Abstract
Objective
The use of goal-directed fluid therapy (GDFT) has been shown to reduce complications and improve prognosis in high-risk abdominal surgery patients. However, the utilization of pulse pressure variation (PPV) guided GDFT in laparoscopic surgery remains a subject of debate. We hypothesized that utilizing PPV guidance for GDFT would optimize short-term prognosis in elderly patients undergoing laparoscopic radical resection for colorectal cancer compared to conventional fluid therapy.
Methods
Elderly patients undergoing laparoscopic radical resection of colorectal cancer were randomized to receive either PPV guided GDFT or conventional fluid therapy and explore whether PPV guided GDFT can optimize the short-term prognosis of elderly patients undergoing laparoscopic radical resection of colorectal cancer compared with conventional fluid therapy.
Results
The incidence of complications was significantly lower in the PPV group compared to the control group (32.8% vs. 57.1%, P = .009). Additionally, the PPV group had a lower occurrence of gastrointestinal dysfunction (19.0% vs. 39.3%, P = .017) and postoperative pneumonia (8.6% vs. 23.2%, P = .033) than the control group.
Conclusion
Utilizing PPV as a monitoring index for GDFT can improve short-term prognosis in elderly patients undergoing laparoscopic radical resection of colorectal cancer.
Registration number
ChiCTR2300067361; date of registration: January 5, 2023.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
By optimizing intraoperative hemodynamic parameters, goal-directed fluid therapy (GDFT) can maximize oxygen delivery to tissues and organs in patients undergoing abdominal surgery while preventing insufficient or excessive fluid infusion [1,2,3]. Multiple studies have demonstrated that GDFT provides the greatest benefit for high-risk abdominal surgery patients [4,5,6]. Therefore, for elderly patients with underlying diseases and organ dysfunction, GDFT can improve patient outcomes, reduce hospital stay duration, and decrease perioperative complications and readmission rates [7].
Traditional monitoring methods of GDFT mainly rely on transesophageal Doppler ultrasound and pulmonary artery catheterization, which accurately reflect cardiac output (CO) but are limited by their invasiveness and high cost [1, 8]. Currently, pulse pressure variation (PPV), a minimally invasive and accurate dynamic indicator for evaluating fluid responsiveness, is recognized by clinicians. Several studies have shown that PPV can guide GDFT effectively and enhance patient prognosis [1, 3, 9, 10].
Currently, laparoscopic radical resection of colorectal cancer is the preferred surgical approach for achieving enhanced recovery after surgery (ERAS) [11]. However, there is conflicting evidence regarding the application of PPV in laparoscopic surgery. The traditional belief suggests that pneumoperitoneum establishment may influence changes in stroke volume (SV) during respiration, affecting PPV measurement accuracy [12]. Nevertheless, it has been demonstrated that PPV can reliably assess fluid responsiveness during laparoscopic surgery when appropriate pneumoperitoneum pressures are maintained [13, 14]. To date, no relevant studies have investigated whether implementing PPV in laparoscopic radical resection of colorectal cancer can enhance rapid recovery after surgery. In this pragmatic study, we hypothesize that GDFT guided by PPV could optimize intraoperative hemodynamic management and reduce postoperative complications among elderly patients undergoing laparoscopic radical resection of colorectal cancer.
Materials and methods
Study design and population
This prospective randomized controlled trial (RCT) was conducted at the First Affiliated Hospital of Chongqing Medical University, China, as a single-center study. The Ethical approval for this study (2022-231) was provided by the Ethical Committee of the First Affiliated Hospital of Chongqing Medical University (Qing Yan) on 12 September 2022 and was registered in the Chinese Clinical Trial Registry (ChiCTR2300067361). Written informed consent was obtained from all eligible participants or their legal representatives.
Inclusion criteria: (1) age between 60 and 90 years; (2) American Society of Anesthesiologists (ASA) physical status score II-III; (3) patients undergoing elective laparoscopic radical resection of colorectal cancer. Exclusion criteria: (1) emergency surgical cases; (2) unplanned reoperations; (3) contraindications for arterial catheterization; (4) pre-existing arrhythmia; (5) congestive heart failure; (6) hepatic and renal insufficiency (creatinine, liver enzymes > 50% of normal value).
Randomization and blinding
Participants were assigned randomly, in a 1:1 ratio, to either PPV guided GDFT or conventional fluid therapy using a random number generated by Microsoft Excel. Randomization was performed by an assistant who had no involvement in the study and the assignment was stored in sealed envelopes with serial numbers. The anesthesiologist was responsible for preoperative and intraoperative management as well as administering the fluid protocol. Only the performing anesthesiologist was aware of each patient’s assignment, while the postoperative follow-up team remained unaware of both randomization and overall intraoperative management. Data collected by both groups of personnel were recorded on specific data collection forms and subsequently transferred to two separate databases — one containing preoperative and intraoperative data, and another containing postoperative data. The combination of these two databases only occurred at the end of the study period. Independent statisticians conducted data assessment and analysis.
Intervention and intraoperative management
The anesthesia management was standardized by the protocols of our department, and the entire procedure was performed by a single anesthesiologist. Blood pressure (BP), electrocardiogram (ECG), oxygen saturation (SpO2), and bispectral index (BIS) were routinely monitored upon patients’ arrival in the operating room. Radial artery puncture and catheterization were conducted before anesthesia administration, followed by monitoring of arterial blood pressure (ABP) and PPV. Anesthesia induction included midazolam at a dose of 0.05 mg/kg, propofol at 2 mg/kg, sufentanil at 0.5 µg/kg, and vecuronium at 0.15 mg/kg. Maintenance of anesthesia involved propofol infusion along with remifentanil administration, while sevoflurane inhalation maintained the minimum alveolar concentration (MAC) between 0.7 and 1. BIS values were maintained within the range of 40–50 throughout the procedure. Ventilator settings comprised tidal volume set at 8–10 ml/kg PBW, inspiratory-to-expiratory ratio (I/E) set as 1:2, respiratory rate (RR) adjusted to maintain end-tidal carbon dioxide partial pressure between 35 and 40 mmHg, while nasopharyngeal temperature was kept above 36 °C using heated liquid and warm fan devices. Additionally, intraoperative pneumoperitoneum pressure was adjusted between 10 and 12 mmHg based on surgical requirements and patient condition.
Fluid management
Control group
Solid food was allowed up to 8 h before surgery, while clear fluids were permitted up to 4 h prior. Rehydration was administered after admission based on physiological requirements, compensating for missed fasting volume, anesthesia volume expansion, and blood loss. The physiological requirements and missed fasting volume were determined using the 4-2-1 rehydration principle [7]. The fluid infusion volume in the first hour was calculated as follows: fasting loss /2 + physiological requirement + anesthesia expansion volume. The fluid infusion volumes in the second and third hours were determined by fasting loss /4 + physiological requirement + additional loss. The blood volume loss caused by bleeding was adjusted based on the actual amount of blood lost. The physiological requirements, the deficit in fasting, and the expansion of anesthesia volume were supplemented with compound electrolytes, and the bleeding amount was supplemented with hydroxyethyl starch.
PPV group
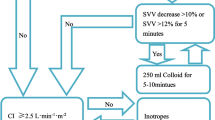
Solid food was allowed up to 8 h before surgery, while clear fluids were permitted up to 4 h prior. a baseline fluid infusion volume of 5 ml/kg/h was administered upon entering the operating room. Fluid infusion was guided by PPV measurements with evaluations conducted every 15 min. No treatment was given when measured PPV ≤ 13%. If PPV > 13%, a rapid infusion of 250 ml hydroxyethyl starch took place within 10 min. After infusion, a reevaluation of PPV occurred. If there was a significant change in PPV (a decrease greater than 2% from baseline), the infusion continued until achieving the aforementioned goals. If there was not a significant change in PPV (a decrease of less than 2% from baseline), intravenous norepinephrine at a dosage range of 0.5 to 5.0 µg/kg/min would be considered until reaching this goal (Fig. 1).
Vital signs management
The dosage of vasoactive drugs was adjusted based on the patient’s hemodynamics, with norepinephrine, phenylephrine, and ephedrine selected according to the patient’s condition. The mean arterial pressure (MAP) was maintained at 20% of the baseline blood pressure, heart rate (HR) between 55 and 100 beats per minute, and urine output greater than 0.5 ml/kg*h. Red blood cells were transfused when hemoglobin (HB) levels dropped below 70 g/L in both groups.
Outcome measures
The primary outcome measure was the occurrence of postoperative complications (including pneumonia, intra-abdominal infection, urinary tract infection, incisional infection, arrhythmia, heart failure, acute myocardial infarction, atelectasis, pulmonary embolism, mechanical ventilation support exceeding 24 h, urinary retention, acute renal failure, intestinal obstruction, anastomotic bleeding, and leakage as well as gastrointestinal dysfunction) within 30 days following laparoscopic radical resection for colorectal cancer in elderly patients. The diagnosis of postoperative complications was established based on a combination of strong clinical suspicion along with supporting evidence from X-ray or ultrasound imaging or laboratory tests (Table 1).
Other outcome measures included the Clavien-Dindo complication score at 30 days post-surgery [15, 16] and the I-FEED score for gastrointestinal dysfunction at 30 days post-surgery [11, 17]. Additionally, various physiological parameters such as HR, MAP, potential of hydrogen (PH), and lactic (Lac) values were assessed at significant intraoperative time points. Intraoperative, postoperative, and perioperative total opioid consumption was measured in morphine equivalents [morphine (iv)1 mg = remifentanil (iv)10 µg = sufentanil (iv)1 µg = tramadol (iv)10 mg] [18]. Postoperative recovery markers evaluated were extubation time, initiation of oral intake, first exhaust time, first defecation time, initial mobilization after surgery, and length of hospital stay (LOS). (Discharge decisions were guided by standardized criteria: patients should be considered ready for hospital discharge when there is tolerance of oral intake, recovery of lower gastrointestinal function, adequate pain control with oral analgesia, ability to mobilize and self-care, and no evidence of complications or untreated medical problems [19]).
Sample size and statistical analysis
The study was designed as an RCT. The PPV group received GDFT guided by PPV, while the control group received conventional fluid therapy. The primary outcome measure assessed in this study was the rate of complications within 30 days. Based on a previous study by Mayer et al., which examined high-risk patients undergoing elective major abdominal surgery, the complication rate was 0.2 in the experimental group and 0.5 in the control group [20]. With a two-sided α = 0.05, a power of 1-β set at 0.9, and a sample size ratio of 1:1 between the PPV and control groups, we calculated that each group would require 48 participants using R language analysis software. Considering 20% of cases of loss to follow-up or refusal to participate, we aimed for a final minimum sample size of 60 participants in both groups, resulting in a total sample size of 120 cases.
An independent statistician utilized SPSS for Windows software (version 26.0; SPSS, Chicago, Illinois, USA) to conduct the statistical analysis. The primary outcome measures were evaluated using either the chi-square test or Fisher’s exact test. The normal distribution of the secondary outcome data was assessed using the Kolmogorov–Smirnov test. For normally distributed data, an independent sample t-test was employed for analysis, and variables were expressed as mean ± standard deviation (SD). Non-normally distributed data were compared using the Mann–Whitney U test and presented as the median and interquartile range (IQR). Count data were analyzed using either the chi-square test or Fisher’s exact test, with further pairwise comparisons conducted utilizing the Bonferroni method where appropriate. Two-factor repeated measures analysis of variance was used to compare HR, MAP, PH, and Lac.
Results
Study population
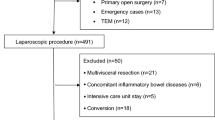
From January 2023 to November 2023, a total of 165 patients were assessed for eligibility, out of which 122 subjects were randomly assigned. Specifically, 61 patients were allocated to the PPV group and another 61 patients to the control group. In the control group, five patients did not receive the assigned intervention due to three cases of consent withdrawal before the procedure initiation and two cases of canceled procedures. Similarly, in the PPV group, three patients did not receive their assigned intervention due to one case of consent withdrawal before surgery and two cases of canceled surgeries. Consequently, a modified intention-to-treat analysis was conducted on the remaining cohort consisting of 114 patients (58 in the PPV group and 56 in the control group) (Fig. 2). Notably, both groups exhibited balanced demographic and perioperative characteristics without any significant differences observed in median operative time, laparoscopic time, or duration of anesthesia as presented in Table 2.
Perioperative management
The crystalloid, colloid, and total fluid intake in the PPV group were significantly lower compared to the control group (P < 0.05). Moreover, a significantly lower number of patients in the PPV group required phenylephrine administration compared to the control group (P < 0.001). Additionally, although not reaching statistical significance (P = 0.056), there was a trend towards fewer cases requiring norepinephrine in the PPV group. No statistically significant differences were observed between the two groups regarding blood loss, urine output, number of blood transfusions, and use of ephedrine (Table 3).
The HR and MAP at T2-T4 in both groups exhibited a significant decrease compared to T1 (P < 0.01). There was no statistically significant difference in HR and MAP between the two groups at T1-T4. The changes in HR and MAP are illustrated in Fig. 3, with precise values provided in Appendix 1.
At T1 and T3, there was no statistically significant difference in Lac levels between the PPV group and the control group. However, at T4, the Lac level in the PPV group was significantly lower than that in the control group (0.59 ± 0.20 mmol/L vs. 0.72 ± 0.30 mmol/L, P = 0.009). Similarly, there was no significant difference in pH between the PPV group and the control group at T1; however, at T3 and T4, the pH value in the PPV group was significantly higher than that in the control group (7.37 ± 0.04 vs. 7.35 ± 0.05, P = 0.012; 7.37 ± 0 0.05 vs 0.7 0.34 ± 0.06, P = 0.004). Lac and PH changes are shown in Fig. 4, with the exact values given in the Supplementary file, Appendix 1.
The intraoperative Lac and pH values. T1, before the administration of anesthesia; T3, 30 min after the initiation of surgery; T4, at the end of surgery. Note: *The difference between the control and PPV groups was statistically significant (P < 0.05). **The difference between the control group the and PPV group was highly statistically significant (P < 0.01)
The opioid consumption (expressed as morphine equivalents) of the two groups is presented in Table 4. There were no statistically significant differences observed in terms of intraoperative morphine equivalents, postoperative morphine equivalents, and perioperative total morphine equivalents between the two groups.
Primary and secondary outcomes
Primary outcomes
Complications occurred in 19 out of 58 patients (32.8%) in the PPV group compared to 32 out of 56 patients (57.1%) in the Control group (P = 0.009). The incidence of gastrointestinal dysfunction was significantly lower in the PPV group compared to the control group (11 vs. 22 cases, P = .017), as well as pneumonia (5 vs. 13 cases, P = 0.033) (Table 5).
Other secondary outcome measures
The distribution of Clavien-Dindo complication scores 30 days after surgery showed a significant difference between the PPV group and the control group (p = 0.032). Furthermore, based on Bonferroni analysis, there were significant differences observed in the distribution of Clavien-Dindo complication scores within the normal function subgroup (PPV group: 40 cases vs. Control group: 25 cases) and grade I (PPV group: 5 cases vs. Control group: 13 cases), while no significant difference was found in the distribution of grade II to V (Fig. 5).
The distribution of I-FEED scores for gastrointestinal dysfunction 30 days after surgery showed a significant difference between the PPV group and the control group (P = 0.001). Furthermore, applying the Bonferroni method revealed significant differences between the two groups in terms of normal function (PPV group: 38 cases vs. Control group: 17 cases) and postoperative intestinal dysfunction (POGD) (PPV group: 11 cases vs. control group: 22 cases), while no significant difference was observed in postoperative gastrointestinal intolerance (POGI) (Fig. 6).
The time to the first postoperative defecation was significantly shorter in the PPV group compared to the control group [58.5 (36.3, 79.0) h vs. 73.5 (41.8, 113.3) h, P = 0.040]. In the PPV group, there was a trend towards shorter durations for extubation, first food intake, and first flatus; however, statistical significance was not reached for these variables. No significant difference was observed between the two groups regarding the time to first ambulation after surgery. Furthermore, LOS in the PPV group was significantly shorter than that in the control group [11.0 (9.0, 14.0) days vs. 13.0 (11.0, 16.0) days, P = 0.007] (Table 6).
Discussion
The key findings of this prospective randomized controlled trial (RCT) demonstrated the efficacy of PPV in GDFT, even during laparoscopic surgery. PPV guided GDFT significantly reduced the incidence of complications and shortened both gastrointestinal recovery time and LOS in patients undergoing laparoscopic radical resection for colorectal cancer.
Due to the impact of pneumoperitoneum on respiratory function, the current application of PPV in laparoscopic surgery remains a subject of controversy [12]. Recent studies have demonstrated that PPV can effectively assess volume responsiveness under appropriate pneumoperitoneum conditions [13]. It has been established that GDFT can optimize early CO, enhance organ oxygen delivery, and contribute to reducing postoperative complications. Its significance is particularly pronounced in high-risk populations such as elderly patients compared to the general population [5, 6]. Therefore, this study selected elderly patients who did not receive perioperative ERAS protocol to investigate whether PPV guided GDFT could improve the prognosis of laparoscopic surgery.
Previous studies have demonstrated that excessive fluid administration can lead to an increase in postoperative complications [21, 22]. Our study revealed that compared with conventional fluid therapy, the PPV group received significantly less fluid volume without a significant difference in urine output when compared with the control group. Furthermore, at the end of surgery, Lac levels were lower and pH values were higher in the PPV group than in the control group, indicating that fluid infusion volume in the PPV group adequately met tissue perfusion needs while excessive fluid administration in the control group may result in volume overload and hinder tissue oxygenation.
Tissue edema resulting from fluid overload can impair the functions of vital organs such as the heart, lungs, and gastrointestinal tract [7, 23,24,25,26,27]. Previous studies have demonstrated that intestinal edema caused by excessive fluid administration may hinder gastrointestinal transit and reduce intestinal contractile activity, leading to gastrointestinal dysfunction[28, 29]. Our study assessed the incidence of postoperative gastrointestinal dysfunction using the I-FEED score and found a significant reduction in its occurrence in the PPV group. Further evaluation of postoperative gastrointestinal function distribution revealed a significantly higher number of patients with completely normal gastrointestinal function in the PPV group than in the control group, which was consistent with the previous study [17, 30]. Additionally, we noted a shorter first postoperative defecation time and trends towards shorter times for extubation, food intake, exhaust time, and defecation time among patients receiving GDFT. The study also observed a decrease in the occurrence of postoperative pneumonia, potentially attributed to the capacity of GDFT to enhance systemic oxygenation, prevent ischemia-reperfusion injury, and mitigate pulmonary edema [31].
Inadequate fluid perfusion can have negative effects on tissue perfusion and tissue oxygenation [32, 33]. Studies conducted by Benes et al. [4] and Chytra et al. [34] have demonstrated that GDFT can decrease Lac levels in surgical patients. However, both of them administered more fluids to the experimental groups compared to the control group, whereas our study found that the PPV group received less fluid due to protocol requirements. Our findings indicate that PPV guided GDFT effectively optimizes perfusion even with reduced fluid administration, thereby preventing compromised tissue oxygenation caused by inadequate or excessive fluids. Additionally, it has been reported that the change in Lac is slow and lacks specificity. Therefore, in future studies, SvO2/ScvO2, PCO2 gap, and PCO2 gap over the arteriovenous difference in oxygen content, an estimate of the respiratory quotient and gastric mucosal pH value could be additionally incorporated for a more comprehensive assessment [35,36,37].
In terms of intraoperative management, both groups exhibited a decrease in MAP and HR following induction, which can be attributed to the vasodilatory effects and myocardial depression induced by anesthetic drugs. Our study demonstrated that GDFT based on PPV could effectively reduce the utilization of vasoactive drugs, aligning with the findings reported by Benes et al. [38] and Goepfert et al. [39]. This may be attributed to PPV’s ability to promptly identify inadequate intravascular volume and guide appropriate fluid administration to enhance CO thereby minimizing reliance on excessive vasoactive drug usage. Furthermore, our study revealed that the PPV group required fewer vasoactive drugs compared to the control group, consequently reducing their impact on tissue and organ perfusion. This observation suggests that decreased reliance on vasoactive drugs might serve as a potential factor contributing to reduced complications within the PPV group.
This study has certain limitations. Firstly, the PPV group in this study solely relied on PPV for guiding volume therapy without monitoring cardiac index (CI) and other indicators, which limited the accurate application of positive inotropic drugs in conjunction with volume therapy. However, recent studies by Benes et al. [38], Cannesson et al. [5], and others have demonstrated that even without CO and SV monitoring, PPV guided GDFT can still yield positive outcomes. The utilization of PPV alone for guiding volume therapy circumvents the need for expensive instruments, reduces monitoring costs, and facilitates the implementation of volume monitoring programs. Secondly, the control group in our study was assigned to a liberal fluid regimen, thus, we did not compare the GDFT with the restrictive fluid regimen. Previous studies have demonstrated that restrictive fluid regimens can mitigate complications associated with fluid overload but may result in renal injury and tissue hypoperfusion [25]. In future investigations, a comparative analysis of the effects of these three regimens on intraoperative management and patient prognosis could be conducted. Additionally, our findings cannot be directly extrapolated to all patients undergoing abdominal surgery due to the limited scope of our study population consisting solely of elderly individuals undergoing laparoscopic radical resection of colorectal cancer while maintaining an intraoperative pneumoperitoneum pressure between 10 and 12 mmHg. Finally, this study had a small sample size, restricted observation time, and lacked long-term survival follow-up; hence, further research is warranted to validate the enduring benefits of PPV guided GDFT in these patients.
Conclusion
This study provides evidence supporting the utilization of PPV as a monitoring indicator for GDFT to facilitate precise fluid management in elderly patients undergoing laparoscopic radical resection of colorectal cancer, thereby optimizing intraoperative hemodynamic control, minimizing complications, and enhancing short-term outcomes.
Data availability
Data can be requested by the corresponding author.
References
Salzwedel C, Puig J, Carstens A, Bein B, Molnar Z, Kiss K, Hussain A, Belda J, Kirov MY, Sakka SG, Reuter DA (2013) Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: A multicenter, prospective, randomized study. Crit Care (London, England) 17(5):R191. https://doi.org/10.1186/cc12885
Stens J, Hering JP, van der Hoeven CWP, Boom A, Traast HS, Garmers LE, Loer SA, Boer C (2017) The added value of cardiac index and pulse pressure variation monitoring to mean arterial pressure-guided volume therapy in moderate-risk abdominal surgery (COGUIDE): A pragmatic multicentre randomised controlled trial. Anaesthesia 72(9):1078–1087. https://doi.org/10.1111/anae.13834
Kan CFK, Skaggs JD (2023) Current commonly used dynamic parameters and monitoring systems for perioperative goal-directed fluid therapy: A review. Yale J Biol Med 96(1):107–123. https://doi.org/10.59249/JOAP6662
Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, Pradl R, Stepan M (2010) Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: Results of prospective randomized study. Crit Care (London, England) 14(3):R118. https://doi.org/10.1186/cc9070
Cannesson M, Ramsingh D, Rinehart J, Demirjian A, Vu T, Vakharia S, Imagawa D, Yu Z, Greenfield S, Kain Z (2015) Perioperative goal-directed therapy and postoperative outcomes in patients undergoing high-risk abdominal surgery: A historical-prospective, comparative effectiveness study. Crit Care (London, England) 19(1):261. https://doi.org/10.1186/s13054-015-0945-2
Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, Hamilton M, Rhodes A (2013) Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Critical Care (London, England) 17(2):209. https://doi.org/10.1186/cc11823
Feng A, Lu P, Yang Y, Liu Y, Ma L, Lv J (2023) Effect of goal-directed fluid therapy based on plasma colloid osmotic pressure on the postoperative pulmonary complications of older patients undergoing major abdominal surgery. World J Surg Oncol 21(1):67. https://doi.org/10.1186/s12957-023-02955-5
Teboul JL, Monnet X, Chemla D, Michard F (2019) Arterial pulse pressure variation with mechanical ventilation. Am J Respir Crit Care Med 199(1):22–31. https://doi.org/10.1164/rccm.201801-0088CI
Malbouisson LMS, Silva JM Jr, Carmona MJC, Lopes MR, Assunção MS, Valiatti JLDS, Simões CM, Auler JOC Jr (2017) A pragmatic multi-center trial of goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery. BMC Anesthesiol 17(1):70. https://doi.org/10.1186/s12871-017-0356-9
Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO Jr, Michard F (2007) Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: A pilot randomized controlled trial. Crit Care (London, England) 11(5):R100. https://doi.org/10.1186/cc6117
Hedrick TL, McEvoy MD, Mythen MMG, Bergamaschi R, Gupta R, Holubar SD, Senagore AJ, Gan TJ, Shaw AD, Thacker JKM, Miller TE, Wischmeyer PE, Carli F, Evans DC, Guilbert S, Kozar R, Pryor A, Thiele RH, Everett S, Grocott M, Perioperative Quality Initiative (POQI) 2 Workgroup (2018) American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative gastrointestinal dysfunction within an enhanced recovery pathway for elective colorectal surgery. Anesth Analg 126(6):1896–1907. https://doi.org/10.1213/ANE.0000000000002742
Myatra SN, Monnet X, Teboul JL (2017) Use of ʽtidal volume challengeʼ to improve the reliability of pulse pressure variation. Crit Care (London, England) 21(1):60. https://doi.org/10.1186/s13054-017-1637-x
Ghoundiwal D, Delaporte A, Bidgoli J, Forget P, Fils JF, Van der Linden P (2020) Effect of pneumoperitoneum on dynamic variables of fluid responsiveness (Delta PP and PVI) during Trendelenburg position. Saudi J Anaesth 14(3):323–328. https://doi.org/10.4103/sja.SJA_737_19
Chen J, Zhao S, Zhu Q (2023) Reliability of stroke volume or pulse pressure variation as dynamic predictors of fluid responsiveness in laparoscopic surgery: a systematic review. J Clin Monit Comput 37(2):379–387. https://doi.org/10.1007/s10877-022-00939-6
Gulin J, Ipavic E, Mastnak DM, Brecelj E, Edhemovic I, Kozjek NR (2023) Phase angle as a prognostic indicator of surgical outcomes in patients with gastrointestinal cancer. Radiol Oncol 57(4):524–529. https://doi.org/10.2478/raon-2023-0060
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Sun Y, Liang X, Chai F, Shi D, Wang Y (2023) Goal-directed fluid therapy using stroke volume variation on length of stay and postoperative gastrointestinal function after major abdominal surgery-a randomized controlled trial. BMC Anesthesiol 23(1):397. https://doi.org/10.1186/s12871-023-02360-1
Li YW, Li HJ, Li HJ, Zhao BJ, Guo XY, Feng Y, Zuo MZ, Yu YP, Kong H, Zhao Y, Huang D, Deng CM, Hu XY, Liu PF, Li Y, An HY, Zhang HY, Wang MR, Wu YF, Wang DX, … Peking University Clinical Research Program Study Group (2021) Delirium in older patients after combined epidural-general anesthesia or general anesthesia for major surgery: A randomized trial. Anesthesiology 135(2):218–232. https://doi.org/10.1097/ALN.0000000000003834
Fiore JF Jr, Bialocerkowski A, Browning L, Faragher IG, Denehy L (2012) Criteria to determine readiness for hospital discharge following colorectal surgery: An international consensus using the Delphi technique. Dis Colon Rectum 55(4):416–423. https://doi.org/10.1097/DCR.0b013e318244a8f2
Mayer J, Boldt J, Mengistu AM, Röhm KD, Suttner S (2010) Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: A randomized, controlled trial. Crit Care (London, England) 14(1):R18. https://doi.org/10.1186/cc8875
Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I (2005) Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 103(1):25–32. https://doi.org/10.1097/00000542-200507000-00008
Brandstrup, B., Tønnesen, H., Beier-Holgersen, R., Hjortsø, E., Ørding, H., Lindorff-Larsen, K., Rasmussen, M. S., Lanng, C., Wallin, L., Iversen, L. H., Gramkow, C. S., Okholm, M., Blemmer, T., Svendsen, P. E., Rottensten, H. H., Thage, B., Riis, J., Jeppesen, I. S., Teilum, D., Christensen, A. M., … Danish Study Group on Perioperative Fluid Therapy (2003) Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann Surg 238(5):641–648. https://doi.org/10.1097/01.sla.0000094387.50865.23
Holte K, Sharrock NE, Kehlet H (2002) Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth 89(4):622–632. https://doi.org/10.1093/bja/aef220
Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M (2008) A rational approach to perioperative fluid management. Anesthesiology 109(4):723–740. https://doi.org/10.1097/ALN.0b013e3181863117
Wuethrich PY, Burkhard FC, Thalmann GN, Stueber F, Studer UE (2014) Restrictive deferred hydration combined with preemptive norepinephrine infusion during radical cystectomy reduces postoperative complications and hospitalization time: A randomized clinical trial. Anesthesiology 120(2):365–377. https://doi.org/10.1097/ALN.0b013e3182a44440
Kang D, Yoo KY (2019) Fluid management in perioperative and critically ill patients. Acute Crit Care 34(4):235–245. https://doi.org/10.4266/acc.2019.00717
Bundgaard-Nielsen M, Secher NH, Kehlet H (2009) Liberal vs restrictive perioperative fluid therapy–a critical assessment of the evidence. Acta Anaesthesiol Scand 53(7):843–851. https://doi.org/10.1111/j.1399-6576.2009.02029.x
Uray KS, Laine GA, Xue H, Allen SJ, Cox CS Jr (2006) Intestinal edema decreases intestinal contractile activity via decreased myosin light chain phosphorylation. Crit Care Med 34(10):2630–2637. https://doi.org/10.1097/01.CCM.0000239195.06781.8C
Uray KS, Laine GA, Xue H, Allen SJ, Cox CS Jr (2007) Edema-induced intestinal dysfunction is mediated by STAT3 activation. Shock 28(2):239–244. https://doi.org/10.1097/shk.0b013e318033eaae
Lee KY, Yoo YC, Cho JS, Lee W, Kim JY, Kim MH (2021) The effect of intraoperative fluid management according to stroke volume variation on postoperative bowel function recovery in colorectal cancer surgery. J Clin Med 10(9):1857. https://doi.org/10.3390/jcm10091857
Dalfino L, Giglio MT, Puntillo F, Marucci M, Brienza N (2011) Haemodynamic goal-directed therapy and postoperative infections: earlier is better. A systematic review and meta-analysis. Crit Care (London, England) 15(3):R154. https://doi.org/10.1186/cc10284
Weinberg L, Ianno D, Churilov L, Chao I, Scurrah N, Rachbuch C, Banting J, Muralidharan V, Story D, Bellomo R, Christophi C, Nikfarjam M (2017) Restrictive intraoperative fluid optimisation algorithm improves outcomes in patients undergoing pancreaticoduodenectomy: A prospective multicentre randomized controlled trial. PLoS ONE 12(9):e0183313. https://doi.org/10.1371/journal.pone.0183313'
Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, Grocott MP, Ahern A, Griggs K, Scott R, Hinds C, Rowan K, OPTIMISE Study Group (2014) Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: A randomized clinical trial and systematic review. JAMA 311(21):2181–2190. https://doi.org/10.1001/jama.2014.5305
Chytra I, Pradl R, Bosman R, Pelnár P, Kasal E, Zidková A (2007) Esophageal Doppler-guided fluid management decreases blood lactate levels in multiple-trauma patients: A randomized controlled trial. Crit Care (London, England) 11(1):R24. https://doi.org/10.1186/cc5703
Monnet X, Teboul JL (2018) My patient has received fluid. How to assess its efficacy and side effects? Ann Intensive Care 8(1):54. https://doi.org/10.1186/s13613-018-0400-z
James JH, Luchette FA, McCarter FD, Fischer JE (1999) Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet (London, England) 354(9177):505–508. https://doi.org/10.1016/S0140-6736(98)91132-1
Kushimoto S, Akaishi S, Sato T, Nomura R, Fujita M, Kudo D, Kawazoe Y, Yoshida Y, Miyagawa N (2016) Lactate, a useful marker for disease mortality and severity but an unreliable marker of tissue hypoxia/hypoperfusion in critically ill patients. Acute Med Surg 3(4):293–297. https://doi.org/10.1002/ams2.207
Benes J, Haidingerova L, Pouska J, Stepanik J, Stenglova A, Zatloukal J, Pradl R, Chytra I, Kasal E (2015) Fluid management guided by a continuous non-invasive arterial pressure device is associated with decreased postoperative morbidity after total knee and hip replacement. BMC Anesthesiol 15:148. https://doi.org/10.1186/s12871-015-0131-8
Goepfert MS, Richter HP, Zu Eulenburg C, Gruetzmacher J, Rafflenbeul E, Roeher K, von Sandersleben A, Diedrichs S, Reichenspurner H, Goetz AE, Reuter DA (2013) Individually optimized hemodynamic therapy reduces complications and length of stay in the intensive care unit: A prospective, randomized controlled trial. Anesthesiology 119(4):824–836. https://doi.org/10.1097/ALN.0b013e31829bd770
Acknowledgements
The authors would like to express their gratitude to colleagues and statisticians at the First Affiliated Hospital of Chongqing Medical University for their invaluable assistance in patient follow-up, data collection, and data analysis.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Qiu-Rong WU served as the lead author and made contributions to the study design, data collection during both preoperative and intraoperative stages, as well as manuscript writing. Ke-Ming Fan and Hui-Ting Cheng were responsible for postoperative data collection. Zi-Zuo Zhao conducted the statistical analysis, while Bin Wang assisted in study design and manuscript review. All authors have thoroughly reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval for this study (2022-231) was provided by the Ethical Committee of the First Affiliated Hospital of Chongqing Medical University (Qing Yan) on 12 September 2022 and was registered in the Chinese Clinical Trial Registry (ChiCTR2300067361). Written informed consent was obtained from all eligible participants or their legal representatives.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, QR., Zhao, ZZ., Fan, KM. et al. Pulse pressure variation guided goal-direct fluid therapy decreases postoperative complications in elderly patients undergoing laparoscopic radical resection of colorectal cancer: a randomized controlled trial. Int J Colorectal Dis 39, 33 (2024). https://doi.org/10.1007/s00384-024-04606-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-024-04606-x