Abstract
Background
Clinical data regarding hypogonadism in very old men with multimorbidity are rare. Hypogonadism can contribute to osteoporosis, anemia and sarcopenia and is therefore a relevant problem for geriatric patients.
Methods
A total of 167 men aged 65–96 years (mean 81 ± 7 years) admitted to an acute geriatric ward were included in a cross-sectional study. Body composition derived from dual-energy X‑ray absorptiometry, bone mineral density, handgrip strength, multimorbidity, polypharmacy and laboratory values were obtained from the routine electronic clinical patient file.
Results
Hypogonadism was present in 62% (n = 104) of the study participants, of whom 83% showed clinical manifestation of hypogonadism (hypogonadism in combination with anemia, sarcopenia and/or low T‑score). The subgroups showed a distribution of 52% primary and 48% secondary hypogonadism. Compared to the eugonadal patients, hypogonadal patients had reduced handgrip strength (p = 0.031) and lower hemoglobin levels (p = 0.043), even after adjustment for age, body mass index and glomerular filtration rate.
Conclusion
Hypogonadism is common in geriatric patients. If chronic anemia, sarcopenia, or osteoporosis are diagnosed, testosterone levels should be determined in geriatric settings.
Zusammenfassung
Hintergrund
Zum Hypogonadismus bei sehr alten multimorbiden Männern gibt es kaum klinische Daten. Hypogonadismus kann zu Osteoporose, Anämie und Sarkopenie beitragen und ist daher ein relevantes Problem für geriatrische Patienten.
Methoden
In diese Querschnittsstudie wurden 167 Männer im Alter von 65–96 Jahren (Mittelwert 81 ± 7 Jahre) aus einer Akutgeriatrie aufgenommen. Die anhand der Dual-Röntgen-Absorptiometrie (DXA) ermittelte Körperzusammensetzung sowie Knochendichtewerte, Handkraft, Multimorbidität, Polypharmazie und Laborwerte wurden den elektronischen Patientenakten entnommen.
Ergebnisse
Bei 62 % (n = 104) der Studienteilnehmer wurde ein Hypogonadismus festgestellt. Von diesen wiesen 83 % eine klinische Manifestation des Hypogonadismus auf (Hypogonadismus in Kombination mit Anämie, Sarkopenie und/oder niedrigem T‑Score). Die Untergruppen zeigten eine Verteilung von 52 % primärem und 48 % sekundärem Hypogonadismus. Im Vergleich zu den eugonadalen Patienten wiesen die hypogonadalen Patienten eine geringere Handkraft (p = 0,031) und niedrigere Hämoglobinwerte (p = 0,043) auf, selbst nach Adjustierung für Alter, Body-Mass-Index und glomeruläre Filtrationsrate.
Schlussfolgerung
Hypogonadismus tritt bei geriatrischen Patienten häufig auf. Wenn in geriatrischen Settings chronische Anämie, Sarkopenie oder Osteoporose diagnostiziert werden, sollten die Testosteronwerte bestimmt werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Testosterone deficiency in men is commonly referred to as hypogonadism [1, 2]. Starting from the third decade of life, there is an annual decline of approximately 0.4–2% in the free testosterone index [3,4,5]. The prevalence of hypogonadism exhibits significant variation across different studies and population groups. Specifically, in healthy outpatient groups of men the prevalence rates of hypogonadism range between 16% and 39% [6,7,8]. Iglesias et al. demonstrated an even higher prevalence of 53% in patients in an acute geriatric ward in Spain (n = 150, mean age 86 years) [9]. These examples show that men are often affected by hypogonadism. Hypogonadism can lead to various consequences, such as decreases in muscle mass and strength, energy levels, mood, libido, erectile function, and bone density [1, 2]. From a clinical perspective, the effects on sarcopenia, osteoporosis, and anemia are particularly relevant as they directly impact mobility, morbidity, and mortality in geriatric patients. Geriatric patients, as a vulnerable group characterized by factors, such as polypharmacy, multimorbidity, and limitations in mobility, require a special clinical focus.
Testosterone plays a crucial role in counteracting sarcopenia. It stimulates mesenchymal multipotent stem cells to differentiate into muscle cells while inhibiting adipogenesis. Additionally, testosterone promotes muscle stem cell replication, activates muscle protein synthesis, and inhibits protein degradation [10]. Clinical data of male kidney transplant recipients showed that decreasing testosterone levels are correlated with significant decline in handgrip strength (n = 144, mean age 72 years) [11]. Auyeung et al. described a positive association between testosterone and handgrip strength in a community-dwelling male cohort (n = 1489, mean age 72 years) [12]. Numerous studies have consistently shown that testosterone replacement increases lean body mass in men of older age [13].
In addition to its effects on the muscles, testosterone also plays a decisive role in maintaining bone mineral density (BMD). Along with estrogens, it stimulates osteoblast proliferation, partially mediated by cytokines and growth factors such as insulin-like growth factor 1 (IGF-1) [14]. Testosterone promotes bone mineralization and supports the maintenance of trabecular bone, while estrogens inhibit osteoclastogenesis. Additionally, testosterone is converted into estradiol via aromatization, which helps prevent bone loss. A recent review highlighted a prevalence of hypogonadism in men with osteopenia or fractures ranging from 7% to 58%, indicating a discrepancy in existing data and a potential diagnostic deficit for hypogonadism and osteoporosis in aging men [14]. Current data demonstrate that testosterone replacement significantly improves BMD, particularly in the lumbar spine region [15, 16]. Therefore, testosterone replacement is considered a treatment option for patients with osteoporosis and hypogonadism.
Chronic anemia in older men is a complex condition with multiple contributing factors. A clinical sign of hypogonadism can be mild anemia [17] as testosterone has the ability to increase erythropoiesis by stimulating erythropoietin production and expanding the number of erythropoietin-responsive cells in the bone marrow [18]. Roy et al. examined the effects of testosterone treatment in 126 men with low testosterone levels and unexplained anemia. After 12 months 54% of the treated patients showed an increase in hemoglobin (Hb) concentrations of at least 1 g/dl, compared to only 15% in the placebo group [19].
In summary, hypogonadism is a common condition that increases with age. Iglesias et al. found a correlation between hypogonadism and high mortality rates in geriatric patients [9]; however, there is a lack of studies investigating the prevalence of clinically evident hypogonadism characterized by clinical findings along with low testosterone levels in geriatric patients. Therefore, this study analyzed the difference between biochemical and clinically relevant hypogonadism, with a focus on sarcopenia, osteoporosis, and anemia in geriatric men.
Methods
The methods part can be found in the supplementary file.
Results
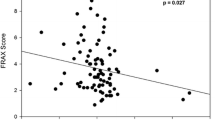
We enrolled a total of 167 men aged 65–96 years (mean 81 ± 7 years) from our acute geriatric ward. In this geriatric cohort, a prevalence of 62% (n = 104) for biochemical hypogonadism was observed. The two groups (hypogonadism vs. eugonadal) differed significantly in handgrip strength, probable sarcopenia and hemoglobin levels. Polypharmacy, characterized by an average use of 11 drugs per patient, and multimorbidity, with an average of 8 different diseases per patient, were observed in both groups. The hormone analysis showed a significant group difference for IGF‑I, but not for LH and FSH (Table 1). The subgroups showed a distribution of 52% primary and 48% secondary hypogonadism (Fig. 1). We could not find any subgroup differences regarding body mass index (BMI), handgrip strength, skeletal muscle mass index (SMI), hemoglobin level, GFR, T‑score, total number of comorbidities and polypharmacy (data not shown). Compensated hypogonadism (normal testosterone levels in combination with elevated LH levels) was present in 22% of all patients (Fig. 1. An extreme testosterone deficit (< 100 ng/dl) was present in 46 (44.2%) patients with hypogonadism (data not shown). Of all hypogonadal patients 83% (n = 86) presented with manifest hypogonadism (hypogonadism in combination with anemia 71%, sarcopenia 33% and/or low T‑score 46%) (Fig. 1). Differences in hemoglobin levels between the two groups (hypogonadism vs. no hypogonadism) remained significant after adjustment for age, BMI, GFR and IGF‑1. Differences in handgrip strength between the two groups (hypogonadism vs. no hypogonadism) remained significant after adjustment for age and BMI, but lost significance after adjusting for IGF‑1 (GFR did not significantly influence model 4). No significant differences were found for SMI and T‑score (Table 2, Fig. 2).
Discussion
The study revealed a high prevalence of hypogonadism, with 62% (n = 104) of geriatric men (mean age 81 years) admitted to a geriatric ward being affected. Among them, a significant majority (83%, n = 86) exhibited manifest hypogonadism, characterized by the coexistence of hypogonadism with anemia, sarcopenia, and/or low T‑score. The complete study cohort exhibited multimorbidity, with an average of eight diseases per patient, and was accompanied by polypharmacy, both common characteristics of geriatric cohorts.
Hypogonadism subgroups
Regarding the subgroups of hypogonadism, the findings showed a distribution of 52% primary and 48% secondary hypogonadism. The European male ageing study, which included 3369 men aged 40–79 years from a single community, reported an increased prevalence of primary and compensated hypogonadism (normal testosterone with elevated LH) with advancing age [20]. Furthermore, this study found associations between both primary and secondary hypogonadism and various comorbidities, such as heart conditions, high blood pressure, cancer, bronchitis, asthma, peptic ulcer, epilepsy, and diabetes [20]; however, data on whether different hypogonadism subgroups lead to distinct clinical consequences are currently lacking. Our study did not reveal any subgroup differences in terms of BMI, handgrip strength, SMI, hemoglobin levels, GFR, T‑score, number of comorbidities, or polypharmacy.
Another relevant subgroup worth considering is compensated hypogonadism, characterized by normal testosterone levels combined with elevated LH/FSH levels. A recent review indicated that compensated hypogonadism is common, affecting approximately 9% of aging men in the general population [21]. In our specific setting, we observed that 22% of participants exhibited compensated hypogonadism, suggesting a higher proportion compared to the general population within the geriatric context. Longitudinal data exposed that compensated hypogonadism is a sign for poor health and increased cardiovascular mortality [21]; however, information on the therapeutic implications of compensated hypogonadism is currently lacking.
Hypogonadism and sarcopenia
The prevalence of sarcopenia in men over 65 years old is approximately 6% [22]. Our assessment showed that handgrip strength was reduced by an average of 3kg compared to the eugonadal group. These findings align with existing literature demonstrating a negative correlation between handgrip strength and testosterone levels in untreated patients [11, 12]. Interventional studies have indicated that testosterone replacement in older men can result in increased muscle strength [12, 23]. The impact of this correlation was modulated by IGF‑1, highlighting the interplay between these factors. Previous studies have established a connection between testosterone and IGF‑1 in the endocrine system. Testosterone can influence the production of IGF‑1, and in turn IGF‑1 levels can affect testosterone secretion [24].
We did not observe any significant correlations for SMI, suggesting that muscle function, as represented by handgrip strength, may be affected earlier than muscle mass. This is a common phenomenon in sarcopenia, where muscle strength tends to decline more rapidly and earlier than muscle mass [25]; however, this emphasizes the importance of recognizing muscle mass and function as separate entities. A study by Van den Beld et al. supported this notion, demonstrating that testosterone was associated with muscle strength but not with muscle mass in a cohort of healthy, independently living older men with a mean age of 78 years [26]. In interventional studies, testosterone treatment has generally shown improvement in lean body mass [27,28,29,30]; however, there is a lack of longitudinal data specifically examining testosterone treatment in geriatric men. Therefore, further studies utilizing the EWGSOP2 definition for sarcopenia are needed to provide more comprehensive insights.
Hypogonadism and osteoporosis
The prevalence of osteoporosis in men in the age group 70–80 years is approximately 4% [31].
We did not find any significant differences in T‑scores between the hypogonadal and eugonadal groups. Interestingly, both groups had a mean T‑score of −2.0, indicating lower bone density. It is important to consider that the methodology of dual-energy X‑ray absorptiometry (DXA) itself may contribute to this finding. Research conducted by our group has shown that DXA loses sensitivity with increasing age, possibly due to confounders, such as aortic calcifications, incorrect positioning, spondylophytes and hip implants [32]. In contrast, peripheral quantitative computed tomography (pQCT) offers several advantages over DXA for assessing BMD in geriatric patients. It enables volumetric assessment, structural analysis, reliable performance in older age, and improved fracture prediction [32].
However, there are measurable effects on bone density under testosterone treatment. Placebo-controlled studies have demonstrated that after 1 year of testosterone replacement, there is an increase in hip and spine BMD [15, 16]. Specifically, there was an improvement in bone strength in the trabecular zone [16]. In a meta-analysis conducted by Isidori et al. involving 1083 participants, it was found that lumbar spine bone density improved by 4% and fat mass was reduced by 6% compared to the placebo group after a minimum of 12–36 months of testosterone replacement [32]; however, in osteoporosis research the likelihood of fractures is a crucial outcome of interest. Therefore, it is important to conduct further studies that not only focus on changes in bone composition but specifically address the incidence of major osteoporotic fractures allied to hypogonadism.
Hypogonadism and anemia
Anemia is a common association with hypogonadism, as testosterone plays a crucial role in stimulating erythropoiesis [33]. Around 30% of all anemia cases in geriatric patients are of unknown etiology and diminished testosterone levels could be a major cause [33]. In our data hemoglobin concentrations differed significantly between hypogonadal and eugonadal patients even after adjustment for GFR. These findings are consistent with the results of Lee et al., who analyzed testosterone and hemoglobin levels in a matched cohort of 444 hypogonadal and 7924 eugonadal men with a mean age of 51 years [34]. Even in this relatively young outpatient group, the hypogonadal participants exhibited lower mean hemoglobin concentrations and a higher incidence of anemia [34]. The relative risk of anemia in the hypogonadal group was 2.4 compared to the eugonadal group [34]. Zhang et al. demonstrated that hemoglobin values significantly increased after long-term (54 weeks) testosterone replacement [35]. Polycythemia, a contraindication of testosterone substitution, occurred less frequently under transdermal replacement [36, 37]. In summary, in geriatric men with unclear and/or unresponsive chronic anemia, the determination of testosterone should be considered.
Strength and limitations
The strengths of our study include the recruitment of a consecutively enrolled high-risk geriatric patient population, with a mean age of 81 years, and the utilization of standardized sarcopenia assessment based on the revised EWGSOP2 criteria using DXA. Considering the challenges associated with conducting clinical studies involving geriatric patients, our study boasts a relatively large sample size. To the best of our knowledge, we are the first to demonstrate the prevalence of manifest hypogonadism in geriatric men, highlighting its clinical significance in relation to anemia, osteoporosis, and sarcopenia. It is important to note that hypogonadism is not merely a laboratory diagnosis but holds significant clinical relevance for this patient group.
However, our study does have several limitations. Firstly, it focused exclusively on hospitalized patients, which introduces the possibility that the observed prevalence of hypogonadism may have been influenced by the acute illnesses. Secondly, the cross-sectional design of our study prevents us from establishing a definitive causal relationship between hypogonadism and the observed outcomes.
Practical conclusion
Hypogonadism is common in geriatric patients. Therefore, if unexplained anemia, sarcopenia, or osteoporosis are diagnosed in geriatric men, testosterone levels should be determined. Hormone treatment might be considered after careful evaluation of risks and benefits and after exclusion of contraindications.
Abbreviations
- BMD:
-
Bone mineral density
- CV:
-
Coefficients of variation
- DXA:
-
Dual-energy X‑ray absorptiometry
- EWGSOP:
-
European Working Group on Sarcopenia in Older People
- FAI:
-
Free androgen index
- FSH:
-
Follicle stimulating hormone
- GFR:
-
Glomerular filtration rate
- Hb:
-
Hemoglobin
- LH:
-
Luteinizing hormone
- pQCT:
-
Peripheral quantitative computed tomography
- SHBG:
-
Sex hormone-binding globulin
- SMI:
-
Skeletal muscle mass index
References
Zitzmann M (2020) Testosterone replacement treatment in older people with and without co-morbidities. Internist 61(6):549–557
Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G et al (2020) European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology 8(5:970–987
Gray A, Feldman HA, McKinlay JB, Longcope C (1991) Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 73(5):1016–1025
Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E et al (2006) Testosterone and estradiol among older men. J Clin Endocrinol Metab 91(4):1336–1344
Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW et al (2008) Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 93(7):2737–2745
Aggarwal V, Menon AS, Verma V (2021) Prevalence of Testosterone Deficiency in Elderly Male and its Association with Frailty and Mobility at a Tertiary Care Centre. Indian J Endocrinol Metab 25(4):337–341
Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C (2006) Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 60(7):762–769
Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD et al (2010) Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 363(2):123–135
Iglesias P, Prado F, Macías MC, Guerrero MT, Muñoz A, Ridruejo E et al (2014) Hypogonadism in aged hospitalized male patients: prevalence and clinical outcome. J Endocrinol Invest 37(2):135–141
Herbst KL, Bhasin S (2004) Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care 7(3):271–277
Gürlek Demirci B, Sezer S, Tutal E, Çolak T, Uyanık S, Haberal M. Hand-Grip Strength Is Associated With Serum Testosterone and Albumin Levels in Male Kidney Transplant Recipients. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation. 2018;16 Suppl 1(Suppl 1):75–9.
Auyeung TW, Lee JS, Kwok T, Leung J, Ohlsson C, Vandenput L et al (2011) Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross-sectional study in 1489 older men. Eur J Endocrinol 164(5):811–817
O’Connell MD, Tajar A, Roberts SA, Wu FC (2011) Do androgens play any role in the physical frailty of ageing men? Int J Androl 34(3):195–211
Shigehara K, Izumi K, Kadono Y, Mizokami A (2021) Testosterone and Bone Health in Men: A Narrative Review. JCM 10(3). https://doi.org/10.3390/jcm10030530
Corona G, Vena W, Pizzocaro A, Giagulli VA, Francomano D, Rastrelli G et al (2022) Testosterone supplementation and bone parameters: a systematic review and meta-analysis study. J Endocrinol Invest 45(5):911–926
Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE et al (2017) Effect of Testosterone Treatment on Volumetric Bone Density and Strength in Older Men With Low Testosterone: A Controlled Clinical Trial. Jama Intern Med 177(4:471–479
Dohle G, Arver S, Bettocchi C, Jones TH, European Association of Urology (2018) (EAU) guidelines on male hypogonadism. J Reprodmed Endokrinol 15(2):71–88
Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S (2008) Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 93(3):914–919
Roy CN, Snyder PJ, Stephens-Shields AJ, Artz AS, Bhasin S, Cohen HJ et al (2017) Association of Testosterone Levels With Anemia in Older Men: A Controlled Clinical Trial. Jama Intern Med 177(4:480–490
Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD et al (2010) Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 95(4):1810–1818
Corona G, Rastrelli G, Dicuio M, Concetti S, Minnetti M, Pivonello R et al (2021) Subclinical male hypogonadism. Minerva Endocrinol 46(3):252–261
Schaupp A, Martini S, Schmidmaier R, Drey M (2021) Diagnostic and therapeutic approach to sarcopenia. Z Gerontol Geriat 54(7):717–724
Chiu HT, Shih MT, Chen WL (2020) Examining the association between grip strength and testosterone. Aging Male 23(5):915–922
Erfurth EM, Hagmar LE, Sääf M, Hall K (1996) Serum levels of insulin-like growth factor I and insulin-like growth factor-binding protein 1 correlate with serum free testosterone and sex hormone binding globulin levels in healthy young and middle-aged men. Clin Endocrinol 44(6):659–664
Cruz-Jentoft AJ, Sayer AA (2019) Sarcopenia. Lancet 393(10191):2636–2646
van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW (2000) Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 85(9):3276–3282
Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G et al (2000) Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 85(8):2839–2853
Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J et al (1996) The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335(1):1–7
Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA et al (1999) Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84(8):2647–2653
Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A (1996) Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 81(12):4358–4365
Golds G, Houdek D, Hypogonadism Osteoporosis ATM (2017) The Effects, Clinical Consequences, and Treatment of Testosterone Deficiency in Bone Health. Int J Endocrinol 2017:4602129
Drey M, Henkel M, Petermeise S, Weiß S, Ferrari U, Rottenkolber M et al (2020) Assessment of Bone and Muscle Measurements by Peripheral Quantitative Computed Tomography in Geriatric Patients. J Clin Densitom 23(4):604–610
Saad F, Röhrig G, von Haehling S, Traish A (2017) Testosterone Deficiency and Testosterone Treatment in Older Men. Gerontology 63(2):144–156
Lee JH, Choi JD, Kang JY, Yoo TK, Park YW (2022) Testosterone deficiency and the risk of anemia: A propensity score-matched analysis. Am J Hum Biol 34(8):e23751
Zhang LT, Shin YS, Kim JY, Park JK (2016) Could Testosterone Replacement Therapy in Hypogonadal Men Ameliorate Anemia, a Cardiovascular Risk Factor? An Observational, 54-Week Cumulative Registry Study. J Urol 195(4 Pt 1:1057–1064
Pastuszak AW, Gomez LP, Scovell JM, Khera M, Lamb DJ, Lipshultz LI (2015) Comparison of the Effects of Testosterone Gels, Injections, and Pellets on Serum Hormones, Erythrocytosis, Lipids, and Prostate-Specific Antigen. Sex Med 3(3):165–173
White J, Petrella F, Ory J (2022) Testosterone therapy and secondary erythrocytosis. Int J Impot Res. https://doi.org/10.1038/s41443-021-00509-5
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (Heisenberg Professorship 325768017) to N.R.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Schluessel, M. Bidlingmaier, S. Martini, M. Reincke, N. Reisch, A. Schaupp, G. Stalla,D. Teupser, R. Schmidmaier and M. Drey declare that they have no competing interests.
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Since this study was retrospective in nature, it did not require informed consent from the participants. The study protocol received approval from the Ethical Review Committee of Ludwig-Maximilians-University under Ethical Vote No. 22‑0305.
Additional information

Scan QR code & read article online
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schluessel, S., Bidlingmaier, M., Martini, S. et al. Hypogonadism is frequent in very old men with multimorbidity and is associated with anemia and sarcopenia. Z Gerontol Geriat 57, 43–49 (2024). https://doi.org/10.1007/s00391-023-02235-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00391-023-02235-7
Keywords
- Testosterone
- Osteoporosis
- Geriatrics
- Dual-energy X-ray absorptiometry
- Primary and secondary hypogonadism