Abstract
Background
The ISAR-REACT 5 trial compared the efficacy and safety of ticagrelor and prasugrel in patients with ACS managed invasively. The present study sought to investigate the impact of ticagrelor and prasugrel on the incidence and pattern of urgent revascularization in acute coronary syndromes (ACS) patients undergoing percutaneous coronary intervention (PCI).
Methods and results
This post-hoc analysis of the ISAR-REACT 5 trial included all ACS patients who underwent PCI. The primary endpoint for this analysis was the incidence of urgent revascularization at 12-month follow-up. Secondary outcome was the pattern of urgent revascularization procedures (namely, urgent target vessel/non-target vessel revascularization – TVR/NTVR). Among 3,377 ACS patients who underwent PCI, 1,676 were assigned to ticagrelor and 1,701 to prasugrel before PCI. After 12 months, the incidence of urgent revascularization was higher among patients assigned to ticagrelor as compared to prasugrel (6.8% vs. 5.2%; hazard ratio [HR] = 1.32, 95% confidence interval [CI] 1.00–1.75; p = 0.051), mostly attributable to significantly more urgent NTVR in the ticagrelor group (3.8% vs. 2.4%; HR = 1.62 [1.09–2.41]; p = 0.017). The risk of urgent TVR did not differ between treatment groups (3.3% vs. 3.0%; HR = 1.13 [0.77–1.65]; p = 0.546).
Conclusions
In ACS patients treated with PCI, the cumulative rate of urgent revascularizations after 12 months is higher with ticagrelor compared to prasugrel, due to a significant increase in urgent revascularizations involving remote coronary vessels.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The percutaneous coronary intervention (PCI) has shown great advancement both in terms of safety and efficacy in the contemporary era [1, 2]. Notwithstanding this, the number of readmissions for repeat revascularization following index PCI remains non-negligible [3, 4] and poses further risk of adverse clinical outcomes particularly in the presence of complex anatomy [5]. Previous studies reported that revascularizations of non-target vessels have a recognized role among subsequent procedures after index PCI [6, 7].
The potent P2Y12-inhibitors ticagrelor and prasugrel represent the antiplatelet therapies of choice in patients presenting with ACS without indication for lifelong oral anticoagulation [8]. The ISAR-REACT 5 randomized trial showed the superiority of prasugrel over ticagrelor in reducing the one-year incidence of ischemic outcomes without excess bleeding both in the overall trial population [9] and in the cohort receiving a PCI of a culprit lesion [10]. Interestingly, latest data lend support to different levels of off-target effects for ticagrelor and prasugrel involving endothelial function, inflammatory parameters, beyond platelet function inhibition [11, 12]. In this regard, whether ticagrelor and prasugrel affect the incidence of urgent revascularization involving both culprit and remote (non-treated) lesions remains unstudied. In fact, the whole spectrum of myocardial revascularizations has not been assessed in the 2 pivotal trials that compared prasugrel [13] or ticagrelor [14] with clopidogrel in patients with ACS. Against this background, the present analysis is the first to report data on the relative merits of ticagrelor versus prasugrel regarding urgent revascularization in patients with ACS managed invasively.
Methods
Study population and design
The current study is a post-hoc analysis of the ISAR-REACT 5 trial. Briefly, the ISAR-REACT 5 trial was an investigator-initiated, multicentre, randomized, open-label clinical trial. Patients with ACS (unstable angina, non-ST-segment elevation myocardial infarction – NSTEMI, or ST-segment elevation myocardial infarction – STEMI) planned to undergo invasive evaluation were eligible for enrolment [9, 15]. Patients were randomized only if they were eligible for both prasugrel and ticagrelor. Ethics committees from each participating institution approved the study protocol and the study complied with the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent before enrolment.
Treatment groups
Patients were randomly assigned to receive either ticagrelor or prasugrel following randomization. Patients in the ticagrelor group received a loading dose of 180 mg, followed by 90 mg twice daily. In the prasugrel group, patients received a loading dose of 60 mg, followed by 10 mg once daily. The loading dose of ticagrelor and prasugrel was administered as soon as possible after randomization and before coronary angiography in patients with STEMI. In patients who had ACS without ST-segment elevation, a prasugrel loading dose was administered once the coronary anatomy was known and before proceeding to PCI. Patients aged ≥ 75 years and those with a body weight < 60 kg received a maintenance dose of 5 mg prasugrel [16]. Finally, an initial single loading dose of 150–300 mg of intravenous or chewed aspirin was administered in both groups, followed by 75–100 mg once daily maintenance dose together with the study drug. A dual antiplatelet therapy with either ticagrelor or prasugrel in addition to aspirin was recommended for at least 12 months.
Study endpoints, follow-up and monitoring
For the current study, the primary endpoint was the incidence of urgent revascularization (percutaneous or surgical). Urgent revascularization was defined as any unplanned hospital readmission and myocardial revascularization procedure due to symptoms or signs of acute ischemia. Secondary endpoint was the incidence of urgent revascularization procedures related to target vessel or non-target vessel. Target vessel revascularization (TVR) was defined according to the Academic Research Consortium (ARC)-2 Criteria as any repeat PCI or surgical bypass of any segment of the target vessel, including the target lesion [17]. Non-target vessel revascularization (NTVR) was defined as any revascularization of a remote vessel with respect to index PCI. Target lesion revascularization was defined as a repeat percutaneous intervention of the target lesion or bypass surgery of the target vessel performed for restenosis or other complication of the target lesion. The composite of death, MI, or stroke and Bleeding Academic Research Consortium (BARC) type 3 through 5 bleeding according to assigned therapy for the cohort of ACS patients who underwent PCI have previously been reported [10] and are shown in Supplemental Table 1. The primary and secondary endpoints of this post-hoc analysis were investigator-reported and were collected in the electronic case report forms, although they were not part of the primary analyses of the trial results. Urgent revascularization procedures associated with the occurrence of main study endpoints (death, MI, stroke and stent thrombosis – ST) were adjudicated by the Central Event Adjudication Committee.
Follow-up was scheduled at 30 ± 10 days, 6 ± 1 months and 12 ± 1 months. Patients were monitored either through telephone calls, structured follow-up letters, outpatient or hospital visits. In case of potential endpoint-related adverse events, we solicited source data from care practitioners in charge for the patient management.
Statistical analysis
The current analysis is post-hoc since it was not pre-specified in the protocol of the parental trial. Continuous variables are expressed as mean ± standard deviation or median with interquartile range (IQR) and were compared between groups using Student’s t-test or the nonparametric Wilcoxon rank-sum test, respectively. Categorical variables are reported as frequencies and percentages and were compared using the χ2 or Fisher’s exact tests. Kaplan–Meier curves were created to estimate the event-free survival for each endpoint of interest. All endpoints were analyzed after accounting for the competing risk of death [18], and cumulative incidence functions were calculated by using the R-package cmprsk [19, 20]. Hazard ratios and 95% confidence intervals were calculated using Cox proportional hazards regression model. The model included the following factor variables as covariates: trial group, participating center, and clinical presentation. We performed a landmark analysis (using the 30-day time point as a landmark) to investigate a potential time dependence of the incidence of urgent revascularization according to assigned antiplatelet therapy. The analysis of the outcomes of interest for this study was mainly performed in the full analysis set according to the intention-to-treat principle. In addition, we assessed the treatment effect for primary outcome on the basis of what treatment the patients actually received, irrespective of the original randomization (on-treatment analysis). Hypothesis testing was performed at two tailed significance levels of 0.05. Statistical analysis was performed using the R 4.1.0 Statistical Package (The R Foundation for Statistical Computing, Vienna, Austria).
Results
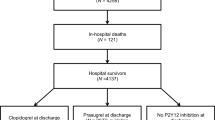
Of 4,018 patients enrolled in the ISAR-REACT 5 trial, 3,377 patients underwent PCI, 553 patients were treated conservatively, 83 patients underwent coronary artery bypass graft procedure and one patient in the ticagrelor group underwent surgery for aortic dissection. Treatment strategy was not available for 4 patients who withdrew consent. Of patients who underwent a CABG procedure after randomization (47 in the ticagrelor and 36 in the prasugrel group), only 5 patients (3 in the ticagrelor and 2 in the prasugrel group) underwent a revascularization procedure during the 1-year follow-up (p = 0.94).
Baseline characteristics
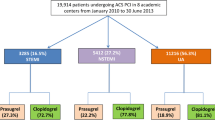
In total, 3,377 of 4,018 patients (84.1%) were included in this analysis. Of them, 1,676 patients were assigned to ticagrelor and 1,701 to prasugrel. Baseline clinical characteristics in the overall cohort and in the treatment groups according to assigned therapy are displayed in Table 1. Of note, 523 out of 3,377 patients (15.5%) had prior MI and 749 (22.2%) had prior PCI, whilst 202 (6.0%) had prior surgical myocardial revascularization. The groups were well-balanced with regard to angiographic and procedural characteristics (Table 2). More than two thirds of participants had multivessel disease and more than half presented with a complex anatomy. Overall, the procedural success rate was 97.8%. Proportion of patients who discontinued treatment drugs and details of therapy at discharge are presented in Supplemental Table 2. The compliance with assigned antiplatelet therapy at different time points of follow-up is shown in Supplemental Fig. 1. The baseline and angiographic characteristics along with drug therapy at discharge for patients who were treated conservatively is shown in Supplemental Table 3.
Clinical outcomes
The breakdown of numbers concerning urgent revascularization after index PCI as per assigned antiplatelet therapy is summarized in Table 3. The clinical outcomes for patients who were treated conservatively are shown in Supplemental Table 4. In this cohort, the risk of urgent revascularization (HR = 0.79 [0.30–2.06], p = 0.625) was not statistically different among treatment groups up to 12-month follow-up.
Urgent revascularization
After 12 months, a total of 113 patients (6.8%) in the ticagrelor group and 88 patients (5.2%) in the prasugrel group underwent urgent revascularization (HR = 1.32 [1.00–1.75], p = 0.051; Fig. 1). In the on-treatment analysis, ticagrelor was associated with a significantly higher risk of urgent revascularization as compared to prasugrel (HR = 1.34 [1.01–1.78], p = 0.044; Supplemental Table 5). There were only 8 cases of myocardial infarction observed within 2 weeks after a revascularization procedure, 5 in the ticagrelor and 3 in the prasugrel group.
Urgent target vessel revascularization
After 12 months, 55 patients (3.3%) in the ticagrelor group and 50 patients (3.0%) in the prasugrel group underwent urgent TVR (HR = 1.13 [0.77–1.65], p = 0.546; Fig. 2A). In the on-treatment analysis, ticagrelor was associated with a comparable risk of urgent TVR as compared to prasugrel (HR = 1.08 [0.73–1.61], p = 0.694; Supplemental Table 5).
Urgent non-target vessel revascularization
After 12 months, urgent NTVR occurred in 63 patients (3.8%) in the ticagrelor group and 40 patients (2.4%) in the prasugrel group (HR = 1.62 [1.09–2.41], p = 0.017; Fig. 2B). The on-treatment analysis confirmed the higher risk for urgent NTVR associated with ticagrelor as compared with prasugrel (HR = 1.73 [1.16–2.59], p = 0.008; Supplemental Table 5).
Urgent target lesion revascularization
After 12 months, a total of 69 patients (4.2%) in the ticagrelor group and 66 patients (3.9%) in the prasugrel group underwent target lesion revascularization (HR = 1.08 [0.77–1.51], p = 0.673). Similarly, the incidence of urgent target lesion revascularization was comparable between the groups (1.9% vs. 1.9%; HR = 1.02 [0.62–1.66], p = 0.939).
Landmark analysis
The result of the landmark analysis for the outcome urgent revascularization according to assigned antiplatelet therapies is displayed in Supplemental Fig. 2. From 0 to 30 days following PCI, 56 out of 1,676 patients (3.4%) in the ticagrelor group and 41 out of 1,701 patients (2.4%) in the prasugrel group underwent urgent revascularization (HR = 1.40 [0.94–2.10], p = 0.100). From 30 days to 12 months, urgent revascularization occurred in 57 patients (3.6%) in the ticagrelor group and 47 patients (2.9%) in the prasugrel group (HR = 1.25 [0.85–1.84], p = 0.256).
Discussion
The key findings of this post-hoc analysis of the ISAR-REACT 5 trial are as follows:
-
i.
Twelve months after index PCI, the incidence of urgent revascularization was higher in patients assigned to ticagrelor as compared to prasugrel.
-
ii.
Approximately one half of urgent revascularizations were attributable to remote coronary vessels, with a significantly higher incidence of urgent NTVR procedures in patients receiving ticagrelor as compared to prasugrel.
-
iii.
The risk of urgent TVR or TLR did not differ significantly between treatment groups.
Although current percutaneous technologies and potent antithrombotic therapies have an indisputable role in reducing adverse events in patients with unstable coronary artery disease (CAD) [1, 2, 21], this is the first study investigating the incidence and pattern of urgent revascularization in ACS patients treated with PCI and assigned to ticagrelor and prasugrel in a randomized trial. Importantly, the present analysis is important because it focuses on the clinically more relevant event of urgent revascularization, performed in both target and non-target vessel localisations. We showed that differences in the efficacy between ticagrelor and prasugrel might go beyond the composite endpoint of death, MI and stroke, the typical endpoint in trials of P2Y12-inhibitors. In our opinion, this study provides unique contribution to the assessment of optimal antiplatelet therapy in patients with ACS, and its results deserve careful discussion.
Firstly, despite improved secondary prevention measures in patients with established CAD, the progression of atherosclerosis in remote sites, unrelated to the culprit lesions, increase significantly the burden of revascularizations in patients with ACS. In keeping with this, our analysis shows a similar rate of urgent NTVR and urgent TVR events. The evidence concerning the proportion, the timing, and the type of adverse events referable to these remote lesions remain controversial [4, 6, 22, 23]. Zellweger et al. [23] reported a higher incidence of target vessel events compared to events attributable to remote vessels from 7 months up to 5 years in patients treated with earlier stent platforms. In a recent pooled analysis of 2 randomized trials, Coughlan et al. [6] reported that events related to non-culprit lesions make up for a higher proportion of total events at 10 years. At variance with our study, this latter analysis showed a higher incidence of target vessel related events up to one year post-PCI. The frequency of urgent NTVR observed in our study might possibly reflect a well-known overall limitation of coronary angiography. Patients with multivessel disease could have possibly presented with angiographically mild non-culprit lesions at the time of index PCI, whereas in reality, these were critical (or vulnerable) lesions with thin-cap fibroatheroma, a large plaque burden and/or stenosis prone to rupture and thrombosis [22, 24]. Furthermore, factors such as vessel geometry that might affect and influence the coronary disease development are not easily assessed through the two-dimensional planar projections of a coronary vessel [25]. A lower threshold to intracoronary imaging with in-vivo plaque-component characterization could be a valuable option to identify intermediate lesions amenable to revascularization independently from the degree of stenosis [26]. However, in the current practice, despite encouragement from consensus documents [27], the acutual penetration of intravascular imaging in clinical practice in Europe and the US remains relatively low [28, 29].
Secondly, it is worth to mention that the evidence of a high proportion of patients undergoing myocardial revascularization after a PCI for ACS has important clinical implications since earlier studies indicate a worse prognosis associated with repeat revascularizations [5, 30]. In fact, in the SYNTAX trial, including patients with complex coronary anatomy such as those with multivessel and/or left main disease, participants who underwent repeat revascularization had a significantly higher rate of adverse clinical events in comparison to those who did not [5]. Consistent with the results of the SYNTAX trial, in the current analysis over half of the patients who underwent myocardial revascularization among those included presented a complex coronary anatomy at the time of PCI of culprit lesions.
Finally in our study, the group of participants receiving ticagrelor had a higher number of urgent revascularization compared with the group receiving prasugrel. This correlates with a previous analysis from the ISAR-REACT 5 trial, in which a large proportion of MI events observed in the ticagrelor group were either type 1 (spontaneous), type 4a (PCI-related) or type 4b (ST-related) [9]. In addition, the higher incidence of urgent revascularization in the ticagrelor group was actually due to significantly more frequent urgent NTVR procedures. Of note, we observed a higher number of patients in the ticagrelor group who discontinued the study medication over the follow-up. This is in keeping with real-life data, in which the adherence to ticagrelor is diminished during maintenance phase [31], mostly because of extra-platelet inhibition side effects such as dyspnoea [32]. However, although interruptions of ticagrelor treatment may be harmful to patients because of the reversible mode of action of this antiplatelet agent, the on-treatment analysis performed for this study, including those patients actually taking the study medications, confirmed a higher risk for urgent revascularization with ticagrelor as compared to prasugrel.
Study limitations
This is a post-hoc analysis of a randomized control trial and as such it suffers the common potential limitations associated with not pre-specified analyses. Accordingly, these results should be regarded as hypothesis generating. Second, information regarding intracoronary imaging or physiology at the time of revascularization was not routinely collected in the electronic case report forms in the primary trial. Thus, we have no data concerning intracoronary imaging or physiologies in the setting of both index and subsequent myocardial revascularization procedures. Third, this analysis did not account for recurrent revascularization events. Fourth, this report lacks detailed information concerning secondary prevention measures among patients with ACS, since this was not routinely collected among participants. In this regard, the association between secondary prevention medications (as those for lipid and glycemic control) and urgent revascularization in ACS patients treated with ticagrelor or prasugrel cannot be investigated. Finally, the ISAR REACT 5 trial was an open-label study, albeit with adjudication of clinical events carried out in a blinded manner.
Conclusions
In patients with ACS treated with PCI, ticagrelor is associated with more urgent revascularizations after 12 months compared to prasugrel, mostly attributable to significantly more urgent revascularizations of remote coronary vessels.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Kastrati A, Mehilli J, Pache J et al (2007) Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med 356(10):1030–1039. https://doi.org/10.1056/NEJMoa067484
Cassese S, Byrne RA, Tada T et al (2014) Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 100(2):153–159. https://doi.org/10.1136/heartjnl-2013-304933
Curtis JP, Schreiner G, Wang Y et al (2009) All-cause readmission and repeat revascularization after percutaneous coronary intervention in a cohort of medicare patients. J Am Coll Cardiol 54(10):903–907. https://doi.org/10.1016/j.jacc.2009.04.076
Stolker JM, Cohen DJ, Kennedy KF et al (2012) Repeat revascularization after contemporary percutaneous coronary intervention: an evaluation of staged, target lesion, and other unplanned revascularization procedures during the first year. Circ Cardiovasc Interv 5(6):772–782. https://doi.org/10.1161/CIRCINTERVENTIONS.111.967802
Parasca CA, Head SJ, Milojevic M et al (2016) Incidence, characteristics, predictors, and outcomes of repeat revascularization after percutaneous coronary intervention and coronary artery bypass grafting: the SYNTAX trial at 5 years. JACC Cardiovasc Interv 9(24):2493–2507. https://doi.org/10.1016/j.jcin.2016.09.044
Coughlan JJ, Aytekin A, Xhepa E et al (2022) Target and non-target vessel related events at 10 years post percutaneous coronary intervention. Clin Res Cardiol. https://doi.org/10.1007/s00392-022-01986-4
Spitaleri G, Moscarella E, Brugaletta S et al (2018) Correlates of non-target vessel-related adverse events in patients with ST-segment elevation myocardial infarction: insights from five-year follow-up of the EXAMINATION trial. EuroIntervention 13(16):1939–1945. https://doi.org/10.4244/EIJ-D-17-00608
Lawton JS, Tamis-Holland JE, Bangalore S et al (2022) 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 145(3):e18–e114. https://doi.org/10.1161/CIR.0000000000001038
Schüpke S, Neumann FJ, Menichelli M et al (2019) Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med 381(16):1524–1534. https://doi.org/10.1056/NEJMoa1908973
Coughlan JJ, Aytekin A, Lahu S et al (2021) Ticagrelor or prasugrel for patients with acute coronary syndrome treated with percutaneous coronary intervention: a prespecified subgroup analysis of a randomized clinical trial. JAMA Cardiol 6(10):1121–1129. https://doi.org/10.1001/jamacardio.2021.2228
Schnorbus B, Daiber A, Jurk K, et al (2020) Effects of clopidogrel vs. prasugrel vs. ticagrelor on endothelial function, inflammatory parameters, and platelet function in patients with acute coronary syndrome undergoing coronary artery stenting: a randomized, blinded, parallel study. Eur Heart J. https://doi.org/10.1093/eurheartj/ehz917
Ariotti S, Ortega-Paz L, van Leeuwen M et al (2018) Effects of ticagrelor, prasugrel, or clopidogrel on endothelial function and other vascular biomarkers: a randomized crossover study. JACC Cardiovasc Interv 11(16):1576–1586. https://doi.org/10.1016/j.jcin.2018.04.022
Wiviott SD, Braunwald E, McCabe CH et al (2007) Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357(20):2001–2015. https://doi.org/10.1056/NEJMoa0706482
Wallentin L, Becker RC, Budaj A et al (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361(11):1045–1057. https://doi.org/10.1056/NEJMoa0904327
Schulz S, Angiolillo DJ, Antoniucci D et al (2014) Randomized comparison of ticagrelor versus prasugrel in patients with acute coronary syndrome and planned invasive strategy–design and rationale of the intracoronary stenting and antithrombotic regimen: rapid early action for coronary treatment (ISAR-REACT) 5 trial. J Cardiovasc Transl Res 7(1):91–100. https://doi.org/10.1007/s12265-013-9527-3
Menichelli M, Neumann FJ, Ndrepepa G et al (2020) Age- and weight-adapted dose of prasugrel versus standard dose of ticagrelor in patients with acute coronary syndromes : results from a randomized trial. Ann Intern Med 173(6):436–444. https://doi.org/10.7326/M20-1806
Garcia-Garcia HM, McFadden EP, Farb A et al (2018) Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation 137(24):2635–2650. https://doi.org/10.1161/CIRCULATIONAHA.117.029289
Austin PC, Lee DS, Fine JP (2016) Introduction to the analysis of survival data in the presence of competing risks. Circulation 133(6):601–609. https://doi.org/10.1161/CIRCULATIONAHA.115.017719
Gray RJ (1988) A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16(3):1141–1154. https://doi.org/10.1214/aos/1176350951
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446):496–509. https://doi.org/10.1080/01621459.1999.10474144
Stone GW, Maehara A, Lansky AJ et al (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364(3):226–235. https://doi.org/10.1056/NEJMoa1002358
Zellweger MJ, Kaiser C, Jeger R et al (2012) Coronary artery disease progression late after successful stent implantation. J Am Coll Cardiol 59(9):793–799. https://doi.org/10.1016/j.jacc.2011.11.024
Erlinge D, Maehara A, Ben-Yehuda O et al (2021) Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet 397(10278):985–995. https://doi.org/10.1016/s0140-6736(21)00249-x
Rampidis G, Rafailidis V, Kouskouras K, et al (2022) Relationship between coronary arterial geometry and the presence and extend of atherosclerotic plaque burden: a review discussing methodology and findings in the era of cardiac computed tomography angiography. Diagnostics (Basel) 12(9). https://doi.org/10.3390/diagnostics12092178
Waksman R, Di Mario C, Torguson R et al (2019) Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet 394(10209):1629–1637. https://doi.org/10.1016/S0140-6736(19)31794-5
Stefanini GG, Alfonso F, Barbato E et al (2020) Management of myocardial revascularisation failure: an expert consensus document of the EAPCI. EuroIntervention 16(11):e875–e890. https://doi.org/10.4244/eij-d-20-00487
Elgendy IY, Ha LD, Elbadawi A et al (2018) Temporal trends in inpatient use of intravascular imaging among patients undergoing percutaneous coronary intervention in the United States. JACC Cardiovasc Interv 11(9):913–915. https://doi.org/10.1016/j.jcin.2018.01.254
Koskinas KC, Nakamura M, Raber L et al (2018) Current use of intracoronary imaging in interventional practice - results of a European association of percutaneous cardiovascular interventions (EAPCI) and Japanese association of cardiovascular interventions and therapeutics (CVIT) clinical practice survey. EuroIntervention 14(4):e475–e484. https://doi.org/10.4244/EIJY18M03_01
Palmerini T, Della Riva D, Biondi-Zoccai G et al (2018) Mortality following nonemergent, uncomplicated target lesion revascularization after percutaneous coronary intervention: an individual patient data pooled analysis of 21 randomized trials and 32,524 patients. JACC Cardiovasc Interv 11(9):892–902. https://doi.org/10.1016/j.jcin.2018.01.277
Zanchin T, Temperli F, Karagiannis A et al (2018) Frequency, reasons, and impact of premature ticagrelor discontinuation in patients undergoing coronary revascularization in routine clinical practice: results from the bern percutaneous coronary intervention registry. Circ Cardiovasc Interv 11(5):e006132. https://doi.org/10.1161/CIRCINTERVENTIONS.117.006132
Zeymer U, Cully M, Hochadel M (2018) Adherence to dual antiplatelet therapy with ticagrelor in patients with acute coronary syndromes treated with percutaneous coronary intervention in real life. Results of the REAL-TICA registry. Eur Heart J Cardiovasc Pharmacother 4(4):205–10. https://doi.org/10.1093/ehjcvp/pvy018
Kirtane AJ, Gupta A, Iyengar S et al (2009) Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 119(25):3198–3206. https://doi.org/10.1161/CIRCULATIONAHA.108.826479
Funding
Open Access funding enabled and organized by Projekt DEAL. The ISAR-REACT 5 randomized control trial was supported by a grant [FKZ 81 × 1 600 501] from the German Centre for Cardiovascular Research and the Deutsches Herzzentrum München, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I.B. reported receiving personal fees from Sysmex Europe GmbH outside the submitted work. B.W. reported receiving grants from ISAReasarch Center during the conduct of the study. W.H. reported receiving personal fees from Bayer Vital, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Novartis, AstraZeneca, and The Medicines Company outside the submitted work. D.S. reported receiving personal fees from Sanofi, Bayer, Daiichi Sankyo, AstraZeneca, and Pfizer during the conduct of the study. D.J.A. declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL-Behring, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi and Vectura, outside the present work. D.J.A. also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and Scott R. MacKenzie Foundation. S.K. reported personal fees from Bristol Myers Squibb, personal fees from AstraZeneca, and personal fees from Translumia outside the submitted work. R.H. reported receiving speaker and consultancy fees from Boston Scientific outside the submitted work. C.V. reported receiving personal fees from Boehringer Ingelheim outside the submitted work. M.J. reported receiving personal fees from Biotronik, OrbusNeich, Boston Scientific, Edwards, Recor, AstraZeneca, and Abbott; and grants from Boston Scientific, Edwards, and Amgen, outside the submitted work. F.J.N. reported receiving grants from Abbott Vascular, Boston Scientific, and Biotronic; personal fees and consultancy fees from Daiichi Sankyo, Amgen, Boston Scientific, Meril, Novartis, and Ferrer, outside the submitted work. H.S. reported receiving personal fees from MSD Sharp & Dohme, Amgen, Bayer Vital GmbH, Boehringer Ingelheim, Daiichi Sankyo, Novartis, Servier, Brahms, Bristol Myers Squibb, Medtronic, Sanofi Aventis, Synlab, Pfizer, and Vifor; and grants from AstraZeneca outside the submitted work. S.S. reported receiving grants from DZHK (German Center for Cardiovascular Research) during the conduct of the study; and grants from Else Kröner-Fresenius-Stiftung (Else Kröner-Memorial grant); personal fees from Bayer Vital GmbH, Daiichi Sankyo, and Biopas Laboratoires outside the submitted work. No other disclosures were reported.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aytekin, A., Scalamogna, M., Coughlan, J.J. et al. Incidence and pattern of urgent revascularization in acute coronary syndromes treated with ticagrelor or prasugrel. Clin Res Cardiol 113, 1060–1069 (2024). https://doi.org/10.1007/s00392-024-02454-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-024-02454-x