Abstract
Background
Although antihyperglycemic pharmacotherapy in frail older adults with type 2 diabetes mellitus (T2DM) is challenging, recommendations from international guidelines are mainly based on indirect evidence from trials not including frail participants.
Objective
This systematic review investigated the effectiveness and safety of pharmacotherapy in frail older adults with T2DM.
Material and methods
Randomized (RCT) and non-randomized prospective clinical trials (non-RCT) were searched in three electronic databases (Medline, Embase, Central) up to October 2018. Trials in older adults with T2DM who were assessed as significantly or severely impaired by defined cut-off scores of assessment instruments on frailty, activities of daily living or physical functional impairment were included.
Results
Two reviewers independently screened 17,391 references for inclusion and assessed risk of bias with ROBINS‑I. Five non-RCTs and no RCT were identified. Treatment of T2DM without insulin compared to insulin could be associated with increased improvement in cardiac functions in patients with cardiac resynchronization therapy and with decreased falls in frail older women. While better glycemic control with low variability and low HbA1c (hemoglobin A1c) values (<7%) was associated with better maintenance of physical function in community-dwelling older persons, higher HbA1c values (8–8.9%) were associated with a reduction in the composite outcome of death or functional decline in community-dwelling diabetic older adults with need for skilled assistance. Due to serious risk of bias in all studies, results should be considered with caution.
Conclusion
Well-designed, large-scale RCTs including this important group of patients are required to assess the effectiveness and safety of pharmacotherapy and HbA1c targets.
Zusammenfassung
Hintergrund
Auch wenn die antihyperglykämische Pharmakotherapie von älteren, gebrechlichen Patienten mit Typ-2-Diabetes anspruchsvoll ist, basieren die Empfehlungen internationaler Leitlinien hauptsächlich auf indirekter Evidenz aus Studien, die gebrechliche Patienten nicht einschließen.
Ziel der Arbeit
Dieser systematische Review untersuchte die Wirksamkeit und Sicherheit medikamentöser Therapien bei gebrechlichen, älteren Patienten mit Typ-2-Diabetes.
Material und Methoden
Bis Oktober 2018 wurden systematische Literatursuchen in drei Datenbanken (Medline, Embase, Central) nach randomisierten (RCTs) und prospektiven nichtrandomisierten kontrollierten Studien (Non-RCTs) durchgeführt. Studien bei älteren Erwachsenen mit Typ-2-Diabetes, die durch vorab definierte Schwellenwerte hinsichtlich Gebrechlichkeit, Aktivitäten des täglichen Lebens oder anderer Instrumente zur Messung der körperlichen Gebrechlichkeit als signifikant oder schwerwiegend funktionell eingeschränkt beurteilt worden waren, wurden eingeschlossen.
Ergebnisse
Zwei Review-Autoren untersuchten unabhängig voneinander die Einschlussfähigkeit von 17.391 Datenbankeinträgen. Es wurden fünf Non-RCTs und keine RCT identifiziert. Die Therapie von Typ-2-Diabetes ohne Insulin könnte im Vergleich zu einer Therapie mit Insulin mit stärkeren Verbesserungen der Herzfunktion bei Patienten mit kardialer Resynchronisation und mit weniger Stürzen bei gebrechlichen älteren Frauen assoziiert sein. In einer Studie war eine bessere glykämische Kontrolle, definiert durch weniger schwankende und niedrigere HbA1c-Werte (<7 %) mit einer besseren Erhaltung der körperlichen Funktion bei zu Hause lebenden gebrechlichen Älteren mit Typ-2-Diabetes assoziiert. In einer anderen Studie waren höhere HbA1c-Werte (8–8,9 %) mit einer Reduktion des zusammengesetzten Endpunkts aus Mortalität oder funktioneller Beeinträchtigung bei älteren, zu Hause lebenden pflegebedürftigen Erwachsenen mit Typ-2-Diabetes assoziiert. Aufgrund eines schwerwiegenden Risikos für Bias in allen Studien sollten die Ergebnisse mit Vorsicht betrachtet werden.
Diskussion
Gut konzipierte, große randomisierte kontrollierte Studien, die gebrechliche, ältere Patienten explizit adressieren sind notwendig, um die Wirksamkeit und Sicherheit der medikamentösen Therapie und der HbA1c-Ziele einzuschätzen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since 1980 the global prevalence of diabetes mellitus (DM) has nearly doubled up to 422 million people worldwide in 2014 and is estimated to redouble in the next 20 years [44]. In high-income countries, DM prevalence was 3 times higher among 65–69 year old patients compared to low income countries and peaked in the 75–79 years age group (22%) [6]. Late complications of DM, such as myocardial infarction, stroke, renal failure, peripheral arterial occlusive disease, diabetic retinopathy and polyneuropathies, negatively impact the quality of life and daily functioning of patients, and represent major costs within healthcare systems [45]. In addition, hypoglycemia has been associated with higher mortality and increased risks for falls and fall-related injuries [23].
In the older diabetic population, type 2 diabetes mellitus (T2DM) has a prevalence of up to 90% [12]. Its antihyperglycemic pharmacotherapy is challenging [35], particularly among frail older adults with impaired physical functions [36]. Diabetes mellitus may increase the risk for frailty [10] and they both share several pathophysiological mechanisms, e.g. chronic low-grade inflammation, insulin resistance and sarcopenia [2, 29], the latter being a major contributing factor to pathophysiology of frailty.
Several national and international medical societies have published guidelines for both T2DM [1, 13, 14, 25, 37, 39] and frail older persons with T2DM [22]. However, recommendations are mainly based on indirect evidence from trials not including frail participants or excluding older adults at all [14, 21].
This research group on medication and quality of life in frail older persons (MedQoL-Group) conducted four extended systematic reviews on drug treatment in hypertension [28], depression, dementia and T2DM. We included trials with older persons who can be characterized as frail in terms of impaired physical functioning, even if the investigators of the primary studies did not explicitly consider the concept of frailty when planning their studies. This article reports a systematic review of randomized (RCT) and non-randomized prospective clinical trials (non-RCT) intended to determine the effectiveness and safety of pharmacotherapy for frail older persons with T2DM characterized as functionally significantly or severely impaired.
Material and methods
This systematic review is reported according to the PRISMA guidelines [20] and has been specified in a protocol registered on PROSPERO (CRD42018108997, https://www.crd.york.ac.uk/prospero).
Search and study eligibility
The databases MEDLINE (via Ovid), Embase (via Ovid) and Cochrane Central Register of Trials (CENTRAL) were searched for randomized and non-randomized prospective clinical trials comparing antihyperglycemic pharmacotherapy versus placebo or other antihyperglycemic medication or comparing different HbA1c targets in older persons with DM (see table S1 and table S2 for Medline search strategies). In addition, reference lists of included studies were screened. Detailed search methods are described in S3. Titles/abstracts and full texts were assessed for eligibility by two reviewers (CB, GT), independently. Conflicts were solved by discussion or a third reviewer (SVR).
RCTs and non-RCTs with control group design reporting on persons aged 65 years or older (or mean age ≥70 years) with T2DM and significant or severe impairment of physical function were included. We assessed functional impairment according to a compilation of predefined thresholds in 51 established indices and scores [4]. For preparation of this compilation, we limited our scope of interest to assessments of frailty and, as proxy indicators, instruments evaluating activities of daily living (ADL) or physical functioning or impairment. With regard to the latter we excluded assessments of cognitive functioning. For each identified instrument, we defined cut-off scores for the categories (1) functionally independent, (2) functionally slightly impaired, (3) functionally significantly impaired or partially dependent and (4) functionally severely impaired or disabled or dependent. In our review, we only considered at least significantly impaired populations.
Data extraction and risk of bias assessment
One author extracted data from each study and another checked data for accuracy and completeness. The two reviewers worked independently on risk of bias assessment. Conflicts were again solved by discussion or consulting the third reviewer. Data extraction was carried out in piloted data forms. The reviewers used the risk of bias in nonrandomized studies of interventions (ROBINS‑I) tool to assess the risk of bias of included prospective cohort studies [40].
Results
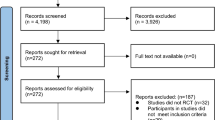
We included five non-RCTs and no RCT involving 1220 older persons with DM. The flow of non-RCT and RCT inclusion is presented in the PRISMA flowcharts (Fig. 1 and S1 Fig).
Flowchart: selection of non-RCTs [26] (For more information visit www.prisma-statement.org)
Characteristics of included studies are mentioned in Table 1. Mean ages ranged from 73.8 to 81.7 years, follow-up periods from 1 to 7 years. Studies were performed from 1986 to 2012. Three trials were supported by public grant [33, 43, 46]. One study reported to have no conflict of interest [32]. One study did not report on conflict of interests [30]. All studies were assessed as serious risk of bias, mainly due to important confounders not considered in the analyses.
Insulin versus oral antihyperglycemics versus no pharmacological antidiabetic treatment
Pham et al. compared 73 diabetic nursing home residents older than 60 years regarding patterns of effective management, DM control and functional dependency [30]. This observational study was conducted in two nursing and two residential homes in France. Items of DM control and follow-up were prospectively recorded. Functional dependency was assessed using the Katz index of activities of daily living [17]. Katz scores were not reported on detail at baseline, but only a minor subsample (n = 17) were classified as functionally independent. Change in DM management from baseline to the 18 months follow-up occurred in participants receiving insulin from 26 to 27, oral antihyperglycemics from 29 to 24, and no treatment or diet alone from 18 to 22. Hypoglycemia occurred in 24 patients: 18 of 27 (67%) on insulin and 6 of 24 (25%) on oral antihyperglycemics. At the end of the study 41 subjects had depression: 15 of 27 (56%) on insulin, 14 of 24 (58%) on oral antihyperglycemics and 12 of 22 (55%) without treatment or diet alone. Associations between DM management and functional dependency were not reported for any subgroup.
Different HbA1c values
Pham et al. furthermore classified the 73 diabetic nursing home residents according to the lowest HbA1c value: 19 participants had values lower than 6.5%; 20 between 6.5% and 8%; 15 higher than 8% [30]. In 19 subjects, HbA1c was never measured during the study period. At the end of the 18-months follow-up, 7 (36.8%), 5 (25%) and 4 (26.7%) participants had died, respectively. In univariate ANOVA analysis and χ2-test no relationships were found between HbA1c range and changes in the Katz index or mortality rate.
Wang and Hazuda evaluated the impact of glycemic control on the maintenance of lower-extremity physical function in 119 diabetic participants aged 71 years or older [43]. Using a latent growth mixture modelling, poor glycemic control class was defined by means of HbA1c > 7% and higher intrasubject variability, better control by means of HbA1c < 7% and lower intra-subject-variability. The comparison of adjusted correlations in the change from baseline scores of the Short Physical Performance Battery (SPPB) at 18 and 36 months revealed that improved glycemic control was associated with a better maintenance of physical function.
Yau et al. evaluated if HbA1c levels predicted functional decline or death in 367 functional severely impaired community-dwelling older adults who were unable to live independently [46]. Functionality was assessed based on five ADLs (bathing, dressing, toileting, transferring and eating) scoring between 0 (completely dependent) and 2 points (independent) in each activity. Two-thirds of participants had a baseline ADL score of 8 or less. Mortality, functional decline plus the composite outcome of mortality and functional decline were reported after 6, 12 and 24 months. After accounting for confounders age, sex, race or ethnicity, time enrolled in the study, baseline activities of daily living, comorbidities (cancer, congestive heart failure, chronic obstructive lung disease, renal disease, dialysis) and medications (none, oral medication, insulin), patients with HbA1c levels of 8–8.9% had the lowest and levels of <7% the highest risk of death, functional decline and composite outcome at 2 years. Only the result for the composite outcome in the 8.0–8.9% group reached statistical significance. Stratification of analysis according to antidiabetic medications (oral antihyperglycemic medications only vs. any insulin) showed similar results in both groups.
Insulin versus no insulin
Schwartz et al. determined risk factors and the risk of falls in 530 insulin treated and 99 non-insulin treated diabetic women aged 65 years or older [33]. At baseline, there were significant differences in characteristics of insulin treated and non-insulin treated participants, e.g. 71% of insulin treated versus 57% of non-insulin treated women had difficulties in instrumental activities of daily living (walking two or three blocks, climbing 10 steps, preparing own meals, doing heavy housework and doing own shopping). Time since diagnosis of diabetes also differed (17.4 ± 11.2 versus 11.3 ± 9.2 years). Functional measures at baseline were grip strength, quadriceps strength, walking speed, 5 chair test, tandem stand and tandem walk. Within the 2‑year follow-up, the incidence of falls per person-year between the insulin and non-insulin group significantly differed in the total study sample (1.12 vs. 0.85) and in age subgroups (70–74 years: 1.26 vs. 0.56; 80–84 years: 1.31 vs. 0.89). Significantly more women taking insulin fell more than once a year (35.4%) compared to women receiving no insulin (25.7%). There was no significant difference in persons who fell more than twice a year (10.6% vs. 15.2%). Tandem walk accounted for 23% of the association between non-insulin-treated DM and falling, which was the largest contributing factor among all risk factors. Together with poor performance on tandem stand, both measures accounted for 30% of the association between non-insulin-treated DM and falling and 26% of the association between insulin-treated DM and falling.
Sardu et al. determined the effects of insulin (n = 12) versus no insulin therapy (n = 18) in diabetic patients with heart failure older than 75 years and receiving a cardiac resynchronization therapy implant [32]. The 6‑min walk improved from baseline to 12 months in the insulin group from 243.2 (SD 52.2) to 259.6 (53.7) m and in the non-insulin group from 254.7 (55.1) to 271.3 (56.4) m. Changes in 6‑min walk differed not significantly between groups. Mean New York Heart Association (NYHA) functional class improved in the insulin group from 3.38 (1.1) to 2.8 (0.7), but significantly more in the non-insulin group from 3.21 (1.0) to 2.05 (0.6). HbA1c values remained stable in both groups compared to baseline. Quality of life was not reported.
Discussion
There is very low evidence that treatment of T2DM without insulin compared to insulin therapy might be associated with improvement in cardiac functions, in older diabetic patients with cardiac resynchronization therapy [32] and with decreased falls, in frail older women [33]; however, subjects might have received insulin due to severe diabetes of longer duration, which may explain adverse outcomes. While better glycemic control with low variability and low HbA1c values (<7%) was associated with better maintenance of physical function in community-dwelling older persons [43], higher HbA1c values (8.0–8.9%) were associated with a reduction in the composite outcome of death or functional decline in community-dwelling diabetic older adults with need for skilled assistance (classified as nursing home eligible [46]).
Strengths and limitations
Strengths of this study were (1) the expanded search (2) the use of up to date and specific tools to assess the risk of bias, and (3) the independent screening and risk of bias assessment by two reviewers. Although we specified assessments with cut-off scores of frailty indicators a priori and dependency in ADL has been validated as frailty proxy [8], the main limitation of our systematic review was the use of proxy indicators and the retrospective identification of the frail population in eligible trials.
Based on the included studies, all with high risk of bias, and the indirect evidence from excluded studies in the research field (S4), we cautiously interpreted that a critical assessment of the need for insulin and its conservative use as well as multimodal strategies to set and reach individual moderate HbA1c targets [47] might have beneficial effects in diabetic frail older adults. Considering the findings that both low and high HbA1c values were associated with increased mortality and cardiac events [7], we recommend to follow current guidelines on T2DM in older persons proposing a defined target range of HbA1c levels (7.5–8.5%) [1, 3]; however, hypothesized positive effects of conservative insulin use and defined HbA1c target ranges are to be evaluated in future rigorous research.
Conclusion
Implications for practice
The very low evidence from the included trials and the additional discussion of indirect evidence from the excluded studies suggest an essential evidence gap so that firm recommendations for antihyperglycemic pharmacotherapy in frail older adults with physical functional impairment cannot be drawn.
Implications for research
Well designed, large-scale RCTs, including not the same team replications [15], are required to establish the effectiveness of implementing internationally established guidelines, based on the most recent position papers managing frailty in DM [38, 41]. To address the well-known challenges in research with frail older persons [34], we suggest a prospective network meta-analysis [42] as methodological framework as well as coordinated practice-based research networks [11, 27] and patient representatives as stakeholder partners to ensure rigorous scientific methods, sufficient recruitment and a patient-centered and practice-oriented study plan [9]. An evidence-based, updated study plan involving networking and methodological expertise [5, 18] of ongoing or recently published RCTs in the field [19, 24, 31] should be established, therefore avoiding waste in research [16].
References
ADA (2019) 12. Older adults: standards of medical care in diabetes – 2019. Diabetes Care 42:S139–S147
Atiénzar P, Abizanda P, Guppy A et al (2012) Diabetes and frailty: an emerging issue. Part 2: linking factors. Br J Diabetes Vasc Dis 12:119–122
Bahrmann A, Bahrmann P, Baumann J et al (2018) S2k-Leitlinie Diagnostik, Therapie und Verlaufskontrolle des Diabetes mellitus im Alter. Diabetol Stoffwechs 13:423–489
Brefka S, Dallmeier D, Muhlbauer V et al (2019) A proposal for the retrospective identification and categorization of older people with functional impairments in scientific studies – recommendations of the Medication and Quality of Life in Frail Older Persons (MedQoL) Research Group. J Am Med Dir Assoc 20:138–146
Ceriello A, Gavin JR 3rd, Boulton AJM et al (2018) The Berlin Declaration: a call to action to improve early actions related to type 2 diabetes. How can specialist care help? Diabetes Res Clin Pract 139:392–399
Cho NH, Shaw JE, Karuranga S et al (2018) IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281
Currie CJ, Peters JR, Tynan A et al (2010) Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 375:481–489
Cuthbertson CC, Kucharska-Newton A, Faurot KR et al (2018) Controlling for frailty in pharmacoepidemiologic studies of older adults: validation of an existing medicare claims-based algorithm. Epidemiology 29:556–561
Forsythe LP, Frank LB, Hemphill R et al (2018) Researchers, patients, and stakeholders evaluating comparative-effectiveness research: a mixed-methods study of the PCORI reviewer experience. Value Health 21:1161–1167
Garcia-Esquinas E, Graciani A, Guallar-Castillon P et al (2015) Diabetes and risk of frailty and its potential mechanisms: a prospective cohort study of older adults. J Am Med Dir Assoc 16:748–754
Goldstein KM, Vogt D, Hamilton A et al (2018) Practice-based research networks add value to evidence-based quality improvement. Healthcare (Amst) 6:128–134
Han Cho N, Whiting D, Guariguata L et al (2013) IDF diabetes atlas, 6th edn. International Diabetes Federation, Brussels
International Diabetes Federation Guideline Group (2014) Global guideline for type 2 diabetes. Diabetes Res Clin Pract 104:1–52
Inzucchi SE, Bergenstal RM, Buse JB et al (2015) Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38:140–149
Ioannidis JP (2012) Scientific inbreeding and same-team replication: type D personality as an example. J Psychosom Res 73:408–410
Ioannidis JP, Greenland S, Hlatky MA et al (2014) Increasing value and reducing waste in research design, conduct, and analysis. Lancet 383:166–175
Katz S, Ford AB, Moskowitz RW et al (1963) Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 185:914–919
Khunti K, Ceriello A, Cos X et al (2018) Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract 137:137–148
Landi F, Cesari M, Calvani R et al (2017) The “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res 29:89–100
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Lipska KJ, Krumholz H, Soones T et al (2016) Polypharmacy in the aging patient: a review of glycemic control in older adults with type 2 diabetes. JAMA 315:1034–1045
Mallery LH, Ransom T, Steeves B et al (2013) Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc 14:801–808
Mattishent K, Loke YK (2016) meta-analysis: association between hypoglycaemia and serious adverse events in older patients. J Diabetes Complications 30:811–818
Mccarthy C, Clyne B, Corrigan D et al (2017) Supporting prescribing in older people with multimorbidity and significant polypharmacy in primary care (SPPiRE): a cluster randomised controlled trial protocol and pilot. Implement Sci 12:99
Meneilly GS, Knip A, Tessier D (2013) Diabetes in the elderly. Can J Diabetes 37:S184–S190
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and neta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Mold JW, Lipman PD, Durako SJ (2012) Coordinating centers and multi-practice-based research network (PBRN) research. J Am Board Fam Med 25:577–581
Muhlbauer V, Dallmeier D, Brefka S et al (2019) The pharmacological treatment of arterial hypertension in frail, older patients—a systematic review. Dtsch Arztebl Int 116:23–30
Perkisas S, Vandewoude M (2016) Where frailty meets diabetes. Diabetes Metab Res Rev 32(Suppl 1):261–267
Pham M, Pinganaud G, Richard-Harston S et al (2003) Prospective audit of diabetes care and outcomes in a group of geriatric French care homes. Diabetes Metab 29:251–258
Rodriguez-Manas L, Laosa O, Vellas B et al (2019) Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J Cachexia Sarcopenia Muscle 10:721–733
Sardu C, Marfella R, Santulli G (2014) Impact of diabetes mellitus on the clinical response to cardiac resynchronization therapy in elderly people. J Cardiovasc Transl Res 7:362–368
Schwartz AV, Hillier TA, Sellmeyer DE et al (2002) Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 25:1749–1754
Sinclair A (2015) Frailty and sarcopaenia trials in primary care—identifying and overcoming key barriers to successful clinician participation. J Frailty Aging 4:129–130
Sinclair A, Dunning T, Rodriguez-Manas L (2015) Diabetes in older people: new insights and remaining challenges. Lancet Diabetes Endocrinol 3:275–285
Sinclair A, Morley J (2013) Frailty and diabetes. Lancet 382:1386–1387
Sinclair A, Morley JE, Rodriguez-Manas L et al (2012) Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc 13:497–502
Sinclair AJ, Abdelhafiz A, Dunning T et al (2018) An international position statement on the management of frailty in diabetes mellitus: summary of recommendations 2017. J Frailty Aging 7:10–20
Sinclair AJ, Paolisso G, Castro M et al (2011) European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 3:27–38
Sterne JA, Hernan MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Strain WD, Hope SV, Green A et al (2018) Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet Med 35:838–845
Vandvik PO, Brignardello-Petersen R, Guyatt GH (2016) Living cumulative network meta-analysis to reduce waste in research: a paradigmatic shift for systematic reviews? BMC Med 14:59
Wang CP, Hazuda HP (2011) Better glycemic control is associated with maintenance of lower-extremity function over time in Mexican American and European American older adults with diabetes. Diabetes Care 34:268–273
Whiting DR, Guariguata L, Weil C et al (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94:311–321
WHO (2016) Global report on diabetes. World Health Organization, Geneva
Yau CK, Eng C, Cenzer IS et al (2012) Glycosylated hemoglobin and functional decline in community-dwelling nursing home-eligible elderly adults with diabetes mellitus. J Am Geriatr Soc 60:1215–1221
Zeyfang A (2016) Individualized diabetes therapy in older persons. Internist 57:502–507
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest
C. Bollig, G. Torbahn, S. Brefka, D. Dallmeier, M. Denkinger, A. Eidam, S. Klöppel and S. Voigt-Radloff for the MedQoL-Group (Medication and Quality of Life in frail older persons) declare that they have no competing interests. J. Bauer: outside the submitted work: personal fees from Fresenius, Nestlé, Nutricia Danone, Novartis, Pfizer and Bayer, transferred to his institution; grants from Nestlé and Nutricia Danone. A. Zeyfang Outside the submitted work: personal fees from Berlin-Chemie AG.
Ethical standards
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bollig, C., Torbahn, G., Bauer, J. et al. Evidence gap on antihyperglycemic pharmacotherapy in frail older adults. Z Gerontol Geriat 54, 278–284 (2021). https://doi.org/10.1007/s00391-020-01724-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00391-020-01724-3