Abstract
Background
Vegetables and fruits are rich sources of a variety of nutrients, including vitamins (E and C), trace minerals, and dietary fibers, and many other classes of biologically active compounds such as carotenoids and polyphenols, which are often assumed to protect against degenerative pathologies such as cardiovascular diseases. Although potato is considered as a starchy food, it is also included in the category of vegetables by its micronutrient content.
Aim of the study
In the present study, we investigated in the rat the effect of a potato-enriched diet on lipid metabolism and antioxidant protection.
Results
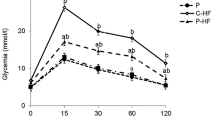
Feeding rats a potato-enriched diet for 3 weeks led to a significant decrease in cholesterol and triglyceride levels in plasma (respectively, −30%, P<0.0001 and −36%, P<0.05) and cholesterol level in liver (−42%, P<0.0001). Antioxidant status was also improved by potato consumption. TBARS levels in heart were decreased and vitamin E/triglycerides ratio in plasma was improved.
Conclusions
Our present results suggest that consumption of cooked potatoes (consumed with skin) may enhance antioxidant defense and improve the lipid metabolism. These effects could be interesting for prevention of cardiovascular disease.


Similar content being viewed by others
References
Block G, Patterson B, Subar A (1992) Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer 18:1–29
Ness AR, Powles JW (1997) Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol 26:1–13
Montonen J, Knekt P, Jarvinen R, Reunanen A (2004) Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 27:362–366
O’Brien MM, Kiely M, Galvin M, Flynn A (2003) The importance of composite foods for estimates of vegetable and fruit intakes. Public Health Nutr 6:711–726
Agudo A, Slimani N, Ocke MC, Naska A, Miller AB, Kroke A, Bamia C, Karalis D, Vineis P, Palli D, Bueno-de-Mesquita HB, Peeters PH, Engeset D, Hjartaker A, Navarro C, Martinez Garcia C, Wallstrom P, Zhang JX, Welch AA, Spencer E, Stripp C, Overvad K, Clavel-Chapelon F, Casagrande C, Riboli E (2002) Consumption of vegetables, fruit and other plant foods in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts from 10 European countries. Public Health Nutr 5:1179–1196
Prynne CJ, Ginty F, Paul AA, Bolton-Smith C, Stear SJ, Jones SC, Prentice A (2004) Dietary acid–base balance and intake of bone-related nutrients in Cambridge teenagers. Eur J Clin Nutr 58:1462–1471
Anderson JW, Bridges SR (1988) Dietary fiber content of selected foods. Am J Clin Nutr 47:440–447
Remesy C, Morand C, Levrat MA, Gamet L, Demigne C (1992) Intérêt nutritionnel des produits végétaux riches en fibres. Cah Nutr Diét 27:370–377
Gallant DJ, Bouchet B, Buleon A, Perez S (1992) Physical characteristics of starch granules and susceptibility to enzymatic degradation. Eur J Clin Nutr 46(Suppl 2):S3–16
Englyst HN, Cummings JH (1987) Digestion of polysaccharides of potato in the small intestine of man. Am J Clin Nutr 45:423–431
Mazur A, Remesy C, Gueux E, Levrat MA, Demigne C (1990) Effects of diets rich in fermentable carbohydrates on plasma lipoprotein levels and on lipoprotein catabolism in rats. J Nutr 120:1037–1045
de Deckere EA, Kloots WJ, van Amelsvoort JM (1995) Both raw and retrograded starch decrease serum triacylglycerol concentration and fat accretion in the rat. Br J Nutr 73:287–298
Cherbut C, Aube A, Mekki N, Dubois C, Lairon D, Barry JL (1997) Digestive and metabolic effect of potato and maize fibres in human subjects. Br. J Nutr 77:33–46
Mathers JC, Dawson LD (1991) Large bowel fermentation in rats eating processed potatoes. Br J Nutr 66:313–329
Lasheras C, Gonzalez S, Huerta JM, Lombardia C, Ibanez R, Patterson AM, Fernandez S (2003) Food habits are associated with lipid peroxidation in an elderly population. J Am Diet Assoc 103:1480–1487
Carr AC, Frei B (1999) Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 69:1086–1107
Breithaupt DE, Bamedi A (2002) Carotenoids and carotenoid esters in potatoes (Solanum tuberosum L.): new insights into an ancient vegetable. J Agric Food Chem 50:7175–7181
Lewis CE, Walker J, Lancaster JE, Sutton KH (1998) Determination of anthocyanins, flavonoids and phenolic acids in potatoes. II: wild, tuberous Solanum species. J Sci Food Agric 77:58–63
Del Mar Verde Mendez C, Delgado M, Rodriguez EMR, Romero CD (2004) Content of free phenolic compound in cultivars of potatoes harvested in Tenerife (Canary Islands). J Agric Food Chem 52:1323–1327
Chu YH, Sun J, Wu X, Liu RH (2002) Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem 50:6910–6916
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22:18–35
Barja G, Lopez-Torres M, Perez-Campo R, Rojas C, Cadenas S, Prat J, Pamplona R (1994) Dietary vitamin C decreases endogenous protein oxidative damage, malondialdehyde, and lipid peroxidation and maintains fatty acid unsaturation in the guinea pig liver. Free Radic Biol Med 17:105–115
Djousse L, Arnett DK, Coon H, Province MA, Moore LL, Ellison RC (2004) Fruit and vegetable consumption and LDL cholesterol: the National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr 79:213–217
Van Hoydonck PG, Schouten EG, Manuel YKB, van Campenhout A, Hoppenbrouwers KP, Temme EH (2004) Does vitamin C supplementation influence the levels of circulating oxidized LDL, sICAM−1, sVCAM-1 and vWF-antigen in healthy male smokers? Eur J Clin Nutr 58:1587–1593
Remesy C, Demigne C (1974) Determination of volatile fatty acids in plasma after ethanolic extraction. Biochem J 141:85–91
Turley SD, Dietschy JM (1978) Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res 19:924–928
Lyan B, Azaïs-Braesco V, Cardinault N, Tyssandier V, Borel P, Alexandre-Gouabau M, Grolier P (2001) Simple method for clinical determination of 13 carotenoids in human plasma using an isocratic high-performance liquid chromatographic method. J Chromato B 751:297–303
Benzie I, Strain J (1996) The ferric reducting ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Lee S, Shoeman D, Csallany A (1992) Urinary response to in vivo lipid peroxidation induced by vitamin E deficiency. Lipids 27:124–128
Rayssiguier Y, Gueux E, Bussiere L, Durlach J, Mazur A (1993) Dietary magnesium affects susceptibility of lipoproteins and tissues to peroxidation in rats. J Am Coll Nutr 12:133–137
Ullrich I (1987) Evaluation of a high-fiber diet in hyperlipidemia: a review. J Am Coll Nutr 6:19–25
Baig MM, Cerda J (1981) Pectin: its interaction with serum lipoproteins. Am J Clin Nutr 34:50–53
Fernandez M (2001) Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr Opin Lipidol 12:35–40
Aprikian O, Duclos V, Guyot S, Besson C, Manach C, Bernalier A, Morand C, Remesy C, Demigne C (2003) Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J Nutr 133:1860–1865
Fernandez M (1995) Distinct mechanisms of plasma LDL lowering by dietary fiber in the guinea pig: specific effects of pectin, guar gum, and psyllium. J Lipid Res 36:2394–2404
Moundras C, Behr SR, Remesy C, Demigne C (1997) Fecal losses of sterols and bile acids induced by feeding rats guar gum are due to greater pool size and liver bile acid secretion. J Nutr 127:1068–1076
Stedronsky ER (1994) Interaction of bile acids and cholesterol with non-systemic agents having hypocholesterolemic properties. Biochim Biophys Acta 1210:255–287
Levrat-Verny MA, Behr S, Mustad V, Remesy C, Demigne C (2000) Low levels of viscous hydrocolloids lower plasma cholesterol in rats primarily by impairing cholesterol absorption. J Nutr 130:243–248
Chen WJ, Anderson JW, Jennings D (1984) Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Proc Soc Exp Biol Med 175:215–218
Wright RS, Anderson JW, Bridges SR (1990) Propionate inhibits hepatocyte lipid synthesis. Proc Soc Exp Biol Med 195:26–29
Valenzuela A (1991) The biological significance of malondialdehyde determination in the assessment of tissue oxidative stress. Life Sci 48:301–309
Lin PH, Aickin M, Champagne C, Craddick S, Sacks FM, McCarron P, Most-Windhauser MM, Rukenbrod F, Haworth L (2003) Food group sources of nutrients in the dietary patterns of the DASH-Sodium trial. J Am Diet Assoc 103:488–496
Wagner JR, Motchnik PA, Stocker R, Sies H, Ames BN (1993) The oxidation of blood plasma and low density lipoprotein components by chemically generated singlet oxygen. J Biol Chem 268:18502–18506
Andlauer W, Stumpf C, Hubert M, Rings A, Fürst P (2003) Influence of cooking process on phenolic marker compounds of vegetables. Int J Vit Nutr Res 73:152–159
Acknowledgements
L. Robert and A. Narcy were supported by CNIPT (Comité National Interprofessionnel de la Pomme de Terre), 9 rue d’Athènes, 75009 Paris, France.
We gratefully acknowledge Catherine Besson, Bernard Lyan, Sylvie Mercier, Pierre Lamby and Marie-Anne Verny for their respective technical assistance and their contribution in animal handling. This work was supported by A.N.R.T. (Association Nationale pour la Recherche et Technique), I.N.R.A. (Institut National de la Recherche Agronomique) and C.N.I.P.T. (Comité National Interprofessionnel de la Pomme de Terre).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robert, L., Narcy, A., Rock, E. et al. Entire potato consumption improves lipid metabolism and antioxidant status in cholesterol-fed rat. Eur J Nutr 45, 267–274 (2006). https://doi.org/10.1007/s00394-006-0594-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-006-0594-y