-022-02927-7
Dennis EA, Norris PC (2015) Eicosanoid storm in infection and inflammation. Nat Rev Immunol 15:511–523. https://doi.org/10.1038/nri3859
Fabian CJ, Kimler BF, Hursting SD (2015) Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res BCR 17:62. https://doi.org/10.1186/s13058-015-0571-6
Rosella O, Sinclair A, Gibson PR (2000) Polyunsaturated fatty acids reduce non-receptor-mediated transcellular permeation of protein across a model of intestinal epithelium in vitro. J Gastroenterol Hepatol 15:626–631. https://doi.org/10.1046/j.1440-1746.2000.02215.x
Usami M, Muraki K, Iwamoto M et al (2001) Effect of eicosapentaenoic acid (EPA) on tight junction permeability in intestinal monolayer cells. Clin Nutr Edinb Scotl 20:351–359. https://doi.org/10.1054/clnu.2001.0430
Usami M, Komurasaki T, Hanada A et al (2003) Effect of gamma-linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutr Burbank Los Angel Cty Calif 19:150–156. https://doi.org/10.1016/s0899-9007(02)00927-9
Willemsen LEM, Koetsier MA, Balvers M et al (2008) Polyunsaturated fatty acids support epithelial barrier integrity and reduce IL-4 mediated permeability in vitro. Eur J Nutr 47:183–191. https://doi.org/10.1007/s00394-008-0712-0
Li Q, Zhang Q, Wang M et al (2008) n-3 polyunsaturated fatty acids prevent disruption of epithelial barrier function induced by proinflammatory cytokines. Mol Immunol 45:1356–1365. https://doi.org/10.1016/j.molimm.2007.09.003
Xiao G, Tang L, Yuan F et al (2013) Eicosapentaenoic acid enhances heat stress-impaired intestinal epithelial barrier function in Caco-2 cells. PloS One 8:e73571. https://doi.org/10.1371/journal.pone.0073571
Xiao K, Liu C, Qin Q et al (2020) EPA and DHA attenuate deoxynivalenol-induced intestinal porcine epithelial cell injury and protect barrier function integrity by inhibiting necroptosis signaling pathway. FASEB J Off Publ Fed Am Soc Exp Biol 34:2483–2496. https://doi.org/10.1096/fj.201902298R
Li E, Horn N, Ajuwon KM (2022) EPA and DHA inhibit endocytosis of claudin-4 and protect against deoxynivalenol-induced intestinal barrier dysfunction through PPARγ dependent and independent pathways in jejunal IPEC-J2 cells. Food Res Int Ott Ont 157:111420. https://doi.org/10.1016/j.foodres.2022.111420
Empey LR, Jewell LD, Garg ML et al (1991) Fish oil-enriched diet is mucosal protective against acetic acid-induced colitis in rats. Can J Physiol Pharmacol 69:480–487. https://doi.org/10.1139/y91-072
Caplan MS, Russell T, Xiao Y et al (2001) Effect of polyunsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatr Res 49:647–652. https://doi.org/10.1203/00006450-200105000-00007
Hudert CA, Weylandt KH, Lu Y et al (2006) Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A 103:11276–11281. https://doi.org/10.1073/pnas.0601280103
Xiao G, Yuan F, Geng Y et al (2015) Eicosapentaenoic acid enhances heatstroke-impaired intestinal epithelial barrier function in rats. Shock Augusta Ga 44:348–356. https://doi.org/10.1097/SHK.0000000000000417
Charpentier C, Chan R, Salameh E et al (2018) Dietary n-3 PUFA may attenuate experimental colitis. Mediators Inflamm 2018:8430614. https://doi.org/10.1155/2018/8430614
Che H, Li H, Song L et al (2021) Orally administered DHA-enriched phospholipids and DHA-enriched triglyceride relieve oxidative stress, improve intestinal barrier, modulate inflammatory cytokine and gut microbiota, and meliorate inflammatory responses in the brain in dextran sodium sulfate induced colitis in mice. Mol Nutr Food Res 65:e2000986. https://doi.org/10.1002/mnfr.202000986
Tao F, Xing X, Wu J, Jiang R (2021) Enteral nutrition modulation with n-3 PUFAs directs microbiome and lipid metabolism in mice. PLoS ONE 16:e0248482. https://doi.org/10.1371/journal.pone.0248482
Fang J, Zhang Z, Cheng Y et al (2022) EPA and DHA differentially coordinate the crosstalk between host and gut microbiota and block DSS-induced colitis in mice by a reinforced colonic mucus barrier. Food Funct 13:4399–4420. https://doi.org/10.1039/d1fo03815j
Du L, Hao Y-M, Yang Y-H et al (2022) DHA-enriched phospholipids and EPA-enriched phospholipids alleviate lipopolysaccharide-induced intestinal barrier injury in mice via a Sirtuin 1-dependent mechanism. J Agric Food Chem 70:2911–2922. https://doi.org/10.1021/acs.jafc.1c07761
Chien Y-W, Peng H-C, Chen Y-L et al (2017) Different dietary proportions of fish oil regulate inflammatory factors but do not change intestinal tight junction ZO-1 expression in ethanol-fed rats. Mediators Inflamm 2017:5801768. https://doi.org/10.1155/2017/5801768
Rubbino F, Garlatti V, Garzarelli V et al (2022) GPR120 prevents colorectal adenocarcinoma progression by sustaining the mucosal barrier integrity. Sci Rep 12:381. https://doi.org/10.1038/s41598-021-03787-7
Mobraten K, Haug TM, Kleiveland CR, Lea T (2013) Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells. Lipids Health Dis 12:101. https://doi.org/10.1186/1476-511X-12-101
Camilleri M (2019) The leaky gut: mechanisms, measurement and clinical implications in humans. Gut 68:1516–1526. https://doi.org/10.1136/gutjnl-2019-318427
Matheus VA, Monteiro L, Oliveira RB et al (2017) Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Exp Biol Med Maywood NJ 242:1214–1226. https://doi.org/10.1177/1535370217708188
Gao Y, Davis B, Zhu W et al (2021) Short-chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation in vitro and in vivo. Am J Physiol Gastrointest Liver Physiol 320:G521–G530. https://doi.org/10.1152/ajpgi.00279.2020
Bach Knudsen KE, Lærke HN, Hedemann MS et al (2018) Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 10:1499. https://doi.org/10.3390/nu10101499
Wang R, Yang X, Liu J et al (2022) Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat Commun 13:2522. https://doi.org/10.1038/s41467-022-30240-8
Acknowledgements
We thank all participants and staff members involved in the LIBRE trial.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study is part of the FiberTAG project (www.fibertag.eu) which was initiated from the European Joint Programming Initiative (JPI) “A Healthy Diet for a Healthy Life” (www.healthydietforhealthylife.eu). Funding for the team from the University of Hohenheim derived from the German Federal Ministry for Education and Research (BMBF; grant no. 01EA1701). The LIBRE study was funded by the German Cancer Aid (Deutsche Krebshilfe; http://www.krebshilfe.de; grant no. 110013), Sphingotec GmbH, Senator Rösner Foundation, Marjan Miklus Foundation, and Waltraut Bergmann Foundation. The funding sources had no contribution in the design, implementation, analysis, and interpretation of the data of the FiberTAG project, the LIBRE study, and the present analysis.
Author information
Authors and Affiliations
Contributions
The authors’ contributions were as follows. MK, SCB: project administration; BS, SCB: designed research; BS, KL, MB, WV: conducted research; BS, MYD: analyzed data, performed statistical analyses, created figures; BS, SCB: wrote paper; SCB had primary responsibility for the final content. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors indicate no competing interests related to the topic of the present work.
Clinical trial registration
The trial has been registered at ClinicalTrials.gov (reference number NCT02087592).
Ethical standards
The ethics review board of the Klinikum Rechts der Isar of the Technical University of Munich approved the study protocol (reference 5686/13) which was in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The trial was registered at ClinicalTrials.gov (reference: NCT02087592).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seethaler, B., Lehnert, K., Yahiaoui-Doktor, M. et al. Omega-3 polyunsaturated fatty acids improve intestinal barrier integrity—albeit to a lesser degree than short-chain fatty acids: an exploratory analysis of the randomized controlled LIBRE trial. Eur J Nutr 62, 2779–2791 (2023). https://doi.org/10.1007/s00394-023-03172-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03172-2