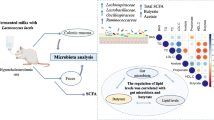
Abstract
Purpose
Several studies highlighted a correlation between folic acid deficiency and high plasma homocysteine concentration, considered a risk factor for multifactorial diseases. Natural folates represent an emerging alternative strategy to supplementation with synthetic folic acid, whose effects are controversial. The present work was, therefore, performed in hyperhomocysteinemic mice to study the impact of supplementation with dairy matrices containing natural folates on plasma homocysteine levels and faecal microbiota composition.
Methods
Forty mice were divided into six groups, two of which fed control or folic acid deficient (FD) diets for 10 weeks. The remaining four groups were fed FD diet for the first 5 weeks and then shifted to a standard control diet containing synthetic folic acid (R) or a FD diet supplemented with folate-enriched fermented milk (FFM) produced by selected lactic acid bacteria, fermented milk (FM), or milk (M), for additional 5 weeks.
Results
Supplementation with dairy matrices restored homocysteine levels in FD mice, although impacting differently on hepatic S-adenosyl-methionine levels. In particular, FFM restored both homocysteine and S-adenosyl-methionine levels to the control conditions, in comparison with FM and M. Next generation sequencing analysis revealed that faecal microbiota of mice supplemented with FFM, FM and M were characterised by a higher richness of bacterial species in comparison with C, FD and R groups. Analysis of beta diversity highlighted that the three dairy matrices determined specific, significant variations of faecal microbiota composition, while hyperhomocysteinemia was not associated with significant changes.
Conclusions
Overall, the results represent a promising starting point for the applicability of food matrices enriched in natural folates to manage hyperhomocysteinemia.





Similar content being viewed by others
Data availability
Raw NGS data are available at the EMBL-EBI European Nucleotide Archive (ENA) [66], under the study accession number PRJEB24434.
References
Strain JJ, Dowey L, Ward M, Pentieva K, McNulty H (2004) B-vitamins, homocysteine metabolism and CVD. Proc Nutr Soc 63(4):597–603. https://doi.org/10.1079/PNS2004390
Bellamy MF, McDowell IF, Ramsey MW, Brownlee M, Bones C, Newcombe RG, Lewis MJ (1998) Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation 98(18):1848–1852
Appel LJ, Miller ER 3rd, Jee SH, Stolzenberg-Solomon R, Lin PH, Erlinger T, Nadeau MR, Selhub J (2000) Effect of dietary patterns on serum homocysteine: results of a randomized, controlled feeding study. Circulation 102(8):852–857
Bissoli L, Di Francesco V, Ballarin A, Mandragona R, Trespidi R, Brocco G, Caruso B, Bosello O, Zamboni M (2002) Effect of vegetarian diet on homocysteine levels. Ann Nutr Metab 46(2):73–79. https://doi.org/10.1159/000057644
Mann NJ, Li D, Sinclair AJ, Dudman NP, Guo XW, Elsworth GR, Wilson AK, Kelly FD (1999) The effect of diet on plasma homocysteine concentrations in healthy male subjects. Eur J Clin Nutr 53(11):895–899
Jamwal S, Sharma S (2018) Vascular endothelium dysfunction: a conservative target in metabolic disorders. Inflamm Res. https://doi.org/10.1007/s00011-018-1129-8
Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB (2016) Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc 5(8):e003768. https://doi.org/10.1161/JAHA.116.003768
Huang X, Li Y, Li P, Li J, Bao H, Zhang Y, Wang B, Sun N, Wang J, He M, Yin D, Tang G, Chen Y, Cui Y, Huang Y, Hou FF, Qin X, Huo Y, Cheng X (2017) Association between percent decline in serum total homocysteine and risk of first stroke. Neurology 89(20):2101–2107. https://doi.org/10.1212/WNL.0000000000004648
Marti-Carvajal AJ, Sola I, Lathyris D, Dayer M (2017) Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev 8:CD006612. https://doi.org/10.1002/14651858.CD006612.pub5
Zhang DM, Ye JX, Mu JS, Cui XP (2017) Efficacy of vitamin B supplementation on cognition in elderly patients with cognitive-related diseases. J Geriatr Psychiatry Neurol 30(1):50–59. https://doi.org/10.1177/0891988716673466
Zhou Z, Liang Y, Qu H, Zhao M, Guo F, Zhao C, Teng W (2018) Plasma homocysteine concentrations and risk of intracerebral hemorrhage: a systematic review and meta-analysis. Sci Rep 8(1):2568. https://doi.org/10.1038/s41598-018-21019-3
Konings EJ, Roomans HH, Dorant E, Goldbohm RA, Saris WH, van den Brandt PA (2001) Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am J Clin Nutr 73(4):765–776
Smith AD, Kim YI, Refsum H (2008) Is folic acid good for everyone? Am J Clin Nutr 87(3):517–533. https://doi.org/10.1093/ajcn/87.3.517
Bailey SW, Ayling JE (2009) The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci USA 106(36):15424–15429. https://doi.org/10.1073/pnas.0902072106
Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, Ulrich CM (2006) Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr 136(1):189–194. https://doi.org/10.1093/jn/136.1.189
Sawaengsri H, Wang J, Reginaldo C, Steluti J, Wu D, Meydani SN, Selhub J, Paul L (2016) High folic acid intake reduces natural killer cell cytotoxicity in aged mice. J Nutr Biochem 30:102–107. https://doi.org/10.1016/j.jnutbio.2015.12.006
Melnyk S, Pogribna M, Miller BJ, Basnakian AG, Pogribny IP, James SJ (1999) Uracil misincorporation, DNA strand breaks, and gene amplification are associated with tumorigenic cell transformation in folate deficient/repleted Chinese hamster ovary cells. Cancer Lett 146(1):35–44. https://doi.org/10.1016/S0304-3835(99)00213-X
Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, Rosenberg IH (2007) A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomark Prev 16(7):1325–1329. https://doi.org/10.1158/1055-9965.EPI-07-0329
Hirsch S, Sanchez H, Albala C, de la Maza MP, Barrera G, Leiva L, Bunout D (2009) Colon cancer in Chile before and after the start of the flour fortification program with folic acid. Eur J Gastroenterol Hepatol 21(4):436–439. https://doi.org/10.1097/MEG.0b013e328306ccdb
Baggott JE, Oster RA, Tamura T (2012) Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol 36(1):78–81. https://doi.org/10.1016/j.canep.2011.05.003
Christensen KE, Mikael LG, Leung KY, Levesque N, Deng L, Wu Q, Malysheva OV, Best A, Caudill MA, Greene ND, Rozen R (2015) High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am J Clin Nutr 101(3):646–658. https://doi.org/10.3945/ajcn.114.086603
Asrar FM, O’Connor DL (2005) Bacterially synthesized folate and supplemental folic acid are absorbed across the large intestine of piglets. J Nutr Biochem 16(10):587–593. https://doi.org/10.1016/j.jnutbio.2005.02.006
LeBlanc JG, Laino JE, del Valle MJ, Vannini V, van Sinderen D, Taranto MP, de Valdez GF, de Giori GS, Sesma F (2011) B-group vitamin production by lactic acid bacteria—current knowledge and potential applications. J Appl Microbiol 111(6):1297–1309. https://doi.org/10.1111/j.1365-2672.2011.05157.x
Saubade F, Hemery YM, Guyot JP, Humblot C (2017) Lactic acid fermentation as a tool for increasing the folate content of foods. Crit Rev Food Sci Nutr 57(18):3894–3910. https://doi.org/10.1080/10408398.2016.1192986
Pompei A, Cordisco L, Amaretti A, Zanoni S, Raimondi S, Matteuzzi D, Rossi M (2007) Administration of folate-producing bifidobacteria enhances folate status in Wistar rats. J Nutr 137(12):2742–2746. https://doi.org/10.1093/jn/137.12.2742
LeBlanc JG, Sybesma W, Starrenburg M, Sesma F, de Vos WM, de Giori GS, Hugenholtz J (2010) Supplementation with engineered Lactococcus lactis improves the folate status in deficient rats. Nutrition 26(7–8):835–841. https://doi.org/10.1016/j.nut.2009.06.023
Iyer R, Tomar SK (2011) Dietary effect of folate-rich fermented milk produced by Streptococcus thermophilus strains on hemoglobin level. Nutrition 27(10):994–997. https://doi.org/10.1016/j.nut.2011.01.003
D’Aimmo MR, Mattarelli P, Biavati B, Carlsson NG, Andlid T (2012) The potential of bifidobacteria as a source of natural folate. J Appl Microbiol 112(5):975–984. https://doi.org/10.1111/j.1365-2672.2012.05261.x
Laino JE, dV HZMJ, de Giori S, Leblanc G JG (2015) Milk fermented with selected strains of lactic acid bacteria is able to improve folate status of deficient rodents and also prevent folate deficiency. J Funct Foods 17:22–32
LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M (2013) Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol 24(2):160–168. https://doi.org/10.1016/j.copbio.2012.08.005
Tidona F, Meucci A, Povolo M, Pelizzola V, Zago M, Contarini G, Carminati D, Giraffa G (2018) Applicability of Lactococcus hircilactis and Lactococcus laudensis as dairy cultures. Int J Food Microbiol 271:1–7. https://doi.org/10.1016/j.ijfoodmicro.2018.02.015
Meucci A, Rossetti L, Zago M, Monti L, Giraffa G, Carminati D, Tidona F (2018) Folates biosynthesis by Streptococcus thermophilus during growth in milk. Food Microbiol 69:116–122. https://doi.org/10.1016/j.fm.2017.08.001
Reeves PG (1997) Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 127(5 Suppl):838S–841S
Tuschl K, Bodamer OA, Erwa W, Muhl A (2005) Rapid analysis of total plasma homocysteine by tandem mass spectrometry. Clin Chim Acta 351(1–2):139–141. https://doi.org/10.1016/j.cccn.2004.08.016
Struys EA, Jansen EE, de Meer K, Jakobs C (2000) Determination of S-adenosylmethionine and S-adenosylhomocysteine in plasma and cerebrospinal fluid by stable-isotope dilution tandem mass spectrometry. Clin Chem 46(10):1650–1656
Krijt J, Duta A, Kozich V (2009) Determination of S-adenosylmethionine and S-adenosylhomocysteine by LC-MS/MS and evaluation of their stability in mice tissues. J Chromatogr B Analyt Technol Biomed Life Sci 877(22):2061–2066. https://doi.org/10.1016/j.jchromb.2009.05.039
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glockner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41(1):e1. https://doi.org/10.1093/nar/gks808
Sacchi CT, Whitney AM, Mayer LW, Morey R, Steigerwalt A, Boras A, Weyant RS, Popovic T (2002) Sequencing of 16S rRNA gene: a rapid tool for identification of Bacillus anthracis. Emerg Infect Dis 8(10):1117–1123. https://doi.org/10.3201/eid0810.020391
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336. https://doi.org/10.1038/nmeth.f.303
Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG (2013) Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10(1):57–59. https://doi.org/10.1038/nmeth.2276
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8(4):e61217. https://doi.org/10.1371/journal.pone.0061217
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2018) Community Ecology Package, Vegan. R Package Version 2.0-9. https://CRAN.R-project.org/package=vegan
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60. https://doi.org/10.1186/gb-2011-12-6-r60
Zakrzewski M, Proietti C, Ellis JJ, Hasan S, Brion MJ, Berger B, Krause L (2017) Calypso: a user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 33(5):782–783. https://doi.org/10.1093/bioinformatics/btw725
Velez-Carrasco W, Merkel M, Twiss CO, Smith JD (2008) Dietary methionine effects on plasma homocysteine and HDL metabolism in mice. J Nutr Biochem 19(6):362–370. https://doi.org/10.1016/j.jnutbio.2007.05.005
Obeid R (2013) The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 5(9):3481–3495. https://doi.org/10.3390/nu5093481
Jones ML, Nixon PF (2002) Tetrahydrofolates are greatly stabilized by binding to bovine milk folate-binding protein. J Nutr 132(9):2690–2694. https://doi.org/10.1093/jn/132.9.2690
Laino JE, dV MJ, de Giori S, Leblanc G JG (2013) Development of a high folate concentration yogurt naturally bio-enriched using selected lactic acid bacteria. LWT Food Sci Technol 54:1–5
Forssen KM, Jagerstad MI, Wigertz K, Witthoft CM (2000) Folates and dairy products: a critical update. J Am Coll Nutr 19(2 Suppl):100S–110S
Eitenmiller RR, Landen WO (1999) Folate. In: Eitenmiller RR, Landen WO (ed) Vitamin analysis for the health and food sciences. CRC Press, Boca Raton, pp 411–466
Said HM, Chatterjee N, Haq RU, Subramanian VS, Ortiz A, Matherly LH, Sirotnak FM, Halsted C, Rubin SA (2000) Adaptive regulation of intestinal folate uptake: effect of dietary folate deficiency. Am J Physiol Cell Physiol 279(6):C1889–C1895. https://doi.org/10.1152/ajpcell.2000.279.6.C1889
Rossi M, Amaretti A, Raimondi S (2011) Folate production by probiotic bacteria. Nutrients 3(1):118–134. https://doi.org/10.3390/nu3010118
Ghoshal K, Li X, Datta J, Bai S, Pogribny I, Pogribny M, Huang Y, Young D, Jacob ST (2006) A folate- and methyl-deficient diet alters the expression of DNA methyltransferases and methyl CpG binding proteins involved in epigenetic gene silencing in livers of F344 rats. J Nutr 136(6):1522–1527. https://doi.org/10.1093/jn/136.6.1522
Miller JW, Nadeau MR, Smith D, Selhub J (1994) Vitamin B-6 deficiency vs folate deficiency: comparison of responses to methionine loading in rats. Am J Clin Nutr 59(5):1033–1039. https://doi.org/10.1093/ajcn/59.5.1033
Janosik M, Kery V, Gaustadnes M, Maclean KN, Kraus JP (2001) Regulation of human cystathionine beta-synthase by S-adenosyl-l-methionine: evidence for two catalytically active conformations involving an autoinhibitory domain in the C-terminal region. Biochemistry 40(35):10625–10633. https://doi.org/10.1021/bi010711p
Ereno-Orbea J, Majtan T, Oyenarte I, Kraus JP, Martinez-Cruz LA (2014) Structural insight into the molecular mechanism of allosteric activation of human cystathionine beta-synthase by S-adenosylmethionine. Proc Natl Acad Sci USA 111(37):E3845–E3852. https://doi.org/10.1073/pnas.1414545111
Zhao R, Diop-Bove N, Visentin M, Goldman ID (2011) Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 31:177–201. https://doi.org/10.1146/annurev-nutr-072610-145133
Mosca A, Leclerc M, Hugot JP (2016) Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol 7:455. https://doi.org/10.3389/fmicb.2016.00455
Vaughan EE, de Vries MC, Zoetendal EG, Ben-Amor K, Akkermans AD, de Vos WM (2002) The intestinal LABs. Antonie Van Leeuwenhoek 82(1–4):341–352
Strozzi GP, Mogna L (2008) Quantification of folic acid in human feces after administration of Bifidobacterium probiotic strains. J Clin Gastroenterol 42(Suppl 3 Pt 2):S179–S184. https://doi.org/10.1097/MCG.0b013e31818087d8
Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, Gervais P (2014) Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr 54(2):175–189. https://doi.org/10.1080/10408398.2011.579361
Fabian E, Majchrzak D, Dieminger B, Meyer E, Elmadfa I (2008) Influence of probiotic and conventional yoghurt on the status of vitamins B1, B2 and B6 in young healthy women. Ann Nutr Metab 52(1):29–36. https://doi.org/10.1159/000114408
Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA (2011) Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 140(3):976–986. https://doi.org/10.1053/j.gastro.2010.11.049
Holmes E, Li JV, Marchesi JR, Nicholson JK (2012) Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab 16(5):559–564. https://doi.org/10.1016/j.cmet.2012.10.007
Leinonen R, Akhtar R, Birney E, Bower L, Cerdeno-Tarraga A, Cheng Y, Cleland I, Faruque N, Goodgame N, Gibson R, Hoad G, Jang M, Pakseresht N, Plaister S, Radhakrishnan R, Reddy K, Sobhany S, Ten Hoopen P, Vaughan R, Zalunin V, Cochrane G (2011) The European nucleotide archive. Nucleic Acids Res 39(Database issue):D28–D31. https://doi.org/10.1093/nar/gkq967
Acknowledgements
The Authors wish to thank Kariklia Pascucci for her kind support in daily lab work, Altero Aguzzi for his help in fermented milk liophylisation and Rita Rami for the excellent care of the animals. This work was funded by the Italian Ministry of Agriculture, Food & Forestry Policies (MiPAAF), with Grant “MEDITO” (DM 12487/7303/11) and with national support to the JPI-HDHL “ENPADASI” project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Consent for publication
All authors read and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
394_2019_1911_MOESM3_ESM.tif
Supplementary material 3 Fig. S1. Per sample rarefaction curves. Each curve shows the average number of Operational Taxonomic Units (OTUs) as a function of the reads abundance subsampled at different depths. Each frame and color represent the experimental treatments, the vertical gray line indicates the lowest number of reads obtained on overall samples (n = 76187) (TIF 13183 KB)
Rights and permissions
About this article
Cite this article
Zinno, P., Motta, V., Guantario, B. et al. Supplementation with dairy matrices impacts on homocysteine levels and gut microbiota composition of hyperhomocysteinemic mice. Eur J Nutr 59, 345–358 (2020). https://doi.org/10.1007/s00394-019-01911-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-01911-y