Abstract
Purpose
To determine the effects of dietary sugar or carbohydrate restriction on physical activity energy expenditure, energy intake, and physiological outcomes across 24 h.
Methods
In a randomized, open-label crossover design, twenty-five healthy men (n = 10) and women (n = 15) consumed three diets over a 24-h period: moderate carbohydrate and sugar content (MODSUG = 50% carbohydrate [20% sugars], 15% protein, 35% fat); low sugar content (LOWSUG = 50% carbohydrate [< 5% sugars], 15% protein, 35% fat); and low carbohydrate content (LOWCHO = 8% carbohydrate [< 5% sugars], 15% protein, 77% fat). Postprandial metabolic responses to a prescribed breakfast (20% EI) were monitored under laboratory conditions before an ad libitum test lunch, with subsequent diet and physical activity monitoring under free-living conditions until blood sample collection the following morning.
Results
The MODSUG, LOWSUG and LOWCHO diets resulted in similar mean [95%CI] rates of both physical activity energy expenditure (771 [624, 919] vs. 677 [565, 789] vs. 802 [614, 991] kcal·d−1; p = 0.29] and energy intake (2071 [1794, 2347] vs. 2195 [1918, 2473] vs. 2194 [1890, 2498] kcal·d−1; P = 0.34), respectively. The LOWCHO condition elicited the lowest glycaemic and insulinaemic responses to breakfast (P < 0.01) but the highest 24-h increase in LDL-cholesterol concentrations (P < 0.001), with no differences between the MODSUG and LOWSUG treatments. Leptin concentrations decreased over 24-h of consuming LOWCHO relative to LOWSUG (p < 0.01).
Conclusion
When energy density is controlled for, restricting either sugar or total dietary carbohydrate does not modulate physical activity level or energy intake over a 24-h period (~ 19-h free-living) despite substantial metabolic changes.
Clinical trials registration ID
NCT03509610, https://clinicaltrials.gov/show/NCT03509610
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbohydrates are a dietary staple for many people, providing ~ 50% of energy intake in high-income countries [1,2,3]. When carbohydrate intake is restricted to the extent where endogenous carbohydrate stores become substantially depleted, liver-derived ketone bodies provide an alternative fuel for the brain and skeletal muscle [4, 5]. Achieving ketosis through dietary carbohydrate restriction is known as a ketogenic diet. Ketogenic diets have become popular as a method of reducing body mass, and there is current discourse on the mechanisms by which carbohydrate restriction may alter body mass or composition [6, 7]. Ultimately, to influence long-term body mass and composition, any nutritional intervention must influence at least one component of energy balance. Of the components of energy balance, one important but often neglected component is physical activity energy expenditure, which is causally affected by nutrition to magnitudes relevant for body composition [8, 9].
An alternative approach to restricting all carbohydrates for altering energy balance and body composition is to specifically restrict sugar intake. Indeed, meta-analysis reveals higher sugar intakes (~ 12% increase) increase energy intake by ~ 265 kcal/d [10], particularly when added sugars are ingested in sugar-sweetened beverages—a major source of sugar intake in high-income countries [11, 12]. Therefore, public health guidelines advocate restriction of added or free sugars, typically to less than 5% of total energy intake [10, 13], despite some limitations with the quality of the evidence [14, 15]. Manipulating dietary sugars, however, results in modest (< 1 kg) changes in body mass, and isoenergetic exchange of free sugars with other sources of carbohydrates does not change body mass [16]. The relatively small decreases in body mass seen with sugar restriction, when taken in concert with the more substantial reduction in energy intake, suggest that other components of energy balance (e.g., physical activity) may be compensating to erode the energy deficit. To the best of the authors knowledge, there is to date, no causal data on the role of dietary sugars on all components of energy balance, especially those most likely to respond to such an intervention like physical activity.
Prior evidence indicates potential for carbohydrate availability to alter physical activity. Alternate day fasting can decrease physical activity by > 100 kcal⋅d−1 [17] and men and women randomized to morning fasting display ~ 440 kcal⋅d−1 lower physical activity than those consuming a carbohydrate-rich breakfast [8]. These dietary stimuli have an immediate impact on physical activity—without the need for weeks of intervention to manifest—but it remains unclear if these effects are specific to restriction of total energy, carbohydrate, or sugar per se. Indeed, evidence from rodents indicates a direct role of hepatic glycogen stores in regulating physical activity [18], which implicates dietary sugars on the basis that fructose potently stimulates hepatic glycogenesis [19]. Only one study has estimated physical activity in response to ketogenic carbohydrate restriction in lean adults (n = 5), demonstrating a 20% reduction in indirectly estimated physical activity with a low-carbohydrate, ketogenic diet versus a high-carbohydrate diet [20]. Therefore, to the best of the authors knowledge, no studies to date, have directly measured the effects of ketogenic carbohydrate, or sugar restriction on physical activity energy expenditure. Accordingly, the present study investigated whether the quantity of carbohydrate and/or sugar consumed alters 24-h physical activity energy expenditure in humans. In addition, we established the acute metabolic responses to these diets and their effects on appetite and energy intake. We hypothesized that carbohydrate restriction would reduce 24-h physical activity energy expenditure compared to higher-sugar and/or higher-carbohydrate intake.
Methods
Study design and sample size
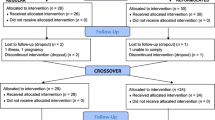
Twenty-five men and women (Table 1) participated in an open-label (participants blinded to primary outcome but not intervention), randomized, crossover design. Participants completed 1 week of habitual diet and physical activity monitoring, before completing 3 laboratory visits in a random order, during which they standardised diet for 24-h prior, then received breakfast in the laboratory with the 4-h postprandial responses measured, before they received an ad libitum lunch. Participants left the laboratory for the remainder of the day immediately following lunch, were provided with ad libitum dinner to consume outside of the laboratory, and returned the following morning. No restrictions were placed on free-living physical activity outside the laboratory, and participants did not know this was the primary outcome as confirmed using an exit questionnaire during the final visit. A schematic of the study design is presented in Fig. 1. The study was approved by the Research Ethics Approval Committee for Health at the University of Bath (EP 17/18 78) and all measures were conducted in accordance with the Declaration of Helsinki with participants providing written informed consent. The study was registered at clinicaltrials.gov (NCT03509610). Trial order randomization was completed using randomizer.org. Inclusion criteria were body mass index 18.5–29.9 kg∙m−2, age 18–65 years, and no anticipated changes in diet and physical activity during the study (e.g., holidays or diet plans). Exclusion criteria were any reported condition or behaviour that might pose undue personal risk or introduce bias, diagnosed metabolic disease (e.g. type 2 diabetes), lifestyle not conforming to standard sleep–wake cycle (e.g. shift worker), and any reported change in body mass greater than 3% in the previous 6 months [21].
The required sample size was estimated based on the Bath Breakfast Project [8] using G*Power 3.1 software [22]. Mean ± SD physical activity energy expenditure (PAEE) for the fasting vs. breakfast groups during the morning (when differences in carbohydrate availability between groups were present) were 311 ± 124 kcal vs. 492 ± 227 kcal. Based on this effect size (d = 0.998), a two-tailed matched-pairs design with 15 participants would provide > 90% chance (power) of detecting the stated effect with an α-level of 0.05. Due to a technical failure, data loss occurred for the first 10 participants for the primary outcome measure. As stated in the original protocol, a rolling recruitment to achieve the sample size of 15 for primary outcome measure (24-h physical activity energy expenditure) was continued which resulted in n = 15 for the primary outcome measure but a total sample of n = 25. The study team were unable to cannulate one participant due to small vasculature so blood samples could not be obtained. The higher sample size than initially planned enabled exploratory analyses of sex differences in response to the different diets.
Diets
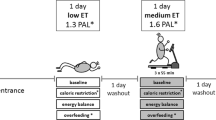
We investigated 3 diets (Fig. 2). The moderate-sugar (MODSUG) diet was designed to be reflective of macronutrient and sugar intake in European populations [1, 23], the low sugar (LOWSUG) diet was designed to meet UK public health guidelines that advocate reducing free sugar intake to < 5% of total energy intake [10, 24], and the low-carbohydrate (LOWCHO) diet was designed to restrict carbohydrate availability and promote ketogenesis, consistent with the definition of a ‘very low-carbohydrate ketogenic diet’ [25]. Estimated caloric values for each nutrient were used to calculate energy intake: carbohydrates 3.75 kcal g−1, sucrose 3.94 kcal g−1, fat 8.94 kcal g−1, protein 4.02 kcal g−1, and alcohol 6.93 kcal g−1 [26]. The UK labelling system currently requires the reporting of ‘total sugars’ rather than ‘free sugars’ [27], therefore whilst we have aimed to manipulate free sugars between the diets, it can only assumed that most of the sugars in the present study are free sugars, so we refer to ‘sugars’ throughout. A description and nutritional information of the breakfast meals given to participants to consume in full within the laboratory is provided in Table 2. A description and nutritional information of the lunch and dinner meals given to participants to consume ad libitum within the laboratory (lunch) and outside of the laboratory (dinner) is provided in Table 3. Photographs of meals provided to participants are shown in Supplemental Fig. 1. The energy content of the breakfast meal was calculated on the first laboratory visit and replicated in subsequent trials. We aimed to provide 20% of total energy requirements as a typically-representative breakfast intake [28] and factor in confinement to the laboratory during the testing phase by using measured resting metabolic rate (RMR) and measured habitual physical activity energy expenditure (PAEE). To achieve this, we estimated energy requirements by combining 8 h of resting metabolic rate (RMR) for sleep, 6 h of RMR for the laboratory component of the trial (14 h total), and combined resting and physical activity energy expenditure (PAEE) for the remainder of waking hours (10 h) using the following equation:
Lunch and dinner were ad libitum with 2000 kcal prepared for each meal. All lunch and dinner meals were prepared the evening before the trial day and refrigerated overnight and all bread was refrigerated but not frozen, as these practices influence resistant starch production and glycaemic responses to the ingested carbohydrates [29, 30]. Palatability of the breakfast and lunch meals was assessed by asking participants to strike a line through a 0–100 mm scale (0 = bad, 100 = good) shortly following ingestion of the meal. A palatability score was calculated by combining mean scores for ‘Visual Appeal’, ‘Smell’, ‘Taste’, and ‘Palatability’.
Preliminary measures
Participants attended the laboratory for eligibility screening and a treadmill walk to calibrate the physical activity monitors. Then, participants completed 7 days of habitual lifestyle monitoring, which comprised a weighed food diary and wearing a combined accelerometer and heart rate monitor to measure free-living physical activity energy expenditure (Actiheart 4™, CamNtech Ltd., UK). Participants were provided with weighing scales to weigh food items (SmartWeigh, China) and food diaries were analysed using diet analysis software (Nutritics, Ireland).
Laboratory visit standardization
Participants chose a menu aligned to the macronutrient intake of the MODSUG diet and were provided with food, weighing scales, and a physical activity monitor for 24 h leading into trial days. Participants were asked to record actual intake for 24 h leading into the first trial and replicate before this each subsequent trial, which was confirmed by writing on a printed menu. Participants were also asked to refrain from strenuous physical activity in the 24 h before each laboratory visit. Median (interquartile range) time between main visits was 13 (7–21) days for males and females using oral contraception, and 28 (28–34) days for menstruating females.
Laboratory visits
Participants arrived at the laboratory following an overnight fast (duration 11:38 ± 00:57 hh:mm). They were asked to consume a glass of water and take an inactive transport mode rather than walk or cycle to the laboratory. Anthropometric measures of height, body mass, waist circumference, and hip circumference were obtained, and body fat percentage was estimated using digital scales (Tanita, Japan). Resting metabolic rate was measured using the Douglas bag technique by averaging three 5-min gas samples, with guidelines for best practice followed [31]. Expired fractions of O2 and CO2 were determined via paramagnetic and infrared analysers (Mini HF 5200, Servomex Group Ltd., UK), respectively, and the volume expired was measured using a dry gas meter (Harvard Apparatus, UK). Inspired O2 and CO2 were measured concurrently to account for ambient fluctuations [32]. Energy expenditure and substrate oxidation in the postprandial period was calculated using stoichiometric equations [33, 34], assuming urinary nitrogen excretion was negligible.
A cannula (BD Venflon™ Pro, Becton Dickenson & Co., Sweden) was inserted into a hand vein or antecubital forearm vein if hand cannulation was unsuitable and the arm was placed in a heated box (University of Vermont, USA) set to 55 °C to arterialize venous blood [35]. Participants completed a computer-based food preference task, which consisted of 18 plates of food individually photographed on a white plate or transparent bowl. Two foods were placed side-by-side and the participant selected which food they would ‘choose to eat right now’. Foods were distinguished into three categories: sweet high-carbohydrate foods, non-sweet high-carbohydrate foods, non-sweet low-carbohydrate foods which were matched at six levels for energy density and content. The task determines relative preference for these 3 food categories at each measurement. Participants completed 0–100 mm visual analogue scales to measure appetite; marking a line through the scale relating to how hungry, full, or thirsty they were and how strong their desire for sweet, savoury, rich, or creamy food with 0 corresponding to ‘not at all’ and 100 corresponding to ‘extremely’. A baseline blood sample was obtained, and the cannula was flushed with 10 mL sterile NaCl 0.9% (B. Braun, Pennsylvania, USA) to maintain patency throughout the trial (repeated at each blood sample). Participants were provided with breakfast at 09:12 ± 00:19 hh:mm and asked to ingest the whole meal within 15 min. A timer was started upon ingestion of the first bite of breakfast and metabolic responses were measured for 4 h. Blood samples were taken at 15-min intervals for the first hour and then every 30 min thereafter. A five-minute expired gas samples was collected within the final 10 min of each hour. Visual analogue scales were repeated hourly. Immediately following the 4-h postprandial period, participants completed a second computer task for food preferences with the hand still being heated. Following this the cannula was removed and participants were served with the lunch meal. Participants remained in the bed whilst bowls of the lunch meal were served to them in ~ 500 kcal portions. They were asked to eat until they were comfortably full. Food was served at 52.8 ± 3.7 °C (mean ± SD). Bowls were replaced at random time intervals with the aim that participants did not finish a portion and so could not estimate the quantity consumed.
Participants left the laboratory following lunch and returned the following morning using a similar mode of transport. Food was provided ad libitum between laboratory visits with no constraints on free-living energy expenditure. Participants were instructed to eat only the food provided and to drink only water for the rest of the day. Upon arrival the following morning, participants completed a visual analogue scale, a food preference task, and a 5-mL arterialized venous blood sample was obtained from an antecubital vein.
Physical activity energy expenditure
Physical activity energy expenditure was measured using branched-equation modelling of heart rate and accelerometry across 24 h. Physical activity monitors were individually calibrated for each participant by using a treadmill protocol modified from Brage et al. [36]; participants attended the laboratory following a minimum 5 h fast and walked at a speed of 5.2 km h−1 for 20 min on a treadmill with incremental 5-min stages at gradients 0, 3, 6 and 9%. Expired gas samples were obtained in the final minute of each stage and analysed via indirect calorimetry to measure energy expenditure. Heart rate was obtained during the final minute of each stage using a chest-worn monitor (Polar Electro, Finland). Sleeping heart rate was measured during the 7 days of preliminary wear time. Mean resting metabolic rate from laboratory visits was entered as energy expenditure at sleeping heart rate. A linear model was fitted for energy expenditure at a range of heart rates from sleeping heart rate to the final stage of the treadmill walk and was extrapolated beyond this point for higher intensity activity. Thresholds for physical activity intensities were defined and calculated for each participant as sedentary < 1.5 METs, light ≥ 1.5–< 3.0 METs, moderate ≥ 3.0–< 6.0 METs, vigorous ≥ 6.0–< 10.2 METs, and very vigorous ≥ 10.2 METs [37, 38].
Blood sampling and analyses
Blood samples were collected into tubes containing clotting activator (Sarstedt, Germany) and left at room temperature for 15 min before being centrifuged at 4000 × g for 10 min at 4 °C. Serum was aliquoted in duplicate into sterile tubes, placed on dry ice, and stored at − 80 °C. Serum glucose, triglycerides (TAG), glycerol, non-esterified fatty acids (NEFA), lactate, beta-hydroxybutyrate (βOHB), total cholesterol, high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) concentrations were measured using an automated analyser (RX Daytona, Randox Laboratories, UK). Reported TAG values in the present paper have been blanked for glycerol based on recommendations for clinical research [39]. Serum insulin and leptin were measured using enzyme-linked immunosorbent assay (ELISA) kits (Mercodia AB, Sweden). Fibroblast growth factor 21 (FGF21) was measured using a U-plex electro-chemiluminescent kit (U-Plex, Mesoscale Discovery, USA). Incremental area under the curve (iAUC) or total area under the curve (tAUC) for postprandial responses were calculated with the trapezoid method using the Time Series Response Analyser [40]. Inter-assay coefficients of variation were < 3% for glucose, < 2% for TAG, < 6% for glycerol, < 7% for NEFA, < 3% for lactate, < 6% for βOHB, < 4% for total cholesterol, < 5% for HDL-c, < 6% for LDL-c, < 7% for insulin, < 6% for leptin, and < 3% for FGF21. All samples for a participant were measured on the same run or plate. Samples producing values below the lower limit of detection were assigned the value of the lower detectable concentration, which was necessary for some samples with βOHB, insulin, and leptin.
Statistical analyses
Descriptive statistics were calculated using Microsoft Excel (Microsoft, Washington, USA). GraphPad Prism was used for other statistical analyses and producing figures (GraphPad Software Inc., California, USA). The distribution of residuals was checked using Shapiro–Wilk tests and visual inspection of residual plots. Single-variable outcomes were analysed using one-way repeated measures ANOVA with post-hoc Bonferroni corrections applied. Outcomes with multiple time-points for each condition were analysed using two-way repeated measures ANOVA or mixed-effects models (depending on missing data points) to detect significant time, condition, or time x condition interactions, with post hoc Bonferroni corrections applied. Pearson correlation coefficients were used to assess linear associations between βOHB and NEFA, and LDL-cholesterol and NEFA across 4 and 24 h in the LOWCHO condition. The larger sample size than originally planned offered the opportunity to perform post-hoc tests exploring sex differences in physiological outcomes. Firstly, we ran two-way ANOVA to detect significant sex x condition effects for summative outcomes (iAUC and tAUC across 4 or across 24 h for outcomes with only 3 time points) and secondly, we disaggregated data by sex and ran the same analyses as the whole cohort to identify changes in interpretation compared to the whole sample. Significance was accepted at P ≤ 0.05. Data are presented as mean and 95% confidence intervals (CI) unless otherwise stated.
Results
Physical activity energy expenditure
Physical activity energy expenditure was not different between conditions (P = 0.29; Fig. 3A). When classified into physical activity intensity thresholds, there was an overall condition effect for vigorous intensity physical activity (P = 0.03) but following adjustment for multiple comparisons, no differences between conditions were apparent (all P > 0.05; Fig. 3B). Pre-trial 24-h physical activity energy expenditure was 1002 (694 to 1309) kcal prior to MODSUG, 870 (700–1040) kcal prior to LOWSUG, and 999 (815–1183) kcal prior to LOWCHO (all P > 0.05).
Physical activity energy expenditure (PAEE) across 24 h of moderate-sugar diet (MODSUG), low-sugar diet (LOWSUG), or a low-carbohydrate diet (LOWCHO) in healthy men and women. Minute-by-minute PAEE (a) and cumulative PAEE split into intensity thresholds (b). Habitual PAEE is included from the preliminary week of monitoring to display the impact of the postprandial laboratory testing. n = 15. Data expressed as mean ± 95% confidence intervals
Energy balance and nutrient intake
Energy intake at lunch, dinner, and across 24 h was similar in all conditions (Fig. 4A). By design, dietary carbohydrate intake was lower in the LOWCHO condition compared to other conditions (Fig. 4B) and dietary fat and saturated fat intake were greater in the LOWCHO condition compared to other conditions (Fig. 4E, F). Dietary sugar intake was greater in the MODSUG condition compared to other conditions (Fig. 4C). Dietary protein intake was well-matched between conditions (Fig. 4G). Dietary fibre intake was 6.2 (2.6–9.8) g higher during LOWSUG vs. MODSUG and 6.1 (2.6–9.7) g higher in LOWSUG vs. LOWCHO (Fig. 4D). Mass of food eaten was 102 (20–185) g greater at dinner and 156 (44–267) g greater across 24 h in the LOWSUG vs. MODSUG (P = 0.01 and P < 0.01, respectively) and was 111 (23–199) g greater at dinner and 123 (15–231) g greater across 24 h in the LOWSUG vs. LOWCHO (P = 0.01 and P = 0.02, respectively; Supplemental Fig. 2A). Time taken to eat breakfast was lower during LOWCHO vs. LOWSUG (P < 0.01; Supplemental Fig. 2B). Time taken to eat lunch did not differ between conditions (all P > 0.05; Supplemental Fig. 2C).
Energy and nutrient intake across 24 h of moderate-sugar diet (MODSUG), low-sugar diet (LOWSUG), or a low-carbohydrate diet (LOWCHO) in healthy men and women. Intakes of energy partitioned by meal (a), carbohydrate (b), sugars (c), fibre (d), fat (e), saturated fat (f), and protein (g). n = 25. Data expressed as mean ± 95% confidence intervals
Substrate oxidation and appetite responses
Following breakfast ingestion, energy expenditure increased (time effect, P < 0.001) in all conditions (time x condition interaction, P = 0.153; Fig. 5A). Postprandial RER was lower during LOWCHO vs. MODSUG and LOWSUG (time x condition interaction, P < 0.001; Fig. 5B). Carbohydrate oxidation rates were lower in the LOWCHO condition vs. MODSUG and LOWSUG (time x condition interaction, P < 0.001; Fig. 5C), whereas fat oxidation rates were greater (time x condition interaction, P < 0.001; Fig. 5D).
Postprandial energy expenditure (a), respiratory exchange ratio (b), carbohydrate (CHO) oxidation (c), and fat oxidation (d) responses to a moderate-sugar diet (MODSUG), low-sugar diet (LOWSUG), or a low-carbohydrate diet (LOWCHO) in healthy men and women. n = 25. Data expressed as mean ± 95% confidence intervals. Annotations: b, P < 0.05 MODSUG vs. LOWCHO; c, P < 0.05 LOWSUG vs. LOWCHO
Ratings of fullness and hunger were not different between conditions (time x condition interaction, P = 0.29; Fig. 6A). Hunger ratings initially declined following breakfast before rising to baseline (time effect, P < 0.001), rising more rapidly with MODSUG vs. LOWCHO (time x condition interaction, P = 0.034; Fig. 6B). Following 24 h of the diets, hunger ratings were lower with LOWSUG vs. LOWCHO (Fig. 6B). Thirst ratings responded similarly in all conditions (time x condition interaction, P = 0.734). There was an initial decrease in desire for sweet foods with MODSUG vs. both LOWSUG and LOWCHO (time x condition interaction, P < 0.01; Fig. 6C). In the fasted state following 24 h of the diets, desire for sweet foods was higher with LOWCHO vs. both LOWSUG and MODSUG (Fig. 6C). Ratings of desire for savoury and rich foods initially decreased following breakfast ingestion before rising (time effect, all P < 0.001; Fig. 6D, E), whereas desire for creamy foods did not demonstrate such a clear postprandial change (P = 0.057; Fig. 6F). Based on the computer task, preference for high carbohydrate savoury food was higher at lunchtime in all conditions (time effect, P < 0.001; time x condition interaction, P = 0.16; Fig. 6I), and preference for high carbohydrate sweet food was lower at lunchtime in all conditions (time effect, P < 0.001; time x condition interaction, P = 0.31; Fig. 6I). The MODSUG breakfast was rated as more palatable than LOWSUG (Fig. 6G), and the LOWSUG lunch/dinner was rated as more palatable than LOWCHO (Fig. 6H).
Postprandial and 24-h appetite and palatability responses to a moderate sugar diet (MODSUG), low-sugar diet (LOWSUG), or a low-carbohydrate diet (LOWCHO) in healthy men and women. Postprandial and 24-h visual analogue scale ratings for fullness (a), hunger (b), desire for sweet (c), desire for savoury (d), desire for rich (e), and desire for creamy (f). Palatability visual analogue scale ratings for breakfast (g) and lunch (h). Relative food preference for high-carbohydrate sweet, high-carbohydrate savoury, or low-carbohydrate savoury foods at baseline, 4 h postprandial, and after 24 h of MODSUG, LOWSUG, and LOWCHO (i). n = 25. Data expressed as mean ± 95% confidence intervals. Annotations: a, P < 0.05 MODSUG vs. LOWSUG; b, P < 0.05 MODSUG vs. LOWCHO; c, P < 0.05 LOWSUG vs. LOWCHO
Systemic metabolic and endocrine responses
Following breakfast ingestion, serum glucose, insulin and lactate concentrations rose to a greater extent with MODSUG vs. LOWSUG (time x condition interaction, both P < 0.001; Fig. 7A, C, E). In contrast, serum glucose, insulin and lactate concentrations remained essentially at fasting concentrations during LOWCHO (Fig. 7A, C, E). This resulted in higher peak serum glucose, insulin, and lactate concentrations with MODSUG vs. LOWSUG and higher with LOWSUG vs. LOWCHO, respectively (Table 4). Postprandial serum glucose and insulin iAUC were higher with MODSUG and LOWSUG vs. LOWCHO with no differences between MODSUG and LOWSUG (Fig. 7B, D). In contrast, postprandial serum lactate iAUC was higher with MODSUG vs. LOWSUG, and higher with LOWSUG vs. LOWCHO (Fig. 7F). In the fasted state 24 h following initiation of the diets, serum glucose concentrations were lower with LOWCHO vs. both MODSUG and LOWSUG (Fig. 7A).
Postprandial and 24-h responses to a moderate sugar diet (MODSUG), low-sugar diet (LOWSUG), or a low-carbohydrate diet (LOWCHO) in healthy men and women. Time course and incremental area under the curves (iAUC) of serum glucose (a, b), insulin (c, d), and lactate (e, f). n = 24. Data expressed as mean ± 95% confidence intervals. Annotations: a, P < 0.05 MODSUG vs. LOWSUG; b, P < 0.05 MODSUG vs. LOWCHO; c, P < 0.05 LOWSUG vs. LOWCHO
Following breakfast ingestion, serum TAG concentrations rose to a greater extent with LOWCHO vs. LOWSUG (time x condition interaction, P < 0.001; Fig. 8A), leading to a serum TAG iAUC which was greater with LOWCHO vs. both MODSUG and LOWSUG (P = 0.02 and P < 0.01, respectively; Fig. 8B). In the fasted state 24 h following initiation of the diets, serum TAG concentrations were lower with LOWCHO vs. both MODSUG and LOWSUG (Fig. 8A). Following breakfast ingestion, serum NEFA and glycerol concentrations both decreased to a greater extent with MODSUG and LOWSUG vs. LOWCHO (time x condition interaction, both P < 0.001; Fig. 8C, E), leading to serum NEFA and glycerol tAUCs which were greater with LOWCHO vs. both MODSUG and LOWSUG (all P < 0.001; Fig. 8D, F). Serum βOHB concentrations began to increase from 180 min following breakfast ingestion (time effect, P < 0.001) only in the LOWCHO condition (time x condition interaction, P < 0.001; Fig. 8G). This resulted in a postprandial βOHB tAUC which was greater with LOWCHO vs. both MODSUG and LOWSUG (both P < 0.001; Fig. 8H). NEFA tAUC was positively correlated with βOHB tAUC across 4- and 24-h (Supplemental Fig. 4A, C) but was not correlated with 4-h and 24-h LDL-cholesterol tAUC (Supplemental Fig. 4B, D).
Postprandial and 24-h responses to a moderate-sugar diet (MODSUG), low-sugar diet (LOWSUG), or a low-carbohydrate diet (LOWCHO) in healthy men and women. Time course and incremental area under the curves (iAUC) of serum triglycerides (a, b), non-esterified fatty acids (c, d), glycerol (e, f), and β-hydroxybutyrate (g, h). n = 24. Data expressed as mean ± 95% confidence intervals. Annotations: a, P < 0.05 MODSUG vs. LOWSUG; b, P < 0.05 MODSUG vs. LOWCHO; c, P < 0.05 LOWSUG vs. LOWCHO
Serum total cholesterol, HDL-cholesterol and LDL cholesterol concentrations all rose across the 24 h period (time effect, P = 0.002, P = 0.021, and P < 0.001, respectively; Fig. 9A–C). The increase in LDL-cholesterol concentrations was greater with LOWCHO vs. both MODSUG and LOWSUG (time x condition interaction, P < 0.001; Fig. 9C). Serum leptin concentrations displayed divergent responses between conditions such that by the end of the 24 h period, leptin concentrations were lower with LOWCHO vs. LOWSUG (time x condition interaction, P < 0.01; Fig. 9D). Following breakfast ingestion, serum FGF21 concentrations decreased in all conditions and remained lower following 24 h of LOWCHO vs. MODSUG (time x condition interaction, P < 0.001; Fig. 9E).
Serum total cholesterol (a), HDL-cholesterol (b), LDL-cholesterol (c), leptin (d) and FGF21 (e) responses to a moderate sugar diet (MODSUG), low-sugar diet (LOWSUG), or a low-carbohydrate diet (LOWCHO) in healthy men and women. n = 24. Data expressed as mean ± 95% confidence intervals. Annotations: b, P < 0.05 MODSUG vs. LOWCHO; c, P < 0.05 LOWSUG vs. LOWCHO
Sex differences
There was a trend towards higher NEFA tAUC (sex effect, P = 0.06, sex x condition interaction, P = 0.08) and βOHB tAUC (sex effect, P = 0.06, sex x condition interaction, P = 0.10) in females compared to males, but analysis of responses within each sex did not change interpretation of the findings (Fig. 10A–D). Females did not display higher LDL-cholesterol iAUC vs. males (sex effect, P = 0.11, sex x condition interaction, P = 0.28), but analysis of responses within each sex revealed the increase in LDL-cholesterol across 24-h in LOWCHO was driven primarily by females (male time x condition interaction, P = 0.12, female time x condition interaction, P < 0.001, Fig. 10E, F). Serum leptin tAUC was higher in females than males (sex effect, P < 0.001, sex x condition interaction, P = 0.008) and analysis of responses within each sex revealed the decrease in leptin across 24-h in LOWCHO was driven primarily by females (male time x condition interaction, P = 0.33, female time x condition interaction, P < 0.001, Fig. 10G, H). Serum TAG iAUC was lower in females than males (sex effect, P < 0.001, sex x condition interaction, P = 0.86), but analysis of responses within each sex did not change interpretation of the findings (Supplemental Fig. 3A, B). Serum lactate iAUC following MODSUG was lower in females than males (sex x condition interaction, P = 0.04; Supplemental Fig. 3C, D) and serum glycerol was greater in females than males (sex effect, P = 0.005, sex x condition interaction, P = 0.85). Analysis of responses within each sex revealed the decrease in FGF21 across 24-h in LOWCHO was driven primarily by females (male time x condition interaction, P = 0.14, female time x condition interaction, P = 0.008, Supplemental Fig. 3E, F). There were no main effects of sex or sex x condition interactions for other outcomes (all P > 0.05, Supplemental Fig. 3) and the interpretation of other physiological outcomes did not change when analysing data within each sex, but some time-points were changed.
Sex-disaggregated postprandial and 24-h responses to a moderate-sugar diet (MODSUG), low-sugar diet (LOWSUG), or a low-carbohydrate diet (LOWCHO) in healthy men and women. Time course of serum non-esterified fatty acids (a, b), β-hydroxybutyrate (c, d), LDL-cholesterol (e, f), and leptin (g, h). Females n = 14, males n = 10. Data expressed as mean ± 95% confidence intervals. Annotations: a, P < 0.05 MODSUG vs. LOWSUG; b, P < 0.05 MODSUG vs. LOWCHO; c, P < 0.05 LOWSUG vs. LOWCHO
Discussion
The present study reveals that total physical activity energy expenditure is not detectably altered by either restricting free sugars to less than 5% of energy intake or restricting overall carbohydrate intake to less than 8% of energy intake in the initial 24 h (~ 19 h free-living), despite distinct physiological responses with ketogenic carbohydrate restriction. Manipulating the type and/or amount of dietary carbohydrate also did not alter energy intake across 24 h. Twenty-four hours of a low-carbohydrate ketogenic diet did, however, reduce serum glucose, insulin, triglyceride, leptin, and FGF21 concentrations, and increased βOHB, NEFA, and LDL-cholesterol concentrations.
In the present study, no detectable effect of carbohydrate or sugar restriction on directly measured physical activity energy expenditure was observed in the initial 24-h of commencing the diets (of which ~ 19 h were free-living). Prior work has indicated that carbohydrate availability may alter physical activity as demonstrated by energy expenditure in response to genetically increased hepatic glycogen stores in mice [18], and lower physical activity with alternate day fasting and breakfast skipping in humans [8, 17]. Indeed, a reduction in the ratio of total energy expenditure to resting metabolic rate has been observed when people consumed a low-carbohydrate ketogenic diet versus a high-carbohydrate diet [20], indicating a potential effect on physical activity. The lack of detectable changes in directly measured physical activity with acute carbohydrate or sugar restriction in the present study indicate that the acute responses seen with intermittent fasting and breakfast skipping may be primarily driven by energy intake rather than carbohydrate intake. The reduction in indirectly measured physical activity with carbohydrate restriction that has previously been observed [20], suggests that either the potential effects of carbohydrate restriction on physical activity occur in the longer-term, and(or) that components of energy expenditure other than physical activity and resting metabolic rate are decreased with carbohydrate restriction. There is debate around the propensity for carbohydrate restriction to alter total energy expenditure, with suggestions that duration might be an important factor [41, 42]. Findings from the present study suggest physical activity is not a large contributor to potential changes in total energy expenditure in the initial 24 h, which agrees with findings from 5 days of carbohydrate-manipulated diet in overweight men [43]—although not to ketogenic levels. Furthermore, it is possible that a decrease in physical activity erodes the potential energy deficit arising from sugar restriction, consequent from adhering to government guidelines, but the present study suggests this does not occur across 24 h, providing initial support for the guidelines regarding physical activity.
The absence of any meaningful differences in physical activity energy expenditure was observed in the presence of similar 24-h energy intake between conditions. In a manner designed to enhance external validity, ad libitum intake of lunch and dinner was permitted and, despite differences in nutrient composition and palatability, 24-h energy intake did not differ between conditions. Energy density was relatively closely matched between conditions. Room calorimetry studies have shown that large differences in energy density (from 1.15 to 1.68 kcal g−1) with high-fat, low-carbohydrate versus high-carbohydrate, low-fat diets increase energy intake linearly (from 2158 kcal d−1 to 2954 kcal d−1) [44]; however, when energy density is matched, dietary macronutrient manipulation typically does not change energy intake [45]. Similarly, it has been proposed that diets high in sugar lead to increases in body mass via increased energy intake, which is thought to be explained, at least in part, by increasing dietary energy density [10]. Therefore, the lack of difference in energy intake in the present study is most likely due to the matching of energy density.
The similar energy intake and physical activity between conditions in the present study were observed in the presence of substantial changes in substrate metabolism and hormone availability linked to energy balance behaviours. Considerable decreases in FGF21 (85 pg mL−1) and leptin (3.5 ng mL−1) concentrations were observed with 24 h of carbohydrate (but not sugar) restriction, alongside markedly increased βOHB (0.35 mmol L−1). Diet-induced ketogenesis achieving βOHB concentrations ~ 0.5 mmol L−1 after 4–8 weeks are associated with increased subjective feelings of satiety and fullness and decreased hunger and desire to eat [46]; however, similar concentrations of βOHB in the present study did not translate into changes in appetite ratings after 24 h. FGF21 concentrations increase postprandially in humans with infusion or ingestion of sugars [47, 48]. Interestingly, however, carbohydrate restriction and not sugar restriction per se lowered 24-h FGF21 and glucose concentrations in the present study, and this decrease in FGF21 concentrations in the LOWCHO condition coincided with reduced fasting glucose and an increase in desire for sweet food after 24 h, consistent with evidence in mice that FGF21 administration inhibits sucrose intake via paraventricular nerve signalling [47]. Leptin is an adipokine involved in the long-term regulation of energy balance and adipose tissue mass [49]. Three days of carbohydrate restriction (from 70 to 35% of energy intake) can decrease leptin concentrations by ~ 1.5 ng mL−1 in postmenopausal women [50], and 4-weeks of ketogenic diet reduce serum leptin concentrations by ~ 1.8 ng mL−1 in men with overweight or obesity compared to an isoenergetic diet containing ~ 50% carbohydrate [51]. We observed substantial decreases in leptin concentrations of ~ 3.5 ng mL−1 after just 24 h of carbohydrate restriction, which is likely due to reduced glycaemic and(or) insulinaemic responses [52]. The lack of changes in energy intake suggests that the changes in ketone body, FGF21 and leptin availability, are insufficient to alter energy intake with carbohydrate restriction in the first 24-h of following such diets.
In the present study, carbohydrate (but not sugar) restriction lowered postprandial glucose and insulin concentrations alongside carbohydrate oxidation rates, whilst raising postprandial TAG, NEFA and glycerol concentrations alongside greater fat oxidation rates. The following morning, fasting serum TAG concentrations were markedly reduced with carbohydrate restriction. The higher immediate postprandial TAG concentrations and the lower fasting TAG concentrations the following morning are likely explained by the increased fat intake (thus greater chylomicron appearance), as well as less suppression of VLDL-TAG due to lower insulinaemia, as insulin suppresses VLDL secretion directly and indirectly [53, 54]. The lower fasting TAG concentrations the next morning likely reflects increased TAG uptake by muscle when carbohydrate availability is low. Whilst this response has been shown following 3 days of reduced-carbohydrate diet [55], here we demonstrate this response can occur within 24 h with severe carbohydrate restriction.
Serum lactate was the only metabolic effect measured, which responded specifically to sugar restriction, whereby postprandial lactate concentrations were lowered by sugar restriction, and lowered further still by total carbohydrate restriction. Whereas ingestion of any carbohydrates in sufficient amounts will stimulate carbohydrate utilization and thus can increase lactate concentrations, the higher lactate concentrations with sugar ingestion are likely to reflect hepatic fructose metabolism [56]. High fructose intake results in rapid and unregulated flux of fructose through the liver, leading to accumulation of triose phosphate, which can be converted into triglycerides, lactate and/or glucose [56]. Therefore the higher lactate concentrations observed with higher sugar intake likely reflect hepatic accumulation of triose phosphate [56], and therefore (under sedentary conditions) might be expected to stimulate de novo lipogenesis and serum triglyceride concentrations. However, while very high fructose ingestion can stimulate de novo lipogenesis and increase plasma triglyceride concentrations [57], our data demonstrate that—at least in healthy men and women, over a short time frame—typical intakes of sugar do not raise serum triglyceride concentrations when compared to very low sugar intakes.
To the best of the authors knowledge, this is the first evidence that a low-carbohydrate ketogenic diet can raise LDL-cholesterol concentrations over such a short timeframe (24 h), despite similar total energy intake between conditions. The reduced insulin concentrations and increased NEFA concentrations observed in the present study are likely to have increased VLDL production [58], and LDL-cholesterol is produced by the hydrolysis of VLDL as VLDL particles become lipid depleted [59]. Therefore, higher VLDL concentrations from increased fatty acid availability across the day is a likely explanation for the increase in LDL-cholesterol. Interestingly, the increase in LDL-cholesterol across 24 h occurred primarily in female participants. The trend towards higher NEFA concentrations in females may have provided more precursor for VLDL production [58]. However, it has also been shown that despite similar postprandial VLDL concentrations between males and females [60], females partition NEFA to βOHB (rather than to VLDL) to a greater extent than males [61]. Our data support this, as NEFA responses were positively correlated with βOHB responses across 4 and 24 h, but not with LDL-cholesterol. Furthermore, females have increased adipose tissue postprandial lipoprotein lipase activity versus males [62]. Altogether, this suggests the increased LDL-cholesterol concentrations driven by females are explained by increased LPL-mediated hydrolysis of VLDL rather than increased VLDL production.
In addition to sex-specific responses with LDL cholesterol, our data demonstrate that carbohydrate restriction causes a reduction in leptin concentrations in females but not in males. Leptin concentrations were considerably lower in male participants than females in the present study, which is consistent with previous literature and appears to be only partly explained by body fat mass [63, 64]. This was an exploratory discovery which provokes consideration of sex when investigating hormonal responses to dietary carbohydrate manipulation.
Whilst a strength of the study is providing real foods in their natural matrix, rather than meal replacements or supplements, it should be noted that the results presented are specific to the context of the foods provided, and not necessarily representative of different foods which fit the same macronutrient profile. This highlights the requirement for multiple studies to validate findings when investigating the effects of macronutrient manipulation per se. Furthermore, the laboratory confinement following the breakfast meal may have impacted the interpretation of 24-h physical activity energy expenditure by influencing a period where physical activity could have varied. Whilst this was deemed necessary for the present study, to provide context about the physiological stimuli of each diet, future studies should include measurement of physical activity in the immediate postprandial period following breakfast.
In summary, these data demonstrate that, when energy density is controlled for, the amount of dietary carbohydrate has little impact on physical activity and energy intake in the initial 24 h (~ 19 h free-living). This is despite rapid, profound changes in circulating metabolites and appetite hormones with total carbohydrate restriction. These data also demonstrate that restricting sugar intake from 20 to 5% of energy intake does not acutely alter postprandial triglyceride or LDL-cholesterol concentrations nor fasting triglyceride or LDL-cholesterol concentrations the following morning. Conversely, carbohydrate restriction lowered triglyceride concentrations after 24 h but markedly increased LDL-cholesterol concentrations. Interestingly, the largest changes in leptin and LDL-cholesterol concentrations occurred in female participants. Together, these data suggest that specifically reducing dietary sugar intake does not acutely alter energy intake or metabolic health markers, at least within the context of a healthy body mass. Since many of the hormonal responses were more clearly observed after 24 h of dietary manipulation, the longer-term effects of these diets require further examination.
References
Roberts C, Steer T, Maplethorpe N, Cox L, Meadows S, Nicholson S, Page P, Swan G (2018) National diet and nutrition survey: results from years 7 and 8 (combined) of the rolling programme (2014/2015 to 2015/2016). Public Health England, London
Shan Z, Rehm CD, Rogers G, Ruan M, Wang DD, Hu FB, Mozaffarian D, Zhang FF, Bhupathiraju SN (2019) Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999–2016. JAMA 322(12):1178–1187. https://doi.org/10.1001/jama.2019.13771
van Rossum CTM, Buurma-Rethans EJM, Dinnissen CS, Beukers MH, Brants HAM, Dekkers ALM, Ocké MC (2020) The diet of the Dutch: results of the Dutch National Food Consumption Survey 2012–2016. RIVM report 2020–2016, Bilthoven
Mikkelsen KH, Seifert T, Secher NH, Grondal T, van Hall G (2015) Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-beta-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab 100(2):636–643. https://doi.org/10.1210/jc.2014-2608
Moller N (2020) Ketone body, 3-hydroxybutyrate: minor metabolite–major medical manifestations. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa370
Hall KD, Farooqi IS, Friedman JM, Klein S, Loos RJF, Mangelsdorf DJ, O’Rahilly S, Ravussin E, Redman LM, Ryan DH, Speakman JR, Tobias DK (2022) The energy balance model of obesity: beyond calories in, calories out. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqac031
Ludwig DS, Aronne LJ, Astrup A, de Cabo R, Cantley LC, Friedman MI, Heymsfield SB, Johnson JD, King JC, Krauss RM, Lieberman DE, Taubes G, Volek JS, Westman EC, Willett WC, Yancy WS, Ebbeling CB (2021) The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqab270
Betts JA, Richardson JD, Chowdhury EA, Holman GD, Tsintzas K, Thompson D (2014) The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr 100(2):539–547. https://doi.org/10.3945/ajcn.114.083402
Kien CL, Bunn JY, Tompkins CL, Dumas JA, Crain KI, Ebenstein DB, Koves TR, Muoio DM (2013) Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am J Clin Nutr 97(4):689–697. https://doi.org/10.3945/ajcn.112.051730
SACN (2015) Carbohydrates and health. Public Health England, London
Sluik D, Engelen AI, Feskens EJ (2015) Fructose consumption in the Netherlands: the Dutch National Food Consumption Survey 2007–2010. Eur J Clin Nutr 69(4):475–481. https://doi.org/10.1038/ejcn.2014.267
Ricciuto L, Fulgoni VL 3rd, Gaine PC, Scott MO, DiFrancesco L (2021) Sources of added sugars intake among the US population: analysis by selected sociodemographic factors using the national health and nutrition examination survey 2011–18. Front Nutr 8:687643. https://doi.org/10.3389/fnut.2021.687643
World Health Organisation (2015) Guideline: sugars intake for adults and children. WHO, Geneva
Erickson J, Sadeghirad B, Lytvyn L, Slavin J, Johnston BC (2017) The scientific basis of guideline recommendations on sugar intake: a systematic review. Ann Intern Med 166(4):257–267. https://doi.org/10.7326/M16-2020
Buyken AE, Mela DJ, Dussort P, Johnson IT, Macdonald IA, Stowell JD, Brouns F (2018) Dietary carbohydrates: a review of international recommendations and the methods used to derive them. Eur J Clin Nutr 72(12):1625–1643. https://doi.org/10.1038/s41430-017-0035-4
Morenga LT, Mallard S, Mann J (2013) Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. Bmj Brit Med J. https://doi.org/10.1136/bmj.e7492
Templeman I, Smith HA, Chowdhury E, Chen YC, Carroll H, Johnson-Bonson D, Hengist A, Smith R, Creighton J, Clayton D, Varley I, Karagounis LG, Wilhelmsen A, Tsintzas K, Reeves S, Walhin JP, Gonzalez JT, Thompson D, Betts JA (2021) A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abd8034
Lopez-Soldado I, Fuentes-Romero R, Duran J, Guinovart JJ (2017) Effects of hepatic glycogen on food intake and glucose homeostasis are mediated by the vagus nerve in mice. Diabetologia 60(6):1076–1083. https://doi.org/10.1007/s00125-017-4240-4
Fuchs CJ, Gonzalez JT, Beelen M, Cermak NM, Smith FE, Thelwall PE, Taylor R, Trenell MI, Stevenson EJ (1985) van Loon LJ (2016) sucrose ingestion after exhaustive exercise accelerates liver, but not muscle glycogen repletion compared with glucose ingestion in trained athletes. J Appl Physiol 120(11):1328–1334. https://doi.org/10.1152/japplphysiol.01023.2015
Bandini LG, Schoeller DA, Dietz WH (1994) Metabolic differences in response to a high-fat vs. a high-carbohydrate diet. Obes Res 2(4):348–354. https://doi.org/10.1002/j.1550-8528.1994.tb00074.x
Stevens J, Truesdale KP, McClain JE, Cai J (2006) The definition of weight maintenance. Int J Obes (Lond) 30(3):391–399. https://doi.org/10.1038/sj.ijo.0803175
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Method 39(2):175–191
Wittekind A, Walton J (2014) Worldwide trends in dietary sugars intake. Nutr Res Rev 27(2):330–345. https://doi.org/10.1017/S0954422414000237
U.S. Department of Agriculture USDoHaHS (2015) Dietary guidelines for Americans, 2015–2020. US Government Printing Office, Washington DC
Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, Accurso A, Frassetto L, Gower BA, McFarlane SI, Nielsen JV, Krarup T, Saslow L, Roth KS, Vernon MC, Volek JS, Wilshire GB, Dahlqvist A, Sundberg R, Childers A, Morrison K, Manninen AH, Dashti HM, Wood RJ, Wortman J, Worm N (2015) Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 31(1):1–13. https://doi.org/10.1016/j.nut.2014.06.011
Elia M, Cummings JH (2007) Physiological aspects of energy metabolism and gastrointestinal effects of carbohydrates. Eur J Clin Nutr 61(Suppl 1):S40-74. https://doi.org/10.1038/sj.ejcn.1602938
Gibson-Moore H, Spiro A (2021) Evolution not revolution–what might the future hold for front-of-pack nutrition labelling in the UK?: A British nutrition foundation roundtable. Nutr Bull 46(3):383–394. https://doi.org/10.1111/nbu.12517
Gaal S, Kerr MA, Ward M, McNulty H, Livingstone MBE (2018) Breakfast consumption in the UK: patterns, nutrient intake and diet quality a study from the international breakfast research initiative group. Nutrients. https://doi.org/10.3390/nu10080999
Robertson TM, Brown JE, Fielding BA, Hovorka R, Robertson MD (2021) Resistant starch production and glucose release from pre-prepared chilled food: the SPUD project. Nutr Bull 46(1):52–59. https://doi.org/10.1111/nbu.12476
Robertson TM, Brown JE, Fielding BA, Robertson MD (2021) The cumulative effects of chilling and reheating a carbohydrate-based pasta meal on the postprandial glycaemic response: a pilot study. Eur J Clin Nutr 75(3):570–572. https://doi.org/10.1038/s41430-020-00736-x
Evidence Analysis Working G, Compher C, Frankenfield D, Keim N, Roth-Yousey L (2006) Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 106(6):881–903. https://doi.org/10.1016/j.jada.2006.02.009
Betts JA, Thompson D (2012) Thinking outside the bag (not necessarily outside the lab). Med sci sports exerc 44(10):2040. https://doi.org/10.1249/MSS.0b013e318264526f
Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiology 55(2):628–634
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26(Suppl 1):S28-37. https://doi.org/10.1055/s-2004-830512
Edinburgh RM, Hengist A, Smith HA, Betts JA, Thompson D, Walhin JP, Gonzalez JT (2017) Prior exercise alters the difference between arterialised and venous glycaemia: implications for blood sampling procedures. Br J Nutr 117(10):1414–1421. https://doi.org/10.1017/S0007114517001362
Brage S, Ekelund U, Brage N, Hennings MA, Froberg K, Franks PW (1985) Wareham NJ (2007) Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J Appl Physiol 103(2):682–692. https://doi.org/10.1152/japplphysiol.00092.2006
College of Sports M American Heart A, Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116(9):1081–1093. https://doi.org/10.1161/CIRCULATIONAHA.107.18
Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SFM, Altenburg TM, Chinapaw MJM, Participants STCP (2017) Sedentary behavior research network (sbrn)–terminology consensus project process and outcome. Int J Behav Nutr Phys Act 14(1):75. https://doi.org/10.1186/s12966-017-0525-8
Cole TG (1990) Glycerol blanking in triglyceride assays: is it necessary? Clin Chem 36(7):1267–1268
Narang BJ, Atkinson G, Gonzalez JT, Betts JA (2020) A tool to explore discrete-time data: the time series response analyser. Int J Sport Nutr Exerc Metab 30(5):374–381. https://doi.org/10.1123/ijsnem.2020-0150
Hall KD, Guo J (2017) Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology 152(7):1718–1727
Ludwig DS, Dickinson SL, Henschel B, Ebbeling CB, Allison DB (2021) Do lower-carbohydrate diets increase total energy expenditure? An updated and reanalyzed meta-analysis of 29 controlled-feeding studies. J Nutr 151(3):482–490. https://doi.org/10.1093/jn/nxaa350
Parr EB, Devlin BL, Callahan MJ, Radford BE, Blankenship JM, Dunstan DW, Hawley JA (2018) Effects of providing high-fat versus high-carbohydrate meals on daily and postprandial physical activity and glucose patterns: a randomised controlled trial. Nutrients. https://doi.org/10.3390/nu10050557
Stubbs RJ, Ritz P, Coward WA, Prentice AM (1995) Covert manipulation of the ratio of dietary fat to carbohydrate and energy density: effect on food intake and energy balance in free-living men eating ad libitum. Am J Clin Nutr 62(2):330–337. https://doi.org/10.1093/ajcn/62.2.330
Stubbs RJ, Harbron CG, Prentice AM (1996) Covert manipulation of the dietary fat to carbohydrate ratio of isoenergetically dense diets: effect on food intake in feeding men ad libitum. Int J Obes Relat Metab Disord 20(7):651–660
Gibson AA, Seimon RV, Lee CM, Ayre J, Franklin J, Markovic TP, Caterson ID, Sainsbury A (2015) Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes Rev 16(1):64–76. https://doi.org/10.1111/obr.12230
von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, Potthoff MJ (2016) FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab 23(2):335–343. https://doi.org/10.1016/j.cmet.2015.12.003
Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E (2015) Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab 4(1):51–57. https://doi.org/10.1016/j.molmet.2014.09.008
Friedman JM (2019) Leptin and the endocrine control of energy balance. Nat Metab 1(8):754–764. https://doi.org/10.1038/s42255-019-0095-y
Koutsari C, Karpe F, Humphreys SM, Frayn KN, Hardman AE (2003) Plasma leptin is influenced by diet composition and exercise. Int J Obes Relat Metab Disord 27(8):901–906. https://doi.org/10.1038/sj.ijo.0802322
Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT, Ravussin E (2016) Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr 104(2):324–333. https://doi.org/10.3945/ajcn.116.133561
Sonnenberg GE, Krakower GR, Hoffmann RG, Maas DL, Hennes MM, Kissebah AH (2001) Plasma leptin concentrations during extended fasting and graded glucose infusions: relationships with changes in glucose, insulin, and FFA. J Clin Endocrinol Metab 86(10):4895–4900. https://doi.org/10.1210/jcem.86.10.7951
Jensen MD, Caruso M, Heiling V, Miles JM (1989) Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 38(12):1595–1601. https://doi.org/10.2337/diab.38.12.1595
Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G (1995) Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest 95(1):158–166. https://doi.org/10.1172/JCI117633
Culling KS, Neil HA, Gilbert M, Frayn KN (2009) Effects of short-term low- and high-carbohydrate diets on postprandial metabolism in non-diabetic and diabetic subjects. Nutr Metab Cardiovasc Dis 19(5):345–351. https://doi.org/10.1016/j.numecd.2007.09.003
Hengist A, Koumanov F, Gonzalez JT (2019) Fructose and metabolic health: governed by hepatic glycogen status? J Physiol. https://doi.org/10.1113/JP277767
Chong MF, Fielding BA, Frayn KN (2007) Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 85(6):1511–1520. https://doi.org/10.1093/ajcn/85.6.1511
Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G (1995) Interaction between free fatty-acids and insulin in the acute control of very-low-density lipoprotein production in humans. J Clin Investig 95(1):158–166. https://doi.org/10.1172/Jci117633
Jackson RL, Morrisett JD, Gotto AM Jr (1976) Lipoprotein structure and metabolism. Physiol Rev 56(2):259–316. https://doi.org/10.1152/physrev.1976.56.2.259
Hodson L, Bickerton AST, McQuaid SE, Roberts R, Karpe F, Frayn KN, Fielding BA (2007) The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitive men and women-studies in the postprandial state. Diabetes 56(10):2433–2441. https://doi.org/10.2337/db07-0654
Marinou K, Adiels M, Hodson L, Frayn KN, Karpe F, Fielding BA (2011) Young women partition fatty acids towards ketone body production rather than VLDL-TAG synthesis, compared with young men. Brit J Nutr 105(6):857–865. https://doi.org/10.1017/S0007114510004472
Votruba SB, Jensen MD (2006) Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab 291(5):E1115–E1123. https://doi.org/10.1152/ajpendo.00196.2006
Couillard C, Mauriege P, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Despres JP (1997) Plasma leptin concentrations: gender differences and associations with metabolic risk factors for cardiovascular disease. Diabetologia 40(10):1178–1184. https://doi.org/10.1007/s001250050804
Hellstrom L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P (2000) Mechanisms behind gender differences in circulating leptin levels. J Intern Med 247(4):457–462. https://doi.org/10.1046/j.1365-2796.2000.00678.x
Acknowledgements
The authors thank the participants for their time and commitment to the study. The authors also thank Isobel Townsend, Drusus Johnson-Bonson, Matthew Farrow, Robert Edinburgh, and Oliver Chrzanowski-Smith for their help on occasional trial days. The authors would also like to thank William Trim and Jariya Buniam for occasional assistance with biochemical analysis. J.T.G, A.H., P.J.R., J.M.B., L.J.C.v.L., F.K., D.T., and J.A.B. designed the research. A.H., J.T.G, R.G.D., J-.P.W., F.K., and J.A.B. conducted the research. P.J.R., and J.M.B., provided essential reagents or materials. A.H., and J.T.G analysed data, performed statistical analysis, and wrote the initial draft of the manuscript. All authors contributed to manuscript editing. J.T.G had primary responsibility for the final content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding and conflict of interest
This work was funded by The Rank Prize Funds and Cosun Nutrition Center. A.H. and R.G.D have received funding from The Rank Prize Funds, and The University of Bath. P.J.R. has received funding for research from Sugar Nutrition UK, provided consultancy services for Coca-Cola Great Britain, and received speaker's fees from the Global Stevia Research Institute, ILSI-Brasil, ILSI-Europe, ILSI-India and the International Sweeteners Association, and other support from industry for travel expenses for workshops and conferences where he presented research on sugar and low-calorie sweeteners J.M.B., D.T., and F.K. are investigators on research grants funded by BBSRC and MRC. L.J.C.vL. has received research grants, consulting fees, speaking honoraria, or a combination of these for research on the impact of exercise and nutrition on muscle metabolism, which include funding from Nestlé, Knowledge centre sugar & nutrition, and Pepsico. A full overview on research funding is provided at: https://www.maastrichtuniversity.nl/l.vanloon. J.A.B. is an investigator on research grants funded by BBSRC, MRC, British Heart Foundation, Rare Disease Foundation, EU Hydration Institute, GlaxoSmithKline, Nestlé, Lucozade Ribena Suntory, ARLA foods and Cosun Nutrition Center; has completed paid consultancy for PepsiCo, Kellogg’s and SVGC; receives an annual stipend as Editor-in Chief of International Journal of Sport Nutrition & Exercise Metabolism; and receives an annual honorarium as a member of the academic advisory board for the International Olympic Committee Diploma in Sports Nutrition. J.T.G. is an investigator on research grants funded by BBSRC, MRC, British Heart Foundation, The Rank Prize Funds, The European Society for Clinical Nutrition and Metabolism (ESPEN), Lucozade Ribena Suntory, ARLA Foods Ingredients and Cosun Nutrition Center; and has completed paid consultancy for PepsiCo and SVGC.
Additional information
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction at https://researchdata.bath.ac.uk.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hengist, A., Davies, R.G., Rogers, P.J. et al. Restricting sugar or carbohydrate intake does not impact physical activity level or energy intake over 24 h despite changes in substrate use: a randomised crossover study in healthy men and women. Eur J Nutr 62, 921–940 (2023). https://doi.org/10.1007/s00394-022-03048-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03048-x