Abstract
Purpose
Whole grain exhibits potential for regulating lipid levels, possibly because of its antioxidant activity. This study aims to investigate the antioxidant activity of whole grain highland hull-less barley (WHLB) and its effect on liver protein expression profiles in rats fed with high-fat diets.
Methods
Antioxidant activity of WHLB was investigated in vitro by analyzing phenolic and pentosan contents and oxygen radical absorbance capacity (ORAC). Proteins involved in lipid regulation were investigated in vivo by analyzing liver protein expression profiles in Sprague–Dawley rats fed with high-fat diet (HFD) with or without WHLB.
Results
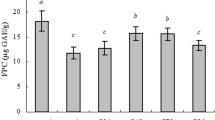
WHLB possessed high total phenolic content (259.90 mg/100 g), total pentosan content (10.74 g/100 g), and ORAC values (418.05 ± 5.65 μmol/g). Rats fed with WHLB diet exhibited significantly (P < 0.05) lower liver lipid levels than those fed with the control HFD diet. Seven differentially expressed proteins were detected through liver proteome analysis and were found to be correlated with 11 pathways, including lipid metabolism, through annotation with Kyoto Encyclopedia of Genes and Genomes. Quantitative real-time polymerase chain reaction analysis showed that rats given with WHLB diet exhibited down-regulated expression of heat shock protein 60 (HSP60) and phosphatidylethanolamine binding protein 1 (PEBP1) and up-regulated expression of enoyl-coenzyme A hydratase (ECH) and peroxiredoxin 6 (PRDX6).
Conclusions
HSP60, PEBP1, ECH, and PRDX6 may be involved in the lipid regulatory effect of WHLB. Moreover, the regulation of PRDX6 may be related to the antioxidant activity of WHLB.



Similar content being viewed by others
References
Tong LT, Zhong K, Liu LY et al (2015) Effects of dietary hull-less barley β-glucan on the cholesterol metabolism of hypercholesterolemic hamsters. Food Chem 169:344–349. doi:10.1016/j.foodchem.2014.07.157
Gong LX, Jin C, Wu XQ et al (2012) Determination of Arabinoxylans in Tibetan Hull-less Barley Bran. Proc Eng 37:218–222. doi:10.1016/j.proeng.2012.04.230
Sette S, D’Addezio L, Piccinelli R et al (2015) Intakes of whole grain in an Italian sample of children, adolescents and adults. Eur J Nutr. doi:10.1007/s00394-015-1097-5
Adom KK, Liu RH (2002) Antioxidant activity of grains. J Agric Food Chem 50:6182–6187. doi:10.1021/jf0205099
Abdel-Aal ESM, Rabalski I (2013) Effect of baking on free and bound phenolic acids in wholegrain bakery products. J Cereal Sci 57:312–318. doi:10.1016/j.jcs.2012.12.001
Wang J, Cao YP, Wang CT et al (2011) Wheat bran xylooligosaccharides improve blood lipid metabolism and antioxidant status in rats fed a high-fat diet. Carbohydr Polym 86:1192–1197. doi:10.1016/j.carbpol.2011.06.014
Domínguez-Avila JA, Alvarez-Parrilla E, López-Díaz JA et al (2015) The pecan nut (Carya illinoinensis) and its oil and polyphenolic fractions differentially modulate lipid metabolism and the antioxidant enzyme activities in rats fed high-fat diets. Food Chem 168:529–537. doi:10.1016/j.foodchem.2014.07.092
Gorinstein S, Leontowicz H, Leontowicz M et al (2003) Seed oils improve lipid metabolism and increase antioxidant potential in rats fed diets containing cholesterol. Nutr Res 23:317–330. doi:10.1016/S0271-5317(02)00532-8
Robert L, Narcy A, Rock E et al (2006) Entire potato consumption improves lipid metabolism and antioxidant status in cholesterol-fed rat. Eur J Nutr 45:267–274. doi:10.1007/s00394-006-0594-y
Yang Y, Yang D, Tang G et al (2013) Proteomics reveals energy and glutathione metabolic dysregulation in the prefrontal cortex of a rat model of depression. Neuroscience 247:191–200. doi:10.1016/j.neuroscience.2013.05.031
Gygi SP, Corthals GL, Zhang YN et al (2000) Evaluation of two-dimensional gel electrophoresis based proteome analysis technology. PNAS 97:9390–9395. doi:10.1073/pnas.160270797
American Association of Cereal Chemists (2003) Approved method of the AACC, 10th edn. AACC, St. Paul
Omwamba M, Hu QH (2010) Antioxidant activity in barley (Hordeum Vulgare L.) grains roasted in a microwave oven under conditions optimized using response surface methodology. J Food Sci 75:C66–C73. doi:10.1111/j.1750-3841.2009.01426.x
Liu Q, Qiu Y, Beta T (2010) Comparison of antioxidant activities of different colored wheat grains and analysis of phenolic compounds. J Agric Food Chem 58:9235–9241. doi:10.1021/jf101700s
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol 299:152–178. doi:10.1016/S0076-6879(99)99017-1
Rouau X, Surget A (1994) A rapid semiautomated method for the determination of total and water-extractable pentosans in wheat flours. Carbohydr Polym 24:123–132. doi:10.1016/0144-8617(94)90022-1
Douglas SG (1981) A rapid method for the determination of pentosans in wheat flour. Food Chem 7:139–145. doi:10.1016/0308-8146(81)90059-5
Garzón GA, Manns DC, Riedl K et al (2015) Identification of phenolic compounds in petals of Nasturtium Flowers (Tropaeolum majus) by high-performance liquid chromatography coupled to mass spectrometry and determination of oxygen radical absorbance capacity (ORAC). J Agr Food Chem 63:1803–1811. doi:10.1021/jf503366c
Choi YM, Jeong HS, Lee JS (2007) Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem 103:130–138. doi:10.1016/j.foodchem.2006.08.004
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Zhang L, Fang G, Zheng L et al (2013) Hypocholesterolemic effect of Capsaicinoids in rats fed diets with or without cholesterol. J Agric Food Chem 61:4287–4293. doi:10.1021/jf304471t
Pakalapati G, Li L, Gretz N et al (2009) Influence of red clover (Trifolium pratense) isoflavones on gene and protein expression profiles in liver of ovariectomized rats. Phytomedicine 16:845–855. doi:10.1016/j.phymed.2009.03.003
Rabilloud T (2012) Silver staining of 2D electrophoresis gels. Methods Mol Biol 893:61–73. doi:10.1007/978-1-61779-885-6_5
Sendegeya P, Li GN, Zhao HH et al (2016) Effect of fluoride on the midgut proteins of the T6 and 734 strains of the silkworm (Bombyx mori). Fluoride 49:102–111
Vega FE, Brown SM, Chen H et al (2015) Draft genome of the most devastating insect pest of coffee worldwide: the coffee berry borer, Hypothenemus hampei. Sci Rep 15:1–17. doi:10.1038/srep12525
Xu QB, Schett G, Perschinka H et al (2000) Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation 102:14–20. doi:10.1016/0022-2860(82)85085-0
Wang L, Li HY, Zhang JF et al (2013) Phosphatidylethanolamine binding protein 1 in vacular endothelial cell autophagy and atherosclerosis. J Physiol 591:5005–5015. doi:10.1113/jphysiol.2013.262667
Wang XS, Phelan SA, Forsman-Semb K et al (2003) Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem 278:25179–25190. doi:10.1074/jbc.M302706200
Henry RJ (1987) Pentosan and (1 → 3), (1 → 4)-β-Glucan concentrations in endosperm and wholegrain of wheat, barley, oats and rye. J Cereal Sci 6:253–258. doi:10.1016/S0733-5210(87)80062-0
Chambel SS, Santos-Gonçalves A, Duarte TL (2015) The dual role of Nrf2 in nonalcoholic fatty Liver disease: regulation of antioxidant defenses and hepatic lipid metabolism. Biomed Res Int 2015:597134. doi:10.1155/2015/597134
Kanehisa M, Furumichi M, Mao T et al (2017) KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45:D353–D361. doi:10.1093/nar/gkw1092
Ohashi K, Burkart V, Flohé S et al (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164:558–561. doi:10.4049/jimmunol.164.2.558
Gong XQ, Zhu YY, Dong J et al (2013) Small hepatitis B surface antigen interacts with and modulates enoyl-coenzyme A hydratase expression in hepatoma cells. Arch Virol 158:1065–1070. doi:10.1007/s00705-012-1581-7
Zhang J, Ibrahim MM, Sun MZ et al (2015) Enoyl-coenzyme A hydratase in cancer. Clin Chim Acta 448:13–17. doi:10.1016/j.cca.2015.01.020
Chatterjee S, Feinstein SI, Dodia C et al (2011) Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem 286:11696–11706. doi:10.1074/jbc.M110.206623
Sharma L, Kaur J, Shukla G (2014) Expression of heat shock protein 90, 70, 60 and 25 in the placenta of Plasmodium berghei infected BALB/c mice. Asian Pac J Trop Med 4:S442–S444. doi:10.1016/S2222-1808(14)60487-4
Acknowledgements
This work was supported by the Science and Technology Support Demonstration Project of Chongqing (CSTC2014JCSF-JCSSX004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors had a personal or financial conflict of interest.
Rights and permissions
About this article
Cite this article
Xia, X., Li, G., Xing, Y. et al. Antioxidant activity of whole grain highland hull-less barley and its effect on liver protein expression profiles in rats fed with high-fat diets. Eur J Nutr 57, 2201–2208 (2018). https://doi.org/10.1007/s00394-017-1494-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1494-z