Abstract
Purpose
With the aim of characterizing the gastrointestinal (GI) microbiota and contextually determine how different prenatal, perinatal, and postnatal factors affected its composition in early childhood, infants were enrolled in a longitudinal-prospective study named “A.MA.MI.” (Alimentazione MAmma e bambino nei primi MIlle giorni; NCT04122612, October 2019).
Methods
Forty-five fecal samples were collected at 12 months of infants’ age, identified as the 3rd follow-up (T3). The evaluated variables were pre-gestational weight and weight gain during pregnancy, delivery mode, feeding, timing of weaning, and presence/absence of older siblings. Fecal alpha and beta-diversities were analyzed. Noteworthy, to determine the impact of the influencing factors, multivariate analyses were conducted.
Results
At T3, all prenatal and perinatal variables did not result to be significant whereas, among the postnatal variables, type of milk-feeding and weaning showed the greatest contribution in shaping the microbiota. Although aged 1 year, infants exclusively breastfed until 6 months were mainly colonized by Lactobacillaceae and Enterobacteriaceae. Differently, Bacteroidaceae characterized the microbiota of infants that were never breastfed in an exclusive way. Moreover, although an early introduction of solid foods determined higher values of Faith’s PD, high abundances of Ruminococcaceae and Faecalibacterium mainly associated with infants weaned after the 4th month of age.
Conclusion
The microbial colonization during the first year of life is likely affected by a simultaneous effect of multiple variables playing a significant role at different times. Therefore, these data contribute to add evidence concerning the complex multifactorial interaction between GI microbiota and various stimuli affecting infants during the early stages of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growing interest in symbiotic microbes inhabiting humans is paired with the increasing knowledge about their great genomic encoding power. From birth, newborns are daily exposed to a large number of influencing factors, rapidly occurring without any interruption, many of which are known to work as drivers for microbial adaptations into the intestinal environment [1]. Perturbations in gut microbiota structure, composition, or activity, broadly defined by the term dysbiosis, are believed to increase the risk of several diseases including metabolic disorders, autoimmune and allergic diseases, bacterial infections, and cancer [2].
It is generally accepted that the human gut is not microbiologically sterile at birth; in fact, the bacterial colonization is a multifaceted process that begins in utero [3, 4] and is influenced by both delivery mode (i.e., cesarean section and vaginally) and feeding (i.e., breast milk, formula, solid foods introduction) [5]. In infancy, evidence suggested that the replacement of breastmilk with formula milk affects the relative abundance of some and specific early gut colonizers, particularly Bacteroides and Bifidobacterium [5, 6]. The presence of oligosaccharides in human milk (hMO) promotes distinct shifts in microbiota composition and metabolism affecting the overall gut development [7]. Noticeably, the gut microbiome undergoes an essential shift to an “adult-type” when solid foods start to be introduced during weaning [8, 9]. The complete transition from an exclusively milk-based diet to a solid food diet is pivotal for microbiota evolution. This depends on the introduction of foods accounting for changes in macronutrient intakes (proteins, fats, carbohydrates, and fibers) different than milk [10]. During this transition phase, based on the ability in metabolizing hMO, the microbiota shifts from a simple community enriched in Bifidobacterium to a much more complex polymicrobial system, able to metabolize starch and other substrates [11].
The association between timing of solid food introduction and childhood overweight/obesity is still under debate [12,13,14]. Nonetheless, some authors suggested that a solid food introduction at the fourth month, or earlier, may increase the risk of childhood obesity [15, 16], whereas some others indicated the food composition, rather than the timing of food introduction, to be related with the highest risk of develop such pathologies [17, 18]. High intakes of energy, simple sugars, and animal proteins in infancy could be associated with an early increase in body weight and fat deposition [17].
During earlier life stages, other peculiar factors may impact the gut microbiota composition. Inside the infants’s family context, some other factors such as the biodiversity in the house and in the neighborhood, the exposures to family members and with other members in close and continuous contact with the newborn, particularly the presence of older siblings, and the overall hygienic practices need to be taken into account [19].
However, although all the mentioned variables demonstrated to have a huge impact in shaping the infant gut microbiota, nutrition remains the most important one in modulating both the composition and the metabolic activity and, at the same time, resulted to be pivotal in maintaining the state of health in future steps of the child growth.
Therefore, the purpose of the present study was to investigate the role of prenatal, perinatal, and postnatal factors, with a particular focus on nutrition, in shaping microbial ratios by determining the presence/absence of specific bacterial taxa in gut microbiota of 1-year-aged children.
Materials and methods
Study design
The present study is part of the longitudinal-prospective study named A.MA.MI (Alimentazione MAmma e bambino nei primi MIlle giorni), ClinicalTrials.gov identifier: NCT04122612. The study was approved by the Human Ethics Committee (EC) of Fondazione IRCCS Policlinico S. Matteo of Pavia (Protocol number: 20180022618; 6/12/2018) and it was conducted on a group of mother-infant pairs (Supplementary Table S1) referred to the Neonatal Unit of the Fondazione IRCCS Policlinico San Matteo, Pavia (Italy) from birth to 1 year of age, according to the Good Clinical Practice guidelines. Written informed consent of the parents/legal guardian was provided. The Human EC of Fondazione IRCCS Policlinico S. Matteo of Pavia approved this procedure after ascertaining its compliance with the dictates of the Declaration of Helsinki (IV Adaptation). The complete study design and the study protocol were previously detailed [20].
Herein, a total of 45 fecal samples were collected and analyzed at 12 months of infants’ age, corresponding to the 3rd sampling time (T3) of the A.MA.MI project. To evaluate variables influencing the early gut microbiota colonization, we investigated maternal factors, such as pre-pregnancy body mass index (BMI) and gestational weight gain (WG), perinatal factors (i.e., type of delivery), and postnatal variables, such as type of feeding, timing of introduction of complementary foods, and environmental influences including the presence of older siblings in the household.
To reduce bias, the administration of antibiotics, probiotics, or prebiotics in the last 3 months before the delivery of the fecal samples was considered among exclusion criteria.
The methodology applied to stratify the anthropometric data (maternal BMI and gestational WG) has been previously detailed [20].
Briefly, maternal anthropometric data, likely height and pre-gestational weight, were used to calculate body mass index (BMI; kg/m2). Based on pre-gestational BMI, women were then stratified as Normal Weight (NW: BMI ≤ 24.9 kg/m2) or Overweight/with Obesity (OW: BMI ≥ 25 kg/m2). The first group outnumbered 35 samples, whereas the latter 10 samples. The gestational weight gain (WG), that is the body weight increase from pre-pregnancy to delivery, was compared with recommended ranges of WG by IOM guidelines, which are 11.5–16 kg for NW, 7–11.5 kg for OW, and 5–9 kg for OB mothers. This determined a stratification of samples in two groups, which were normal gestational WG (NWG, that included 28 samples) or excessive gestational WG (EWG, that included 17 samples). The collection of infants’ delivery mode determined that the group of vaginally delivered (VD) was composed of 40 samples, whereas those born via cesarean section (CS) were 5 samples. The type of milk-based feeding was stratified in exclusively breastfed (EBF; n = 27) against infants not following an exclusive breastfeeding (NeBF; n = 18). To note that the latter group included both exclusively formula-fed and infants following a mixed milk-feeding (breast and formula). The time weaning was stratified based on the solid food introduction, if before or after 4 months of age (named ≤ 4 or > 4, respectively) and outnumbering 34 vs 11 samples, respectively. A presence of older siblings was collected for 21 infants (nFB), while 24 samples belonged to firstborns (FB).
In a second step, infants were split in a further subgrouping based on both type and duration of milk-feeding. Specifically, 16 samples belonged to infants aged 1 year that were exclusively breastfed till 6 months of age (EBF6m); 16 samples belonged to infants aged 1 year that were fed with breast milk at least once/day up to 1 year of age (ut1Y); 13 samples belonged to infants aged 1 year that never followed an exclusively breastfeeding (neverEBF).
Questionnaires and interviews
At T3, concomitantly to sample delivery, mothers/parents were interviewed by trained personnel of the research group to investigate the dietary habits in terms of diversity and frequency of food groups consumed by the infant at 1 year of age, by means of a previously validated questionnaire [21], re-adapted according to our new cohort [22]. Briefly, the questionnaire was composed of two sections, the first of which investigates the infant feeding during the first year of life, while the second is composed of a Food Frequency questionnaire (FFQ) useful in the assessment over the past 28 days. Survey questions were formulated in each one of sections, respecting fixed categories: always, often, sometimes, never. According to the National Dietary Guidelines [23, 24], the score assigned to each response ranges from 0 to 3, with the maximum value assigned to the healthiest one.
Sample processing for gut microbiota analysis
After the collection, the fecal samples were stored at the Neonatal Intensive Care Unit of Fondazione IRCCS Policlinico San Matteo (Pavia, Italy) at − 80 °C until the processing started. Samples were then shipped, on dry ice, to Genomix4Life Srl (C/O Laboratory of Molecular and Genomic Medicine—Campus of Medicine and Surgery, Baronissi, Salerno, Italy, a spin-off of the University of Salerno, Fisciano, Italy) where 16S rDNA gene amplicon analysis was carried out. Total DNA was extracted from stool with the Invimag Stool kit (Stratec Molecular GmbH, Berlin, Germany) following the manufacturer's instructions and using sterilized distilled water as extraction negative control. To guarantee privacy, no clinical or personal information was included, with the only exception of the ID number assigned to samples. The 16S rDNA gene amplification was performed with primers: Forward 5′-CCTACGGGNGGCWGCAG-3′ and Reverse 5′-GACTACHVGGGTATCTAATCC-3′, which targeted the hypervariable V3-V4 regions of the 16S rDNA gene. Followed protocols useful to obtain raw sequence files (fast files) and filter them according to a quality control check (FastQC) were previously detailed [25]. The 16S rDNA metabarcoding analysis was carried out by QIIME2, and the SILVA 138 taxonomic database used to define the bacterial taxonomy.
Phylogenetic investigation of communities by reconstruction of unobserved states
The PICRUSt was carried out as previously described [26]. Per sample, the metabolic pathway predictions from 16S rRNA marker gene data were obtained using the ‘Phylogenetic Investigation of Communities by Reconstruction of Unobserved States’ Picrust2 software, that was run as a plugin within the QIIME2 library. Per sample MetaCyc pathway abundances were used as input for a two-side Welch test between groups.
Statistical analyses
Data were summarized using descriptive statistics, such as means and standard deviations (± SD) or median with interquartile range (IQR), as appropriate, for quantitative variables and relative frequencies for qualitative ones. Alpha diversity indices were compared according to a two-tailed Mann–Whitney test computing the exact p values (qval or exact pval) after correction for multiple comparisons using GraphPad Prism version 8.0.1 (GraphPad Software, San Diego, California, USA). As previously adopted [25], the multivariable association between 16S rDNA-seq abundances and variables (metadata) was performed using the MaAsLin2 R package (https://huttenhower.sph.harvard.edu/maaslin/; accessed 27 Sept 2021), which allows to investigate metadata as fixed or random effects (Tables 1 and 2, respectively).
A principal component analysis (PCA) was carried out to evaluate the beta diversity occurring between samples after a specific stratification of our cohort, based on time and duration of breastfeeding. The dudi.pca function within the “ade4” R package (https://cran.r-project.org/web/packages/ade4/; accessed 27 Sept 2021) was used to perform a PCA of data frames. The resulting PCA and dudi class objects were plotted with the “factoextra” R package (https://cran.r-project.org/web/packages/factoextra/index.html; accessed 27 Sept 2021). The PICRUSt2 data were analyzed following the Mann–Whitney U test and applying a Sidak-Bonferroni correction for multiple comparisons. For all statistical analyses, the p values < 0.05 were noticed, even if the significance was only reached for corrected p values (reported as qval or exact pval) < 0.05.
Results
Questionnaires
The analysis of FFQ administered to the respective parents showed that almost all children had dietary habits in line with the recommendations given by the National Dietary Guidelines for a healthy diet [23, 24]. The only food category with a consumption lower than the recommended value was ‘beans’ first and foremost classified as a legume. The consumption of legume was screened as a part of the protein-based food group comprising fish, poultry, meat, and eggs. In detail, only 18% of children weekly consumed legume at least 3 times (Supplementary Table S2). Nonetheless, in our study was not possible to evaluate the energy and the protein intake, necessary to assess adherence to a healthy dietary pattern according to the National Dietary Guidelines above mentioned.
Gut microbiota analysis
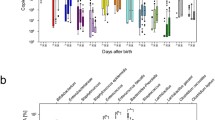
The amplicon 16S rRNA sequencing analysis was performed on the 45 fecal samples collected at T3 and determined 88,253.38 ± 17,055.61 (mean ± SD) reads assigned taxonomy that passed the quality check (QC) filter per sample. Of these, 96.86 ± 3.51% (mean ± SD) was assigned at least at the genus level. To investigate factors influencing the early microbiota colonization during the first year of life of our set of infants, samples were grouped according to different variables (metadata). Specifically, maternal pre-pregnancy BMI and maternal gestational WG have been collected as prenatal variables, while the type of delivery was used as the perinatal determinant. Moreover, the type of feeding, the time of weaning, and the presence/absence of older siblings in the household have been collected as postnatal factors. To describe the alpha diversity characterizing our fecal samples, the Faith’s phylogenetic diversity (Faith’s PD) index and Shannon’s index have been used. Concerning the number of OTU (Fig. 1A), no significant differences were found. Evaluating the Faith’s PD index (Fig. 1B), a close significance was found for the type of milk-feeding. As a matter of the fact, although results were collected from 1-year-aged infants, results deriving from those exclusively breastfed for the first six months of life showed lower but not significant (exact pval = 0.054) indices than peers that took formula at least one/day for the first 6 months after birth. Hence, the only significant difference was found with respect to the Faith’s PD index and concerned infants weaned preterm (before 4 months of age, ≤ 4) compared to those that started to introduce solid foods after the 4th month (exact pval = 0.022). Nonetheless, the comparison of the same pair did not result to be statistically significant when we evaluated the Shannon’s values (Fig. 1C).
Alpha diversity. The number of operational taxonomic units (OTU), the Faith’s phylogenetic diversity index, and the Shannon’s diversity index (respectively A, B, and C panel) were used to describe the alpha diversity in fecal samples of infants at 1 year of age grouped according to different pre-, peri-, and postnatal variables (metadata). In detail, used metadata were: (i) maternal pre-pregnancy BMI (blue boxplots) within recommended values (NW: BMI < 25 kg/m2) or higher (OW: BMI ≥ 25 kg/m2); (ii) gestational WG (red boxplots), if optimal (NWG) or excessive (EWG) according to IOM reference values; (iii) type of delivery (green boxplots), if cesarean section (CS) or vaginally delivered infants (VD); (iv) type of feeding (pink boxplots), according to an exclusively breastfed for the first 6 months of age (EBF) or not (including both mixed and exclusively formula-fed, NeBF); (v) milk-feed up to the year of age (purple boxplots), based on infant that took at least one breastfeed up to the year of age (UT1Y), infants exclusively breastfed till 6 months of age (EBF 6 m), and infants that never were exclusively breastfed (neverEBF); (vi) time of weaning (orange boxplots), if solid foods have been given before (≤ 4) the 4th month of age or after (> 4); (vii) absence/presence of older siblings in the household (FB and nFB, respectively; black boxplots). Reporting (*), it means a significant difference (as exact p value < 0.05) according to the two-tailed Mann–Whitney’s test corrected for multiple comparisons
Factors affecting early microbial colonization of infant gut microbiota
Maternal pre-pregnancy BMI (NW vs OW) and the presence/absence of older siblings in the household (FB vs nFB) were variables that did not report significant differences (Table 1).
Only slight differences were found in Actinobacteria and Firmicutes abundances when maternal gestational WG was evaluated. In fact, both phyla did not reach significance after correction for multiple comparisons (qval > 0.05). MaAsLin2 analysis showed that Actinobacteria seemed to be more associated with an EWG (pval = 0.021) while Firmicutes were more correlated to NWG (pval = 0.034). No other differences were detected at deeper taxonomic levels, specifically at the family and genus levels, when EWG was compared vs NWG.
When delivery modes were compared, Pasteurellaceae were found to be slightly higher in infants born via cesarean section (CS; pval = 0.011) than infants vaginally delivered. However, also in this case, the adjusted p value was not significant (qval > 0.05).
Concerning the feeding, 1-year-aged infants exclusively breastfed during the first 6 months of life (EBF) were poorly colonized by Acidaminococcaceae (pval = 0.019, qval > 0.05). Differently, Lactobacillaceae mainly colonize the microbiota in EBF (pval = 0.02, qval > 0.05). To conduct a deeper investigation, infants were further split by merging type and duration of breastfeeding. Hence, three different groups were obtained as previously detailed: (i) infants who were once breastfed at least day up to 1 year of age (ut1Y), (ii) never exclusively breastfed (neverEBF), and (iii) infants who were exclusively breastfed up to 6 months of age (EBF6m). The intergroup comparison of neverEBF with both the other two groups revealed a gut microbiota characterized by a lower abundance of Firmicutes and mainly colonized by Bacteroidetes (Table 1; pval ≤ 0.028, qval > 0.05). In line with the relative abundance of Bacteroidetes, a positive association between neverEBF and Bacteroidaceae (and Bacteroides at genus level; pval = 0.005, qval > 0.05) was found. Differently, Lactobacillaceae and Enterobacteriaceae showed a negative association with neverEBF (pval = 0.049 and 0.031, respectively). Nonetheless, the above-reported differences were not confirmed after the p value adjustment (qval > 0.05).
To investigate the impact of diet-related factors, weaning was also evaluated. Infants grouped in early-weaned (≤ 4 months of age) or normal ones (> 4 months) did not show differences at phylum level. At deeper taxonomic levels, infants who were weaned after the 4th month of age showed a significant increase of Ruminococcaceae (pval = 0.0001) and Faecalibacterium (pval < 0.0001, qval ≤ 0.003; Fig. 2). The GI microbiota of infants early weaned (≤ 4 months) was enriched in Veillonellaceae (pval = 0.015) but also in this case the significance was lost after adjusting for multiple comparisons (qval > 0.05).
Therefore, to evaluate the significant multivariable association between our infants and all their relative metadata, we ran the MaAsLin2 R package by adopting a random effect for prenatal and perinatal variables. This comparison did not show significant results (Table 2). At the same, this approach was adopted for postnatal factors revealing how all the three variables (feeding, weaning, and the presence of older siblings) showed at least a tendency to drive specific bacterial taxa. In detail, in absence of older siblings (reported as firstborn, FB) infants reported an increased abundance of “Other” phyla (that is, phyla with a relative abundance less than 0.1% within all fecal samples) and Proteobacteria (pval < 0.05, qval > 0.05). At family level, the presence of older siblings likely favored the abundance of Streptococcaceae (pval < 0.016), while the exclusive breastfeeding group harbored Veillonellaceae, Lactobacillaceae, Enterobacteriaceae (pval ≤ 0.04) and a decreased abundance of Bacteroidaceae (pval = 0.035). However, all the above-mentioned results, including the high abundance of Veillonellaceae in preterm weaned infants (pval = 0.002), did not result significantly after multiple comparison correction. Differently, Ruminococcaceae and Faecalibacterium showed a significant association with infants weaned after the 4th month of age (pval < 0.001, qval ≤ 0.03). The latter taxon was statistically significant also when all collected metadata (prenatal, perinatal, and postnatal) have been investigated under a combined form, confirming the strict association of Faecalibacterium with infants weaned after the 4th month of age (pval < 0.001, qval = 0.046).
Principal component analysis (PCA) of breast milk consumption until the first year of life
On the basis of the obtained results for the breastfed child group, we further assessed whether those bacterial families that reported a p value < 0.05, were able to discriminate samples based on the duration of breastfeeding. With this purpose, we used the previous subgrouping of infants in EBF6m, neverEBF, and ut1Y.
Looking at the PCA plot (Fig. 3), more than 84% of the variance was described by summing the two principal components (PC1 and PC2). Infants labeled as neverEBF were mainly colonized by Bacteroidaceae, as shown by the relative vector. Instead, the abundance of Lactobacillaceae was negatively associated with neverEBF. The size of the elliptic cloud corresponded to a higher possibility to cluster and differentiate samples belonging to EBF6m, neverEBF, or ut1Y. Therefore, it clearly appears how no differences occurred between ut1Y and EBF6m. The overlapping of ut1Y and EBF6m clouds was markedly determined by positive scores of both Lactobacillaceae and Enterobacteriaceae. Noteworthy, although some samples belonging to EBF6m or ut1Y moved toward Bacteroidaceae, the weighted clouds noticed a negative association of both groups (EBF6m or ut1Y) with Bacteroidaceae.
Principal component analysis (PCA) of one-year-old infants (T3) sub-grouped according to the duration of breast-feeding, exclusively breast-fed up to six months of age (EBF6m), never exclusively breast-fed (never-EBF), and infants fed with breast milk at least once/day up to 1 year of age (ut1Y), based on the abundance of bacterial families showing p values < 0.05 (i.e., Bacteroidaceae, Lactobacillaceae, and Enterobacteriaceae). The size of blue, yellow, and gray ellipses (including EBF6m, never-EBF, and ut1Y samples, respectively) have been weighted based on the highest probability to find a related sample into the PCA plot
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt)
Based on obtained results, which emphasized postnatal variables and, more in-depth, breastfeeding and weaning as the main affecting factors, we further used the PICRUSt approach to predict metabolic pathways. The comparison of infants exclusively breastfed (EBF) against those who were non-exclusively breastfed (NeBF) during the first 6 months of life (Fig. 4A) determined a significant difference in the relative predicted abundances of the superpathway of sulfolactate degradation and superpathway of taurine degradation. Both these pathways were higher in NeBF than EBF (qval ≤ 0.014).
Following the previously described stratification of samples, a more in-depth investigation was then conducted to investigate differences determined by the combined form of type and duration of milk-feeding. Samples were again grouped into the three different groups of neverEBF, EBF6m, and ut1Y. No differences were found between EBF6m and ut1Y. Comparing neverEBF to ut1Y (Fig. 4B), the above-reported superpathway of sulfolactate degradation and superpathway of taurine degradation were confirmed to have a higher relative frequency in neverEBF (qval ≤ 0.045). From the comparison between neverEBF with EBF6m (Fig. 4B), EBF6m showed an increased frequency of adenosyl-cobalamin biosynthesis II (cobalt incorporation; qval = 0.049), catechol degradation to 2-oxopent-4-enoate II (qval = 0.043), and catechol degradation II (qval = 0.038).
The PICRUSt investigation was also useful in assessing differences determined by the timing of solid food introduction (Fig. 4C). This allows us to notice that the group of infants weaned after the 4th month of age showed an increased predicted frequency of adenosylcobalamin biosynthesis II (qval = 0.038), androstenedione degradation (qval = 0.029), catechol degradation II (qval = 0.031), catechol degradation to 2-oxopent-4-enoate II (qval = 0.034), creatinine degradation I (qval = 0.026), meta cleavage pathway of aromatic compounds (qval = 0.049), and superpathway of aerobic toluene degradation (qval = 0.043).
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) based on 16S rRNA-seq of fecal samples of one-year-old infants (T3). Comparisons were made according to different sub-grouping, i.e., the type of milk-feeding followed during the first six months after birth (i.e., exclusively breastfed, EBF, and non-exclusively breastfed, neverEBF), milk-feeding evaluated till 1 year of age (i.e., never exclusively breastfed, neverEBF, exclusively breastfed till 6 months of age, EBF-6 m, ones taking breast milk at least once/day till 1 year of age, ut1Y), and time of weaning (i.e., early-weaned, ≤ 4, or weaned after the 4th month of age, > 4)
Discussion
Many authors agree on the consideration that infants start to show an “adult-like microbiota” between 1 and 3 years of age, the phase of life when breastfeeding is no longer exclusive and solid foods are widely introduced [1, 27, 28]. An appropriate complementary nutrition, as well as a balanced diet in childhood, are both important for the growth, development, and health. The timing of introduction and the type/order of complementary foods have been associated with an increased risk of obesity in pediatric age, also affecting early sexual maturation [16]. In line with this, the “Green Paper” published by the European Commission recognized that “lifestyle choices that are important in determining health risks in adulthood are made during childhood and adolescence; it is, therefore, essential that children are oriented to adopt healthy behaviors” [29]. The promotion and the improvement of healthy by correct eating behaviors in children are widely accepted as well as the physical activity, which is suggested as an additional intervention useful to counteract the current epidemic incidence of obesity [30]. Due to the low discrepancy in dietary habits among our cohort, even according to different sub-grouping, the other collected and analyzed variables (metadata) increased the specificity of the observed difference in microbiota composition. In our study, infants showed dietary habits aligned with the recommendations of the National Dietary Guidelines for a healthy diet [23, 24]. Only legume were observed to be lower than the recommended frequencies of consumption (at least three times per week). Even though the consumption of legumes is recommended and supported by all the public health agencies around the world, they tend to be consumed sporadically. Oppositely, animal protein intake in infancy, especially dairy products and meat, has been observed to be the primary source of protein, although it is known that the consumption of an excessive amount of protein has been associated to many health issues and later obesity [31]. In this context, our study is in line with previous European and national studies that assessed a higher animal protein intake in infants, in which meat constitutes one of the principal protein sources of the diet, followed by dairy products and fish [32].
With the purpose of increasing knowledge on early gut colonizers able to drive or reduce the onset of non-communicable diseases (NCDs), the gut microbiota of 1-year-old infants (T3 of the A.MA.MI project) has been here characterized by evaluating the impact of prenatal, perinatal, and postnatal factors. In adult microbiota, high values of microbial diversity and richness (alpha diversity) highlight ecosystem resistance, resilience, and health [33]. In the early stages of life, various factors contribute to increase the values of alpha diversity (i.e., born via cesarean section, formula-fed, and those having older siblings in the household) [34,35,36], even if not all of them are positively linked to healthy outcomes later in life. Herein, an early solid food introduction was able to significantly increase Faith’s PD index, while the type of milk-feeding (if exclusively breastfeeding or not for the first 6 months of life) was close to be statistically significant up to 1 year of age. Our results for weaning are in line with previous studies that found an increase of alpha diversity indices in already weaned children [9, 37]. However, literature is not concordant in pushing toward an earlier solid food introduction [12, 13], differently, it has been widely stated that breastfeeding decreases alpha diversity due to Bifidobacterium that markedly colonized the intestinal lumen [34, 35].
Our previous findings on gut microbiota resulted from analyzing infants at birth (T0), 1 month (T1), and 6 months (T2) [20, 25] and showed that prenatal and perinatal variables were the main affecting factors. Differently, the inspection of prenatal and perinatal factors at T3 revealed that these became low significance. After grouping samples according to maternal pregestational BMI, no differences at any taxonomic level were found. When samples were grouped according to maternal gestational WG, only slight differences were observed in Actinobacteria and Firmicutes abundances. However, the statistical comparison showed that both phyla did not reach the threshold of significance after p value correction. This allows us to speculate about the greatest impact of “more recent” postnatal factors, which were also able to reduce the influence of “older” ones, likely prenatal and perinatal variables. In fact, the comparison between the different delivery modes showed that Pasteurellaceae were only partially associated with infants born via CS (not statistically significant). This evidence was further confirmed when prenatal and perinatal variables have been analyzed admitting the possibility of confounders and observing no significant results (Table 2).
Herein, the main differences were found among postnatal variables. In accordance with recommendations given by the WHO, exclusive breastfeeding is the gold standard of nutrition at least during the first 6 months after birth [38]. The comparison within our set of infants supported that Acidaminococcaceae mainly colonized the gut microbiota of individuals fed with formula at least once/day. Oppositely, the exclusively breastfeeding harbored high abundances of taxa belong to the family of Lactobacillaceae. This result confirms what has been widely demonstrated by literature regarding the prebiotic effect of hMOs [39]. Differently, evidence concerning the relationship between diet and Acidaminococcaceae are mainly restricted to mouse models or in vitro experiments. In fact, Jones et al. [40] previously described a greater increase of Acidaminococcaceae in a group of infants consuming a lactose-reduced formula with added corn syrup solids. Meanwhile, Gallier and co-workers positively correlated increases of Acidaminococcaceae to high amounts of propionate, instead of butyrate, evaluating the final metabolites obtained when different kinds of milks (human, cow, goat) were fermented with infants’ feces [41].
We further investigated differences related to a long-term not exclusively milk-feeding and found a clear difference in abundances of Bacteroidaceae, Lactobacillaceae, and Enterobacteriaceae between infants who were never breastfed (neverEBF), those exclusively breastfed up to 6 months of age (EBF6m), and those that were breastfed once/day up to 1 year of age (ut1Y). In fact, plotting the PCA, we assessed that the cloud of neverEBF positively correlated with Bacteroidaceae and negatively with Lactobacillaceae and Enterobacteriaceae. Instead, an almost complete overlap was found for clouds of EBF6m and ut1Y, suggesting a long-term contribution of breastfeeding in driving the early microbiota colonization. Low abundances of Lactobacillaceae and Enterobacteriaceae, probably determined by a not-exclusive breastfeeding, may have allowed an increased abundance of Bacteroidaceae, also considering that a large number of Bacteroides spp. are able to colonize the human gut [42]. Besides, considering the ability of Bacteroides to rapidly adapt to changes in nutrient availability [43], it is not surprising that in our analysis the Bacteroidaceae resulted to be more abundant as a consequence of the gradual cessation of milk-feeding and progression in complementary feeding [44]. Furthermore, the absence of competitors, likely Lactobacillaceae and Enterobacteriaceae, for the available substrates within the gut has led to the possibility of overgrowth for Bacteroidaceae. Not all species of Bacteroidaceae are opportunistic pathogens but a great number of them are lipopolysaccharide (LPS) producers [45] and, due to the strict relationship between LPS and both histone acetylation and methylation processes [46], some Bacteroidaceae sub-taxa could contribute to the onset of NCDs later in life [47]. This is an additional point of view concerning the strength of breastfeeding and its immunoglobulin content, which directly acts as a major driver of gut microbiota composition favoring the growth of health promoting bacteria and indirectly avoiding the overgrowth of potentially pathogenic microbes [48].
Also, the solid food introduction undoubtedly worked as a driver of the gut microbiota composition. Previous evidence correlated the time of solid food introduction to the development of childhood overweight and obesity and assessed an increased risk of disease in infants weaned before 4 months of age [49, 50]. Herein, high abundance of Ruminococcaceae (at the family level) and Faecalibacterium (at genus level) was markedly determined by weaning. For both taxa, the significance (qval < 0.05) was reached even when all postnatal factors have been investigated maintaining the possibility of confounders. Furthermore, Faecalibacterium was also discriminant among all collected prenatal, perinatal, and postnatal variables. In a previous study [51], Faecalibacterium was found to be higher in infants having older siblings and, for this reason, authors speculated about an early microbiota maturation mediated by the presence of the “adult”-like genus Faecalibacterium. In the present study, high abundances of Faecalibacterium, as well as Ruminococcaceae, were linked to infants who were exposed to solid foods from 4 months of age. A great interest grew about the role of Faecalibacterium [52, 53], being one of the main early gut colonizers and due to its involvement in lactose metabolism [54]. Low abundance of Faecalibacterium was found in the gut microbiota of children with allergic asthma [55], querying also its potential role in diabetes onset [56]. The contribution of weaning toward the presence of Faecalibacterium is still unclear; we can suppose that it may be correlated to a longer milk-feeding that had determined a gut microbial community more stable than the gut microbiota characterizing infants who started to reduce milk-feeding earlier. The gut microbiota of infants that were early weaned probably underwent a marked perturbation driven by the introduction of solid foods that could have influenced the resilience of Faecalibacterium. In fact, due to the low number of enrolled subjects, in our set of children it is hard to evaluate differences occurring in weaned before or later the 4th month sub-stratified based on different delivery modes, rather than based on different milk-feeding as suggested by Coker and co-workers [57]. Undoubtedly, exclusively breastfeeding remains the gold standard of nutrition for at least the first 6 months of life but additional evidence about the combined effect of breastfeeding and different timing of solid food introduction might be able to increase the knowledge behind this complex interplay.
Taking into account the suggestion derived from the “hygiene hypothesis” [58], we evaluated the presence of older siblings. No differences were found when this comparison was performed as a fixed effect (Tab. 1). Differently, some bacterial taxa, such as Proteobacteria (at phylum level) and Streptococcaceae (at the family level), showed at least of a tendency due to the fact that these differences were not confirmed by adjusting the p values. The absence of significant results could be probably determined by an increase of interactions between the firstborns and the environmental contaminants, which may have reduced the resilience of microbial colonizers characterizing the first months after birth [59, 60].
To deeper investigate the diet-related factors, we performed a prediction of the metabolic pathways inferred from 16S rDNA gene abundances. This analysis aimed to evaluate if differences, in terms of microbiota metabolism, may have been determined by a cumulative effect of hierarchically different taxa. The predicted over-presence of superpathways involved in sulfolactate and taurine degradation in neverEBF underlined a higher abundance of microbes involved in the metabolism of sulfur compounds. These findings allow us to speculate about a conspicuous presence of Proteobacteria, as widely cited previously [35, 61, 62]. Differently, the increased adenosyl-cobalamin biosynthesis in EBF6m suggested the presence of specific taxa able to contribute to the biosynthesis of vitamin B12. Noteworthy, lactic acid bacteria (LAB), which were higher in breastfed infants than non-exclusively breastfed, were previously detected as cobalamin-producers [63, 64]. Noteworthy, even if being exposed to solid foods for a less time than peers, infants weaned after the 4th month of age had a higher frequency of various predicted pathways when compared with infants weaned < 4 months. Those findings suggested that the microbiota of infants weaned after the 4th month of age might be characterized by a more stable microbial community, but only a transcriptomic analysis might confirm or deny this assumption.
Strengths and limitations
Among the strengths of the A.MA.MI study, authors include the adopted multifactorial approach based on collecting of various babies’ exposures (variables) that allowed to provide a deeper framework into the field of early gut microbial colonizers. Data analysis requires multidisciplinary expertise, which can be facilitated by a team encompassing microbiology, clinical nutrition, pediatrics, bioinformatics, and biostatistics sciences. However, as previously reported, the number of enrolled subjects did not allow us to evaluate factors by coupling them together and, therefore, further stratify our cohort. For this reason, the number of enrolled children remains the greatest limitation that needs to be acknowledged at the same time as the lack of groups having the same number of samples.
Conclusion
According to literature, the first year of life is crucial for healthy growth due to several exposures able to affect gut microbiota composition. Herein, it was mainly found that at 1 year of age prenatal and perinatal factors exerted a lower impact, whereas postnatal ones markedly contributed to modulate some specific microbial patterns. Overall, dietary factors are likely the major modifiable factors contributing to shape the infant gut microbiota. This study confirmed the statement that exclusively breastfeeding remains the gold standard of milk-based nutrition at least for the 6 months of life. At the same time, this study confirms a long-term contribution of breastfeeding up to the year of life. Nonetheless, additional evidence is needed to evaluate the interplaying between milk-feeding and weaning. Furthermore, this study recommends further research considering different factors that could play a role as co-variables during the first steps of babies’ gut microbiota shaping.
Change history
05 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00394-023-03180-2
References
Yatsunenko T, Rey FE, Manary MJ et al (2012) Human gut microbiome viewed across age and geography. Nature 486:222–227. https://doi.org/10.1038/nature11053
Turroni F, Milani C, Duranti S et al (2020) The infant gut microbiome as a microbial organ influencing host well-being. Ital J Pediatr 46:16. https://doi.org/10.1186/s13052-020-0781-0
Stiemsma LT, Michels KB (2018) The role of the microbiome in the developmental origins of health and disease. Pediatrics 141:e20172437. https://doi.org/10.1542/peds.2017-2437
Satokari R, Grönroos T, Laitinen K et al (2009) Bifidobacterium and Lactobacillus DNA in the human placenta. Lett Appl Microbiol 48:8–12. https://doi.org/10.1111/j.1472-765X.2008.02475.x
Bäckhed F, Roswall J, Peng Y et al (2015) Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17:690–703. https://doi.org/10.1016/j.chom.2015.04.004
Baumann-Dudenhoeffer AM, D’Souza AW, Tarr PI et al (2018) Diet and maternal gestational weight gain predict metabolic maturation of infant gut microbiomes. Nat Med 24:1822–1829. https://doi.org/10.1038/s41591-018-0216-2
Walsh C, Lane JA, van Sinderen D, Hickey RM (2020) Human milk oligosaccharides: shaping the infant gut microbiota and supporting health. J Funct Foods 72:104074. https://doi.org/10.1016/j.jff.2020.104074
Fallani M, Amarri S, Uusijarvi A et al (2011) Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiol Read Engl 157:1385–1392. https://doi.org/10.1099/mic.0.042143-0
Homann C-M, Rossel CAJ, Dizzell S et al (2021) Infants’ first solid foods: impact on gut microbiota development in two intercontinental cohorts. Nutrients 13:2639. https://doi.org/10.3390/nu13082639
Johnson CL, Versalovic J (2012) The human microbiome and its potential importance to pediatrics. Pediatrics 129:950–960. https://doi.org/10.1542/peds.2011-2736
Moore RE, Townsend SD (2019) Temporal development of the infant gut microbiome. Open Biol 9:190128. https://doi.org/10.1098/rsob.190128
Hawkins SS, Cole TJ, Law C (2009) An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK Millennium Cohort Study. J Epidemiol Community Health 63:147–155. https://doi.org/10.1136/jech.2008.077917
Neutzling MB, Hallal PRC, Araújo CLP et al (2009) Infant feeding and obesity at 11 years: prospective birth cohort study. Int J Pediatr Obes 4:143–149. https://doi.org/10.1080/17477160802453530
Yan J, Liu L, Zhu Y et al (2014) The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 14:1267. https://doi.org/10.1186/1471-2458-14-1267
Miranda RA, Pietrobon CB, Bertasso IM et al (2020) Early weaning leads to specific glucocorticoid signalling in fat depots of adult rats. Endocrine 67:180–189. https://doi.org/10.1007/s12020-019-02080-y
Calcaterra V, Cena H, Regalbuto C et al (2021) The role of fetal, infant, and childhood nutrition in the timing of sexual maturation. Nutrients 13:419. https://doi.org/10.3390/nu13020419
Iozzo P, Sanguinetti E (2018) Early dietary patterns and microbiota development: still a way to go from descriptive interactions to health-relevant solutions. Front Nutr 5:5. https://doi.org/10.3389/fnut.2018.00005
Thompson AL (2020) Evaluating the pathways linking complementary feeding practices to obesity in early life. Nutr Rev 78:13–24. https://doi.org/10.1093/nutrit/nuz057
Hasegawa K, Linnemann RW, Mansbach JM et al (2017) Association of household siblings with nasal and fecal microbiota in infants. Pediatr Int Off J Jpn Pediatr Soc 59:473–481. https://doi.org/10.1111/ped.13168
Raspini B, Porri D, De Giuseppe R et al (2020) Prenatal and postnatal determinants in shaping offspring’s microbiome in the first 1000 days: study protocol and preliminary results at one month of life. Ital J Pediatr 46:45. https://doi.org/10.1186/s13052-020-0794-8
Turconi G, Bazzano R, Roggi C, Cena H (2010) Reliability and relative validity of a quantitative food-frequency questionnaire for use among adults in Italian population. Int J Food Sci Nutr 61:846–862. https://doi.org/10.3109/09637486.2010.495329
International Fetal and Newborn Growth Consortium (2009). The International Fetal and Newborn Growth Standards for the 21st Century (INTERGROWTH-21st) Study Protocol. https://www.medscinet.net/Intergrowth/patientinfodocs/Intergrowth%20Protocol%20Sept%202009.pdf; Accessed 27 Sept 2021
CREA (2017). Linee Guida per una Sana Alimentazione, ed. 2017. https://www.crea.gov.it/documents/59764/0/Dossier+LG+2017_CAP10.pdf/627ccb4d-4f80-cc82-bd3a-7156c27ddd4a?t=1575530729812; Accessed 27 Sept 2021
INRAN (2003). Linee Guida per una Sana Alimentazione Italiana, ed. 2003. http://www.piramidealimentare.it/files_allegati/guida.pdf; ; Accessed 27 Sept 2021.
Raspini B, Vacca M, Porri D et al (2021) Early life microbiota colonization at six months of age: a transitional time point. Front Cell Infect Microbiol 11:590202. https://doi.org/10.3389/fcimb.2021.590202
Carroccio A, Celano G, Cottone C et al (2021) WHOLE-meal ancient wheat-based diet: effect on metabolic parameters and microbiota. Dig Liver Dis. https://doi.org/10.1016/j.dld.2021.04.026
Palmer C, Bik EM, DiGiulio DB et al (2007) Development of the human infant intestinal microbiota. PLoS Biol 5:e177. https://doi.org/10.1371/journal.pbio.0050177
Derrien M, Alvarez A-S, de Vos WM (2019) The gut microbiota in the first decade of life. Trends Microbiol 27:997–1010. https://doi.org/10.1016/j.tim.2019.08.001
Commission of the European Communities. Promoting healthy diets and physical activity: a European dimension for the prevention of overweight, obesity and chronic diseases.https://ec.europa.eu/health/ph_determinants/life_style/nutrition/documents/nutrition_gp_en.pdf ; Accessed 27 Sept 2021
WHO. Guidelines on physical activity, sedentary behaviour and sleep for children under 5 years of age. https://apps.who.int/iris/bitstream/handle/10665/311664/9789241550536-eng.pdf?sequence=1&isAllowed=y; Accessed 27 Sept 2021
Drummen M, Tischmann L, Gatta-Cherifi B et al (2018) Dietary protein and energy balance in relation to obesity and co-morbidities. Front Endocrinol 9:443. https://doi.org/10.3389/fendo.2018.00443
Bradbury KE, Tong TY, Key TJ (2017) Dietary intake of high-protein foods and other major foods in meat-eaters, poultry-eaters, fish-eaters, vegetarians, and vegans in UK Biobank. Nutrients 9(12):1317. https://doi.org/10.3390/nu9121317
Shade A, Peter H, Allison SD et al (2012) Fundamentals of microbial community resistance and resilience. Front Microbiol. https://doi.org/10.3389/fmicb.2012.00417
Bezirtzoglou E, Tsiotsias A, Welling GW (2011) Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 17:478–482. https://doi.org/10.1016/j.anaerobe.2011.03.009
Fan W, Huo G, Li X et al (2013) Diversity of the intestinal microbiota in different patterns of feeding infants by Illumina high-throughput sequencing. World J Microbiol Biotechnol 29:2365–2372. https://doi.org/10.1007/s11274-013-1404-3
Laursen MF, Zachariassen G, Bahl MI et al (2015) Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol 15:154. https://doi.org/10.1186/s12866-015-0477-6
Savage JH, Lee-Sarwar KA, Sordillo JE et al (2018) Diet during pregnancy and infancy and the infant intestinal microbiome. J Pediatr 203:47-54.e4. https://doi.org/10.1016/j.jpeds.2018.07.066
WHO (2002). Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. https://apps.who.int/iris/bitstream/handle/10665/42519/9241562110.pdf; Accessed 27 Sept 2021
Jost T, Lacroix C, Braegger C, Chassard C (2015) Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr Rev 73:426–437. https://doi.org/10.1093/nutrit/nuu016
Jones RB, Berger PK, Plows JF et al (2020) Lactose-reduced infant formula with added corn syrup solids is associated with a distinct gut microbiota in Hispanic infants. Gut Microbes 12:1813534. https://doi.org/10.1080/19490976.2020.1813534
Gallier S, Van den Abbeele P, Prosser C (2020) Comparison of the bifidogenic effects of goat and cow milk-based infant formulas to human breast milk in an in vitro gut model for 3-month-old infants. Front Nutr 7:608495. https://doi.org/10.3389/fnut.2020.608495
Arumugam M, Raes J, Pelletier E et al (2011) Enterotypes of the human gut microbiome. Nature 473:174–180. https://doi.org/10.1038/nature09944
Lee SM, Donaldson GP, Mikulski Z et al (2013) Bacterial colonization factors control specificity and stability of the gut microbiota. Nature 501:426–429. https://doi.org/10.1038/nature12447
Laursen MF (2021) Gut microbiota development: influence of diet from infancy to toddlerhood. Ann Nutr Metab. https://doi.org/10.1159/000517912
McEneany VL, Coyne MJ, Chatzidaki-Livanis M, Comstock LE (2018) Acquisition of MACPF domain-encoding genes is the main contributor to LPS glycan diversity in gut Bacteroides species. ISME J 12:2919–2928. https://doi.org/10.1038/s41396-018-0244-4
Angrisano T, Pero R, Peluso S et al (2010) LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol 10:172. https://doi.org/10.1186/1471-2180-10-172
De Santis S, Liso M, Vacca M et al (2021) Dysbiosis triggers ACF development in genetically predisposed subjects. Cancers 13:283. https://doi.org/10.3390/cancers13020283
Rogier EW, Frantz AL, Bruno ME et al (2014) Lessons from mother: Long-term impact of antibodies in breast milk on the gut microbiota and intestinal immune system of breastfed offspring. Gut Microbes 5:663–668. https://doi.org/10.4161/19490976.2014.969984
Wang J, Wu Y, Xiong G et al (2016) Introduction of complementary feeding before 4months of age increases the risk of childhood overweight or obesity: a meta-analysis of prospective cohort studies. Nutr Res N Y N 36:759–770. https://doi.org/10.1016/j.nutres.2016.03.003
Differding MK, Doyon M, Bouchard L et al (2020) Potential interaction between timing of infant complementary feeding and breastfeeding duration in determination of early childhood gut microbiota composition and BMI. Pediatr Obes 15:e12642. https://doi.org/10.1111/ijpo.12642
Laursen MF, Laursen RP, Larnkjær A et al (2017) Faecalibacterium gut colonization is accelerated by presence of older siblings. mSphere 2:e00448-e517. https://doi.org/10.1128/mSphere.00448-17
Miquel S, Martín R, Bridonneau C et al (2014) Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes 5:146–151. https://doi.org/10.4161/gmic.27651
Ferreira-Halder CV, de Faria AVS, Andrade SS (2017) Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol 31:643–648. https://doi.org/10.1016/j.bpg.2017.09.011
Cheng L, Kiewiet MBG, Logtenberg MJ et al (2020) Effects of different human milk oligosaccharides on growth of bifidobacteria in monoculture and co-culture with Faecalibacterium prausnitzii. Front Microbiol 11:569700. https://doi.org/10.3389/fmicb.2020.569700
Demirci M, Tokman HB, Uysal HK et al (2019) Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol (Madr) 47:365–371. https://doi.org/10.1016/j.aller.2018.12.009
Ganesan K, Chung SK, Vanamala J, Xu B (2018) Causal relationship between diet-induced gut microbiota changes and diabetes: a novel strategy to transplant faecalibacterium prausnitzii in preventing diabetes. Int J Mol Sci 19:E3720. https://doi.org/10.3390/ijms19123720
Coker MO, Laue HE, Hoen AG et al (2021) infant feeding alters the longitudinal impact of birth mode on the development of the gut microbiota in the first year of life. Front Microbiol 12:642197. https://doi.org/10.3389/fmicb.2021.642197
Strachan DP (1989) Hay fever, hygiene, and household size. BMJ 299:1259–1260. https://doi.org/10.1136/bmj.299.6710.1259
Penders J, Gerhold K, Thijs C et al (2014) New insights into the hygiene hypothesis in allergic diseases: mediation of sibling and birth mode effects by the gut microbiota. Gut Microbes 5:239–244. https://doi.org/10.4161/gmic.27905
Milani C, Duranti S, Bottacini F et al (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev MMBR 81:e00036-e117. https://doi.org/10.1128/MMBR.00036-17
Yasmin F, Tun HM, Konya TB et al (2017) Cesarean section, formula feeding, and infant antibiotic exposure: separate and combined impacts on gut microbial changes in later infancy. Front Pediatr 5:200. https://doi.org/10.3389/fped.2017.00200
Davis EC, Dinsmoor AM, Wang M, Donovan SM (2020) Microbiome composition in pediatric populations from birth to adolescence: impact of diet and prebiotic and probiotic interventions. Dig Dis Sci 65:706–722. https://doi.org/10.1007/s10620-020-06092-x
Kleerebezem M, Hugenholtz J (2003) Metabolic pathway engineering in lactic acid bacteria. Curr Opin Biotechnol 14:232–237. https://doi.org/10.1016/s0958-1669(03)00033-8
Taranto MP, Vera JL, Hugenholtz J et al (2003) Lactobacillus reuteri CRL1098 produces cobalamin. J Bacteriol 185:5643–5647. https://doi.org/10.1128/JB.185.18.5643-5647.2003
Acknowledgements
The authors are very grateful to Fondazione Umberto Veronesi for supporting Dr. Benedetta Raspini with the research Grant “Post-doctoral Fellowships 2019”. We would also thank Ms Emanuela Piazza, Ms Elettra Cesare, and Mr Andrea Quadrelli for their help on the enrollment of dyads (questionnaires and interviews administration, samples collection and data entry), Dr. Micol Angelini and Prof Andrea Frontini for their kind help in the sample collection and storage, and all the nursing staff for their help and collaboration during the recruitment and follow-up phases for their help in the data collection and data entry.
Funding
This study has not received any funding.
Author information
Authors and Affiliations
Contributions
MV and BR equally contributed to the conception and design of the study, results’ interpretation, and drafted the manuscript. DP and RD contributed to the conception, design of the study, and revised the manuscript. MC and ML contributed to results’ interpretation and revised the manuscript. RC, EC, and FG, contributed to the conception and design of the study and revised the manuscript. FMC conducted bioinformatics analyses. MV and FMC conducted the statistical analysis, interpreted results, and revised the manuscript. MDA and HC drafted, revised, and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have neither conflicts nor competing interests.
Ethical approval
The studies involving human participants were reviewed and approved by the Human Ethics Committee (EC) of Fondazione IRCCS Policlinico S. Matteo of Pavia. Written informed consent to participate in this study was provided by the participants’ legal guardians.
Consent for publication
All authors have read and approved this version of the manuscript and declare that the content has not been published elsewhere. All authors contributed to the article and approved the submitted version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vacca, M., Raspini, B., Calabrese, F.M. et al. The establishment of the gut microbiota in 1-year-aged infants: from birth to family food. Eur J Nutr 61, 2517–2530 (2022). https://doi.org/10.1007/s00394-022-02822-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02822-1