Abstract
Background
Patients with chronic conditions, such as heart failure, swim regularly and most rehabilitation exercises are conducted in warm hydrotherapy pools. However, little is known about the acute effects of warm water immersion (WWI) on cardiac haemodynamics in patients with chronic heart failure (CHF).
Methods
Seventeen patients with CHF (NYHA I and II; mean age 67 years, 88% male, mean left ventricular ejection fraction 33%) and 10 age-matched normal subjects were immersed up to the neck in a hydrotherapy pool (33–35 °C). Cardiac haemodynamics were measured non-invasively, and echocardiography was performed at baseline, during WWI, 3 min after kicking in the supine position and after emerging.
Results
In patients with CHF, compared to baseline, WWI immediately increased stroke volume (SV, mean ± standard deviation; from 65 ± 21 to 82 ± 22 mL, p < 0.001), cardiac output (CO, from 4.4 ± 1.4 to 5.7 ± 1.6 L/min, p < 0.001) and cardiac index (CI, from 2.3 ± 0.6 to 2.9 ± 0.70 L/min/m², p < 0.001) with decreased systemic vascular resistance (from 1881 ± 582 to 1258 ± 332 dynes/s/cm5, p < 0.001) and systolic blood pressure (132 ± 21 to 115 ± 23 mmHg, p < 0.001). The haemodynamic changes persisted for 15 min of WWI. In normal subjects, compared to baseline, WWI increased SV (from 68 ± 11 to 80 ± 18 mL, p < 0.001), CO (from 5.1 ± 1.9 to 5.7 ± 1.8 L/min, p < 0.001) and CI (from 2.7 ± 0.9 to 2.9 ± 1.0 L/min/m², p < 0.001).In patients with CHF, compared to baseline, WWI caused an increase in left atrial volume (from 57 ± 44 to 72 ± 46 mL, p = 0.04), without any changes in left ventricular size or function or amino terminal pro B-type natriuretic peptide.
Conclusions
In patients with CHF, WWI causes an acute increase in cardiac output and a fall in systemic vascular resistance.
Clinical trial registration
ClinicalTrials.gov (Identifier: NCT02949544) https://clinicaltrials.gov/ct2/show/NCT02949544?cond=NCT02949544&rank=1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise training is recommended for patients with chronic heart failure (CHF) [1,2,3,4], not only to improve exercise capacity but also for its beneficial effects on morbidity and mortality [5,6,7]. Although symptom-limiting exercise is safe in patients with heart failure [8], factors such as advanced age [9, 10], and comorbidities such as osteoarthritis, hinder exercise training on a treadmill or a cycle in patients with CHF. Swimming is common in the United Kingdom and has the advantage of providing buoyancy to reduce impact on joints [11]. Swimming in patients with arthritis improves mobility, strength and cardiovascular fitness without any joint discomfort [12].
Whether swimming is safe in patients with CHF has not been answered comprehensively in previous studies of water immersion or swimming [13]. When normal subjects are immersed up to the neck in water, the hydrostatic pressure forces approximately 700 mL of blood pooled in the periphery to the cardiothoracic space [14]. The increased preload to the heart increases stroke volume by 34–50% in normal subjects [14, 15]. However, this increased preload to the failing heart may precipitate pulmonary oedema. Currently, only the Scottish guidelines on the management of CHF mention swimming to warn against it in patients with New York Heart Association (NYHA) class III and IV symptoms [4].
The aim of the present study was to assess the acute hemodynamic, echocardiographic and amino terminal pro B-type natriuretic peptide (NTproBNP) changes during warm water immersion (WWI) in patients with CHF.
Methods
Ambulatory patients with an established diagnosis of CHF, on stable treatment for more than 3 months were enrolled from a community heart failure clinic. Patients with severe symptoms (NYHA class IV), weight over 120 kilograms, hospitalised within last 6 weeks or with a contraindication to WWI (epilepsy, recent hypoglycaemia, intravenous line or urinary catheter) were excluded from the study. Controls were normal subjects, over 60 years of age, who were already consented to take part as healthy volunteers in a local observational research program. Normal subjects had to have a left ventricular ejection fraction (LVEF) ≥ 50% on echocardiography.
The research conforms to the Helsinki declaration and ethics approval was granted by an external research ethics committee. The trial was registered on the ClinicalTrials.gov website (Identifier: NCT02949544) and all participants gave their written informed consent.
Water immersion protocol
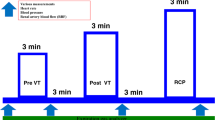
The study was conducted in a hydrotherapy pool on the hospital site. The pool temperature was maintained between 33 and 35 °C and the outside temperature was maintained at 21 °C. A metal bed designed to be immersed in water was attached to a manual hoist fixed next to the pool. Participants remained in supine position on the bed throughout the study and the patients were immersed and removed from the water using the hoist. (Fig. 1) Supine position was chosen as this was the best position to mimic swimming posture and also enable non-invasive haemodynamic measurements and echocardiography whilst immersed.
Study equipment. Echocardiography machine: GE Vivid E9, Hatfield, Hertfordshire, UK. Non-invasive haemodynamic monitoring device (Nexfin, BMeye, Amsterdam, The Netherlands) with an inflatable finger cuff was attached to the mid finger of participant’s left hand. Hydrotherapy pool (maintained at a temperature between 33 and 35 °C)
Baseline measurements, including a blood sample for NTproBNP analysis, and echocardiography (GE Vivid E9, Hatfield, Hertfordshire, UK) were conducted after participants were comfortable on the bed for 10–15 min. A non-invasive haemodynamic monitoring device (Nexfin, BMeye, Amsterdam, The Netherlands) was used to continuously monitor cardiac haemodynamics. This device has been validated against invasive methods of monitoring cardiac haemodynamics in studies of patients with heart failure and critically ill patients [16,17,18]. An inflatable finger cuff was attached to the mid finger of participant’s left hand (Fig. 1) and during immersion the left hand was rested on a floater to ensure the device remained out of the water. The device continuously recorded cardiac haemodynamics from baseline to the end of the study. The reference level for the non-invasive haemodynamic device was at the surface of the water throughout, and thus very slightly higher than the level of the heart. The measurements outside the pool were made with the reference level in the same relative position before immersion. A non-invasive device (VENUS 2000 CVP monitor, Mespere LifeScience Inc, Waterloo, Canada) was used to measure central venous pressure (CVP) using a small adhesive neck sensor placed over the external jugular vein. The device uses near infrared spectroscopy to determine the pressure in the external jugular vein and its clinical use has been validated in critically ill patients with cardiovascular conditions, and in out-patients setting for patient with heart failure [19,20,21].
The participants were then gradually immersed up to their neck into the hydrotherapy pool in supine position. Echocardiography was repeated at 1 and 15 min following WWI with the ultrasound probe covered with a light polythene bag to ensure it remained water proof. After 15 min of WWI, the participants were asked to perform gentle exercise (3 min of kicking at an speed of 40 repetitions per minute) whilst remaining in supine position on the bed. The exercises performed was hip extension and flexion with a straight leg. At the end of 3 min of exercise, echocardiography was repeated. Once all the echocardiographic and haemodynamic readings were recorded the participants were lifted out of the water. Haemodynamic readings, symptom scores, blood sample for NTproBNP and echocardiography were repeated whilst participants remained in supine position on the bed 3 min after emerging from the hydrotherapy pool.
All the electric devices used were checked by University engineers to ensure they were safe for use near water. Additional circuit breaking sockets were installed in case of any power surges or short circuits.
From the non-invasive haemodynamic and central venous pressure monitoring devices, the following variables were recorded at baseline, 1 min WWI, 15 min WWI, 3 min after exercise and after 3 min recovery: heart rate (HR), blood pressure (BP), stroke volume (SV), cardiac output (CO), cardiac index (CI), systemic vascular resistance (SVR) and central venous pressure. An average of five measured values for each haemodynamic variable was taken. All echocardiographic exams were performed by the lead cardiac sonographer in the Department of Academic Cardiology (AB), subsequently stored on DVDs, and finally reviewed off-line by a single experienced operator blind to the various phases of the study (PS).The following echocardiographic variables were measured: left ventricular end diastolic (EDV) and end systolic volumes (ESV), left ventricular ejection fraction (LVEF), left atrial diameter (LAD) and volume (LAV) tricuspid annular plane systolic excursion (TAPSE), systolic tricuspid regurgitation (TR) pressure gradient, inferior vena cava (IVC) diameter. Patients’ modified Borg score, Canadian cardiovascular society angina grading scale, fatigue symptom inventory symptom scores were taken at baseline and recovery.
Statistical analysis
Categorical data are presented as numbers and percentages; normally distributed continuous data as mean ± standard deviation (SD) and non-normally distributed continuous variables as median and interquartile range. The repeated measures ANOVA was used to assess the overall difference between related means of each variable and the Bonferroni correction was used for multiple testing errors. Log transformation of NTproBNP was used, given its not normal distribution. Primary and secondary endpoints are shown in graphs. All analyses were performed on SPSS (V.23.0), and statistical significance was assumed at p < 0.05 (two tailed).
Results
Baseline characteristics of patients with CHF and normal subjects
Baseline characteristics of patients with CHF and normal subjects are shown in Table 1. All but one of the patients had NYHA class II symptoms. Normal subjects were of a similar age, 90% were male and baseline cardiac haemodynamics were similar to patients with CHF.
Haemodynamic changes with warm water immersion and exercise
In patients with CHF, and compared to baseline measurements, WWI caused a significant, immediate (1 min) and sustained (after 15 min) increase in SV, CO, CI and decrease in BP and SVR, with no change in HR or CVP. The 3 min of kicking led to a further increase in HR, BP, CI and CO, with no change in SV, SVR or CVP (Figs. 2, 3).
Cardiac haemodynamic changes during WWI measured by non-invasive haemodynamic monitoring device (Nexfin, BMeye, Amsterdam, The Netherlands). Cardiac haemodynamic changes were measured at baseline, 1 min WWI, 15 min WWI, 3 min after WW exercise, at recovery. HR heart rate, SBP systolic blood pressure, SVR systemic vascular resistance, SV stroke volume, CO cardiac output, CI cardiac index, WWI warm water immersion, WW warm water, CHF chronic heart failure
In normal subjects, WWI caused a significant, immediate (1 min) and sustained (15 min) increase in SV, CO and CI with no change in HR, BP, SVR or CVP. The 3 min of kicking led to an increase in HR, CO and CVP with no change in BP, SV, CI or SVR (Figs. 2, 3).
Echocardiographic changes with warm water immersion and exercise
In patients with CHF, WWI for 15 min led to a significant increase in LAV. The 3 min of kicking led to an increase in estimated pulmonary artery systolic pressure (Figs. 4, 5).
Left cardiac variable changes during WWI. Left cardiac variables changes were measured at baseline, 1 min WWI, 15 min WWI, 3 min after WW exercise, at recovery. EDV End diastolic volume, ESV end systolic volume, LVEF left ventricular ejection fraction, LA left atrial, WWI warm water immersion, WW warm water, CHF chronic heart failure
Right cardiac variable changes during WWI. Right cardiac variables changes were measured at baseline, 1 min WWI, 15 min WWI, 3 min after WW exercise, at recovery. TAPSE: tricuspid annular plane systolic excursion, TR tricuspid regurgitation, IVC inferior vena cava, WWI warm water immersion, WW warm water, CHF chronic heart failure
In normal subjects, WWI for 15 min significantly increased left ventricular EDV. There were no significant changes in echocardiographic variables after 3 min of kicking (Figs. 4, 5).
NTproBNP and symptom changes with warm water immersion and exercise
There was no significant difference in NTproBNP levels between baseline and recovery in patients with CHF [558 (IQR: 323–1140) to 642 (IQR: 309–1187) ng/L, p = 0.21] or normal subjects [82 (42–119) to 84 (43–118) ng/L, p = 0.08]. None of the subjects experienced any adverse events during water immersion. None of the patients reported any change in any symptom (shortness of breath, chest pain or fatigue) during immersion or recovery.
Discussion
We found that WWI in patients with CHF increases cardiac output. The mechanism for the haemodynamic changes is presumably that hydrostatic pressure increased cardiac preload and atrial volumes. WWI caused systemic vasodilation, leading to a fall in blood pressure which, in turn, causes a decreased left ventricular afterload.
Our results are similar to those reported by other researchers. In a study of nine patients with CHF, WWI (34 °C) up to the xiphoid process increased central venous pressure, and as in our study, increased left atrial diameter (by echocardiography), CI and SV index with a decrease in SVR [22]. In a study of 13 patients with CHF (mean LVEF 32%) and more severe symptoms (77% in NYHA class 3), WWI (33–34 °C) up to the sternal notch increased LVEF, SV and CO but also worsened left ventricular diastolic function (increasing trans mitral Doppler E/A ratio), with no effect on blood pressure [23]. 5 min of seated reciprocal unilateral knee-extensions in water increased CO, SBP and HR, and was tolerated by patients [23]. Similarly, in a study of 18 patients with CHF (LVEF 31%, 50% in NYHA class 3), WWI (34 °C) significantly increased SV and CO but decreased HR, BP and SVR. WWI also led to an increase in left ventricular end diastolic and systolic volumes, estimated pulmonary capillary wedge pressure and left ventricular ejection fraction [24].
All the previous studies of WWI on cardiac haemodynamic had patients in sitting or standing positions during WWI. We positioned the participants in supine position because this better replicates the posture during swimming [22,23,24].
Warmer or colder water temperatures might lead to more pronounced, or different effects, on cardiac haemodynamics and heart function. Hot water immersion (41 °C) for 10 min significantly decreased left ventricular and atrial size, increased LVEF and decreased mitral regurgitation in a study of 34 patients with CHF and severe symptoms (LVEF 25%, 94% in NYHA class III/IV). The changes persisted for 30 min after emerging from water [25]. Mean pulmonary artery pressure, mean pulmonary capillary wedge pressure, and mean right atrial pressure rose substantially during hot water immersion but did not cause any symptoms. In two studies, cold-water immersion (12–22°C) for a few seconds increased cardiac output and blood pressure but change in heart rate was variable [26, 27].
Swimming is a popular form of exercise in the United Kingdom [11]. Whether swimming is safe in patients with heart failure is still not known [13, 14]. Swimming-induced pulmonary oedema (SIPE) is a rare occurrence seen in athletes undertaking strenuous exercise [28, 29]. In all studies of water immersion in patients with CHF there have been no reports of SIPE, although the time of water immersion and exercise has been limited to a few minutes in a controlled environment.
In previous studies, warm water immersion up to the neck in standing position for 5–10 min, has increased central venous pressure [30, 31]. We found that central venous pressure did not increase and pulmonary artery systolic pressure increases only after 3 min of gentle exercise in patients with CHF (without precipitating any symptoms), but not in normal subjects. The pulmonary artery systolic pressure rapidly returned to baseline after WWI. Reassuringly, the haemodynamic changes did not lead to an increase in NTproBNP plasma levels. In studies looking at the effect of WWI on natriuretic peptides in normal subjects, atrial natriuretic peptide significantly increased without any change in brain natriuretic peptide [32, 33]. There have been no studies looking at the acute change in natriuretic peptides during water immersion in patients with heart failure. Whether prolonged immersion or swimming causes a more sustained increase in systolic pulmonary pressure and symptoms in patients with CHF requires further studies.
Limitations
Our study was conducted in a controlled, indoor thermoneutral hydrotherapy pool, and therefore, the results cannot be translated to swimming in different environmental conditions. The studied population was small, and patients had only mild symptoms; thus, results of this study cannot be generalised to all patients with CHF, particularly to those with more severe disease. Although majority of the patients swim in prone, we were limited to investigate patients in supine position to enable echocardiography. The workload was not standardized and not adjusted for maximal exercise capacity. It was chosen to allow all participants to be able to conduct 3 min of exercise with continuous haemodynamic monitoring. We did not measure NTproBNP levels after a few hours from completing the study, or troponin levels, which might have provided further information about any delayed effect of WWI with exercise on the myocardium.
Conclusion
In patients with CHF, WWI causes an acute increase in cardiac output and a fall in vascular resistance. The changes were well-tolerated. Whether swimming can be recommended as alternative to other forms of exercise or rehabilitation in patients with CHF needs to be studied further.
References
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members; Document Reviewers (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37:2129–2200. https://doi.org/10.1002/ejhf.592
National Institute for Health and Clinical Excellence (2010) Chronic heart failure: Management of chronic heart failure in adults in primary and secondary care. In: Clinical Guideline, vol 108. NICE, London. https://www.nice.org.uk/guidance/cg108/resources/chronic-heart-failure-in-adults-management-pdf-35109335688901
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR (2013) 2013 ACCF/AHA Guideline for the Management of Heart Failure A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 128:240–327. https://doi.org/10.1161/CIR.0b013e31829e8776
Scottish Intercollegiate Guidelines Network (2007) Management of chronic heart failure. SIGN, Edinburgh. https://www.sign.ac.uk (SIGN Guideline no. 95)
O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pinã IL, HF-Action Investigators (2009) Efficacy and safety of exercise training in patients with chronic heart failure: HFACTION randomized controlled trial. JAMA 301:1439–1450. https://doi.org/10.1001/jama.2009.454
Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD (2015) Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 8:33–40. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001615
Kawauchi TS, Umeda IIK, Braga LM, Mansur AP, Rossi-Neto JM, Guerra de Moraes Rego Sousa A, Hirata MH, Cahalin LP, Nakagawa NK (2017) Is there any benefit using low-intensity inspiratory and peripheral muscle training in heart failure? A randomized clinical trial. Clin Res Cardiol 106:676–685. https://doi.org/10.1007/s00392-017-1089
Voss F, Schueler M, Lauterbach M, Bauer A, Katus HA, Becker R (2016) Safety of symptom-limited exercise testing in a big cohort of a modern ICD population. Clin Res Cardiol 105:53–58. https://doi.org/10.1007/s00392-015-0885-5
Mosterd A, Hoes AW (2007) Clinical epidemiology of heart failure. Heart 93:1137–1146. https://doi.org/10.1136/hrt.2003.025270
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355:251–259. https://doi.org/10.1056/NEJMoa052256
(2010) Evaluation of the impact of free swimming. Year 1 report—main report. Department for Culture, Media & Sport. https://www.gov.uk/government/publications/free-swimming-programme-2009-10-annual-report
Hall J, Skevington SM, Maddison PJ, Chapman K (1996) A randomized and controlled trial of hydrotherapy in rheumatoid arthritis. Arthritis Care Res 9:206–215. https://doi.org/10.1002/1529-0131(199606)9:3%3C206
Shah P, Pellicori P, Macnamara A, Urbinati A, Clark AL (2017) Is swimming safe in heart failure? A systematic review. Cardiol Rev 25:321–325. https://doi.org/10.1097/CRD.0000000000000154
Hall J, Bisson D, O’Hare P (1990) The physiology of immersion. Physiotherapy 76:517–521. https://doi.org/10.1016/S0031-9406(10)63019-2
Weston DFM, O’Hare JP, Evans JM, Corrall RJM (1987) Haemodynamic changes in man during immersion in water at different temperatures. Clin Sci 73:613–616. https://doi.org/10.1042/cs0730613
Ameloot K, Van De Vijver K, Broch O, Van Regenmortel N, De Laet I, Schoonheydt K, Dits H, Bein B, Malbrain ML (2013) Nexfin noninvasive continuous hemodynamic monitoring: validation against continuous pulse contour and intermittent transpulmonary thermodilution derived cardiac output in critically ill patients. Sci World J 2013:51980. https://doi.org/10.1155/2013/519080
Sokolski M, Rydlewska A, Krakowiak B, Biegus J, Zymlinski R, Banasiak W, Jankowska EA, Ponikowski P (2011) Comparison of invasive and non-invasive measurements of haemodynamic parameters in patients with advanced heart failure. J Cardiovasc Med 12:773–778. https://doi.org/10.2459/JCM.0b013e32834cfebb
Martina JR, Westerhof BE, van Goudoever J, de Beaumont EM, Truijen J, Kim YS, Immink RV, Jöbsis DA, Hollmann MW, Lahpor JR, de Mol BA, van Lieshout JJ (2012) Noninvasive continuous arterial blood pressure monitoring with Nexfin®. Anesthesiology 116:1092–1103. https://doi.org/10.1097/ALN.0b013e31824f94ed
Hoyt J, Koelling TM (2013) Non-invasive assessment of central venous pressure using near infrared spectroscopy. J Card Fail 19:S51
Sathish N, Singh NG, Nagaraja PS, Sarala BM, Prabhushankar CG, Dhananjaya M, Manjunatha N (2016) Comparison between noninvasive measurement of central venous pressure using near infrared spectroscopy with an invasive central venous pressure monitoring in cardiac surgical Intensive Care Unit. Ann Card Anaesth 19:405–409. https://doi.org/10.4103/0971-9784.185520
Pellicori P, Clark AL, Kallvikbacka-Bennett A, Zhang J, Urbinati A, Monzo L, Dierckx R, Anker SD, Cleland JGF (2017) Non-invasive measurement of right atrial pressure by near-infrared spectroscopy: preliminary experience. A report from the SICA-HF study. Eur J Heart Fail 19:883–892. https://doi.org/10.1002/ejhf.825
Gabrielsen A, Sorensen VB, Pump B, Galatius S, Videbaek R, Bie P, Warberg J, Christensen NJ, Wroblewski H, Kastrup J, Norsk P (2000) Cardiovascular and neuroendocrine responses to water immersion in compensated heart failure. Am J Physiol Heart Circ Physiol 279:1931–1940. https://doi.org/10.1152/ajpheart.2000.279.4.H1931
Cider A, Svealv BG, Tang MS, Schaufelberger M, Andersson B (2006) Immersion in warm water induces improvement in cardiac function in patients with chronic heart failure. Eur J Heart Fail 8:308–313. https://doi.org/10.1016/j.ejheart.2005.08.001
Sveälv BG, Cider A, Täng MS, Angwald E, Kardassis D, Andersson B (2009) Benefit of warm water immersion on biventricular function in patients with chronic heart failure. Cardiovasc Ultrasound 7:33. https://doi.org/10.1186/1476-7120-7-33
Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N (1995) Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91:2582–2590. https://doi.org/10.1161/01.CIR.91.10.2582
Radtke T, Poerschke D, Wilhelm M, Trachsel LD, Tschanz H, Matter F, Jauslin D, Saner H, Schmid JP (2016) Acute effects of Finnish sauna and cold-water immersion on haemodynamic variables and autonomic nervous system activity in patients with heart failure. Eur J Prev Cardiol 23:593–601. https://doi.org/10.1177/2047487315594506
Schmid JP, Morger C, Noveanu M, Binder RK, Anderegg M, Saner H (2009) Haemodynamic and arrhythmic effects of moderately cold (22 °C) water immersion and swimming in patients with stable coronary artery disease and heart failure. Eur J Heart Fail 11:903–909. https://doi.org/10.1093/eurjhf/hfp114
Moon RE, Martina SD, Peacher DF, Potter JF, Wester TE, Cherry AD, Natoli MJ, Otteni CE, Kernagis DN, White WD, Freiberger JJ (2016) Swimming-induced pulmonary edema: pathophysiology and risk reduction with sildenafil. Circulation 133:988–996. https://doi.org/10.1161/CIRCULATIONAHA.115.019464
Weiler-Ravell D, Shupak A, Goldenberg I, Halpern P, Shoshani O, Hirschhorn G, Margulis A (1995) Pulmonary oedema and haemoptysis induced by strenuous swimming. BMJ 311:361–362. https://doi.org/10.1136/bmj.311.7001.361
Risch WD, Koubenec HJ, Beckmann U, Lange S, Gauer OH (1978) The effect of graded immersion on heart volume, central venous pressure, pulmonary blood distribution, and heart rate in man. Pflugers Arch 374:115–118
Gabrielsen A, Johansen LB, Norsk P (1993) Central cardiovascular pressures during graded water immersion in humans. J Appl Physiol 75:581–585
Anderson JV, Millar ND, O’Hare JP, Mackenzie JC, Corrall RJ, Bloom SR (1986) Atrial natriuretic peptide: physiological release associated with natriuresis during water immersion in man. Clin Sci 71:319–322. https://doi.org/10.1042/cs0710319
Kurabayashi H, Tamura K, Tamura J, Kubota K (2001) The effects of hydraulic pressure on atrial natriuretic peptide during rehabilitative head-out water immersion. Life Sci 69:1017–1021. https://doi.org/10.1016/S0024-3205(01)01193-6
Funding
The research leading to these results has received funding from the Hull & East Riding Cardiac Trust Fund (Registered with the Charity Commission No. 252918).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shah, P., Pellicori, P., Kallvikbacka-Bennett, A. et al. Warm water immersion in patients with chronic heart failure: a pilot study. Clin Res Cardiol 108, 468–476 (2019). https://doi.org/10.1007/s00392-018-1376-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1376-2