Abstract
Purpose
Myocardial injury after noncardiac surgery (MINS) is associated with increased mortality and postoperative complications. In patients with colorectal cancer (CRC), postoperative complications are a risk factor for cancer recurrence and disease-free survival. This study investigates the association between MINS and long-term oncological outcomes in patients with CRC in an ERAS setting.
Methods
This retrospective cohort study was conducted at Zealand University Hospital, Denmark, between June 2015 and July 2017. Patients undergoing CRC surgery were included if troponin was measured twice after surgery. Outcomes were all-cause mortality, recurrence, and disease-free survival within five years of surgery.
Results
Among 586 patients, 42 suffered MINS. After five years, 36% of patients with MINS and 26% without MINS had died, p = 0.15. When adjusted for sex, age and UICC, the hazard ratio (aHR) for 1-year all-cause mortality, recurrence, and disease-free survival were 2.40 [0.93–6.22], 1.47 [0.19–11.29], and 2.25 [0.95–5.32] for patients with MINS compared with those without, respectively. Further adjusting for ASA status, performance status, smoking, and laparotomies, the aHR for 3- and 5-year all-cause mortality were 1.05 [0.51–2.15] and 1.11 [0.62–1.99], respectively. Similarly, the aHR for 3- and 5-year recurrence were 1.38 [0.46–4.51], and 1.49 [0.56–3.98] and for 3- and 5-year disease-free survival the aHR were 1.19 [0.63–2.23], and 1.19 [0.70–2.03].
Conclusion
In absolute numbers, we found no difference in all-cause mortality and recurrence in patients with and without MINS. In adjusted Cox regression analyses, the hazard was increased for all-cause mortality, recurrence, and disease-free survival in patients with MINS without reaching statistical significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improvements in diagnostics, screening, and treatment have led to an increase in survival after colorectal cancer in the past decades [1]. In the same time period, the development and use of the Enhanced Recovery After Surgery (ERAS) protocol has been established [2, 3]. The ERAS protocol consists of evidence-based guidelines that include a multimodal approach to preoperative, intraoperative, and postoperative care, including surgical, anesthetic, and nursing recommendations. The ERAS protocol is associated with improved recovery and reduced complications after surgery [3, 4]. Nonetheless, a recent global study reports that postoperative complications occur in more than 45% of patients with colorectal cancer [5] and in Denmark, more than 20% do not survive the first five years after surgery [6]. Cardiovascular complications including myocardial infarction, congestive heart failure, and stroke are responsible for a significant number of deaths during surgery, making up for at least one-third of the total [7,8,9]. Knowledge about the occurrence and consequences of myocardial injury after noncardiac surgery (MINS) in an ERAS setting is sparse. MINS is most commonly defined as at least one elevated postoperative cardiac troponin due to a presumed ischemic event [9,10,11]. In patients undergoing colorectal cancer surgery in an ERAS setting, MINS is seen in approximately 7% of patients and is associated with postoperative complications [12]. Moreover, postoperative troponin levels among patients undergoing noncardiac surgery are associated with both increased short- and long-term mortality [8, 10, 13]. In surgical patients with colorectal cancer, postoperative complications are independent risk factors for all-cause mortality, disease-free survival and worsen the risk of cancer recurrence [14]. Interestingly, clinical studies have shown an association between cancer and cardiovascular morbidity [15, 16]. The aim of this study was thus to estimate the effect of MINS during the first seven days after surgery for colorectal cancer on long-term all-cause mortality, recurrence, and disease-free survival in an ERAS setting.
Materials & methods
Study design, data sources, and ethics
We performed a retrospective cohort study including adults undergoing elective surgery for colorectal cancer at Zealand University Hospital, Denmark, between June 2015 and July 2017. In this period, troponin I was scheduled to be measured daily on all patients undergoing surgery in the department until discharge as part of the screening for an international multicenter randomized controlled trial, the MANAGE study [17]. However, due to either early discharge of the patient or missed collection of blood samples, not all patients had two or more troponin I measurements [12]. All patients were treated in accordance with existing national guidelines. Clinical care at the Department of Surgery follows the ERAS society guidelines to ensure an optimal perioperative course [18]. The local ERAS protocol has been described in detail previously and includes elements such as patient education, using minimally invasive techniques, and early mobilization [19,20,21].
Patients whose troponin levels were measured twice within seven days of surgery were included. Data were obtained from electronic patient records and included patient demographics, comorbidities, medication, WHO performance status [22], Revised Cardiac Risk Index (RCRI) [23], Preoperative Score to Predict Postoperative Mortality (POSPOM) score [24], and treatment characteristics including details regarding the surgery, anesthesia, length of hospitalization, intensive care unit stay, and postoperative complications as defined in a previous paper [12]. As part of a quality assurance study at Department of Surgery at Zealand University Hospital, five-year follow up were conducted on patients and according to Danish Legislation no ethical approval was required. The study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Exposure of interest
The exposure of interest was the postoperative occurrence of MINS. It was defined in accordance with the MANAGE trial and has been described thoroughly in previous publications [12, 17]. The Department of Clinical Biochemistry at Zealand University Hospital measured cardiac troponin I and in the study period, they used the Siemens Healthcare Dimension Vista® (Siemens, Munich, Germany) with the LOCI® cardiac troponin I assay. The limit of detection of troponin I for this assay was 15 ng per liter with a cut-off ≥ 45 ng per liter for cardiac ischemia (99th percentile for the upper reference limit for mean troponin from a normal reference population, 10% coefficient of variation at 40 ng per liter) [25].
Outcome of interest
Our outcomes of interest were all-cause mortality, recurrence and disease-free survival. All outcomes were investigated at postoperative year one, three, and five. All-cause mortality was defined as patients not alive at the respective time points. Recurrence was defined as either a local recurrence or a remote metastasis appearing more than 180 days after surgery. Metastases found within 180 days after surgery were interpreted as undetected metastases at the time of surgery and thus not classified as recurrence. Disease-free survival was defined as no occurrence of either recurrence or death in the specified follow-up period.
Statistical methods
Continuous data were expressed as mean (standard deviation) and categorical data as numbers with percentages. Patient data were analyzed using either the Pearson’s Chi-squared test or Fisher’s exact test for dichotomous variables and the Student’s t-test or Wilcoxon rank-sum test for continuous data. All-cause mortality, recurrence, and disease-free survival were examined by the Kaplan–Meier estimator, including the log-rank test and Cox regression models. Unadjusted and adjusted cox regression models were performed. Results were expressed as hazard ratios (HR) with 95% confidence intervals. The one-year model was adjusted for sex, age groups (below 70 or 70 and above), and UICC stages (1 and 2 or 3 and 4), whereas the three- and five-year models additionally were adjusted for ASA status (1 and 2 or 3 and 4), WHO performance status (0, 1, 2 or above), smoking status (never smoked, previous smoker, and current smoker), and laparotomies. The variables were chosen based on clinical hypotheses focusing on adjusting for underlying bias such as age and sex and disease severity. The age cut-off was based on guidelines from Danish Colorectal Cancer Group regarding the adjuvant therapy. In the cox regression model, the Schoenfeld test were used to determine if the models fulfill the proportionality assumption. The models were adjusted for a maximum of ten events per variable in order to avoid overfitted models [26, 27]. The method proposed by Fine and Gray were performed for competing risk analyses [28]. The statistical analyses were performed using SAS 9.4.
Results
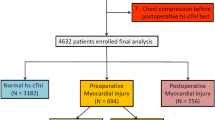
A total of 586 patients with five-year follow-up were included in our study, of which 42 patients suffered MINS within 7 days of surgery. Patients in the MINS group were significantly older, had a lower proportion of non-smokers, and higher ASA score, and higher WHO performance status. The patients with MINS had more comorbidities, but there was no difference in cancer stages nor in the pre-surgical and surgical treatment or procedures. The MINS group experienced more postoperative complications including a longer length of stay and intensive care unit stays. Baseline and perioperative data are presented in Table 1.
All-cause mortality
During the first postoperative year, 12% of patients with MINS and 7% of patients without MINS died, p = 0.20. At postoperative year three, 21% with MINS and 18% without MINS had died, p = 0.58, and at postoperative year five 36% and 26% had died in the two groups, p = 0.15, respectively. The Kaplan–Meier plots showed a higher all-cause mortality in the patient group with MINS at all three time points, however, not statistically significant, see Fig. 1. When adjusted for age, sex, and UICC stage in the Cox regression model of one-year all-cause mortality, the adjusted HR for MINS was 2.40 (0.93 – 6.22). At year three and five, we additionally adjusted for ASA status, performance status, smoking and laparotomies, the adjusted HRs for MINS were 1.05 (0.51 – 2.15), and 1.11 (0.62 – 1.99), respectively. The unadjusted HRs for MINS are shown in Table 2.
Recurrence
In the first postoperative year, the recurrence rate was the same for patients with MINS and those without MINS (2% vs. 3%, p = 1.00). Similarly, no difference at recurrence rate in patients with MINS and in those without were found at postoperative year three (10% and 8%, p = 0.77, respectively) nor at postoperative year five (12% and 9%, p = 0.58, respectively). The adjusted HR for MINS at one year was 1.47 (0.19 – 11.29), at three years 1.38 (0.46 – 4.51), and five years 1.49 (0.56 – 3.98). The unadjusted HRs for MINS were all statically non-significant, see Table 2. Furthermore, the competing risk model proposed by Fine and Gray showed similar associations – data not presented.
Disease-free survival
When adjusting for age, sex, and UICC stage in a Cox regression model of either recurrence or mortality, the adjusted HR for the MINS group at one year was 2.25 (0.95 – 5.32), at three years 1.19 (0.63 – 2.23), and five years 1.19 (0.70 – 2.03). The unadjusted HRs for MINS are presented in Table 2 and shows a non-significant decreased risk of disease-free survival.
Discussion
This retrospective cohort study included patients with troponin measurements after surgery for colorectal cancer in an ERAS setting and patients were followed for five years. Patients with MINS had an increased all-cause mortality and decreased disease-free survival at one, three, and five years after surgery; however, the absolute differences in all-cause mortality and disease-free survival between groups did not reach statistical difference. Recurrence rates were similar for patients with and without MINS.
Throughout the adjusted analyses for all-cause mortality, recurrence, and disease-free survival, patients with MINS had higher HRs compared to those without MINS, but none were statistically significant. Patients with UICC stage 3 and 4 had significantly higher HRs for all-cause mortality, recurrence, and disease-free survival in all adjusted analyses. In the same cohort, we have previously shown no significant difference in 90-day mortality between the MINS group and non-MINS group [12]. In the previous paper, we found that patients with MINS had a higher incidence of postoperative complications. Additionally, we observed that a higher POSPOM score was associated with an increased risk of MINS, and MINS was associated with a higher likelihood of postoperative complications [12].
Bleeding, hypoxemia, and hypotension are all associated with surgery [29,30,31], and in combination with the surgical stress response, it may lead to an oxygen supply–demand mismatch in the myocardium resulting in ischemic myocardial injury [11, 30, 31]. Furthermore, surgery induces acute endothelial dysfunction in the early postoperative period, which could add to the risk of myocardial injury [32,33,34]. Studies have shown that having a cancer is independently associated with a significantly increased risk for cardiovascular morbidity [15]. The other way around, it has also been shown that patients with cardiovascular disease have a higher risk of cancer than the general population [16]. One study has shown that low-grade inflammation is a risk factor for the development of cancer in patients with cardiovascular disease [35]. This implies that inflammation may be a shared underlying factor between cancer and cardiovascular disease.
In a Korean study, elevated high-sensitivity C-reactive protein concentrations at discharge appeared to be associated with increased long-term mortality in patients with MINS [36]. Other studies have shown that in patients with MINS, nonelective surgery is associated with long-term mortality [8]. Interestingly, in patients undergoing orthopedic surgery, trauma surgery was a predictor for long-term mortality after MINS [37]. In patients undergoing open radical cystectomy, there was a significantly lower one-year survival in patients with MINS compared to those without MINS (29% vs 12%) [38]. Elevated C-reactive protein concentrations itself indicate systemic inflammation [39], whereas non-elective/trauma surgery and open surgery all induce a larger surgical stress response causing more inflammation [40, 41]. The use of ERAS reduces postoperative inflammation following colorectal cancer surgery [42] and in our cohort the majority of the patients underwent minimally invasive surgery in an ERAS setting. Reduced postoperative inflammation might explain why we do not find any significant difference in short- or long-term mortality between patients with and without MINS compared to the findings of other studies with more prominent results [13, 43, 44]. However most importantly, our cohort has a low number of patients and limited statistical power to detect meaningful differences between the exposed and unexposed groups, even if a true association between MINS and long-term oncological outcomes exists. Our findings suggest a trend towards an increased risk of mortality and recurrence, which is consistent with the hypothesis of MINS negatively affecting the trajectory of a patient with cancer undergoing surgery. To our knowledge, no other studies have investigated associations between MINS and long-term oncological outcomes in patients undergoing colorectal cancer surgery, but in a small study of patients with head and neck cancer, no difference in three-year overall survival was found in patients with and without postoperative troponin elevations [45].
Our study is a retrospective study with both strengths and limitations. Firstly, unmeasured confounding is inevitable. Secondly, the cohort itself is relatively small and only 42 out of 586 patients developed MINS. During the study period, patients that had less than two troponin measurements were excluded, affecting the cohort size and the power of the study. The 2022 guidelines from European Society of Cardiology recommend to measure troponin concentration before intermediate- and high-risk noncardiac surgery and again at 24 h and 48 h after surgery in patients with cardiovascular disease or symptoms suggestive of it or with cardiovascular risk factors including age 65 or above [46]. Due to the nature of the retrospective study and the limited time-period in which troponin screening was performed at the department, we did not perform a sample size calculation prior to the study, and the study might not be adequately powered to determine significance in terms of long-term outcomes. Even though we did not have any missing data on outcomes of interest, we did have missing data on variables that could have been of interest such as details of the anesthetic techniques, drugs and peroperative monitoring. Furthermore, the limited sample size also restricts the number of covariates that can be adjusted for in a statistical analysis. On the other hand, the study also has several strengths. It examined a homogenous population following the ERAS protocol, ensuring the treatment of all patients was equally good and beneficial. In addition, a complete follow-up was performed on all patients, reducing selection bias and risk of misclassification of the exposure.
In conclusion, the absolute difference in long-term all-cause mortality between patients with and without MINS did not reach statistical significance. In both univariate and adjusted analyses, hazards ratios for all-cause mortality indicated an increased risk for patients with MINS but were not significant. Similarly, the hazards ratios for recurrence were not significant. The trends in colorectal cancer warrants greater focus on identifying patients that may be at higher risk of developing postoperative complications and/or early recurrence. Further studies exploring the association between MINS and long-term oncological outcomes in patients undergoing surgery for colorectal cancer is thus greatly needed. The findings of this study need to be supported by larger cohort studies with sufficient statistical power.
Data Availability Statement
Due to Danish and European Data Protection Regulations and due to the fact, that the individual patients of this study have not give written consent for their data to be shared publicly, supporting data is not available.
References
Iversen LH, Green A, Ingeholm P et al (2016) Improved survival of colorectal cancer in Denmark during 2001–2012 - The efforts of several national initiatives. Acta Oncol Stockh Swed 55(Suppl 2):10–23. https://doi.org/10.3109/0284186X.2015.1131331
Kehlet H (1997) Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 78:606–617. https://doi.org/10.1093/bja/78.5.606
Kehlet H (2008) Fast-track colorectal surgery. The Lancet 371:791–793. https://doi.org/10.1016/S0140-6736(08)60357-8
Ljungqvist O, Scott M, Fearon KC (2017) Enhanced Recovery After Surgery: A Review. JAMA Surg 152:292–298. https://doi.org/10.1001/jamasurg.2016.4952
GlobalSurg Collaborative and National Institute for Health Research Global Health Research Unit on Global Surgery (2021) Global variation in postoperative mortality and complications after cancer surgery: a multicentre, prospective cohort study in 82 countries. Lancet Lond Engl 397:387–397. https://doi.org/10.1016/S0140-6736(21)00001-5
Danish Colorectal Cancer Group: Annual report 2021. Available online on https://dccg.dk/arsrapporter/ or direct link to the PDF is https://dccg.dk/wpcontent/uploads/2023/07/DCCG-Aarsrapport-2021-offentliggjort-version-20221003.pdf
Devereaux PJ, Sessler DI (2015) Cardiac Complications in Patients Undergoing Major Noncardiac Surgery. N Engl J Med 373:2258–2269. https://doi.org/10.1056/NEJMra1502824
Puelacher C, Lurati Buse G, Seeberger D et al (2018) Perioperative Myocardial Injury After Noncardiac Surgery: Incidence, Mortality, and Characterization. Circulation 137:1221–1232. https://doi.org/10.1161/CIRCULATIONAHA.117.030114
Botto F, Alonso-Coello P, Chan MTV et al (2014) Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 120:564–578. https://doi.org/10.1097/ALN.0000000000000113
Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM et al (2017) Association of Postoperative High-Sensitivity Troponin Levels With Myocardial Injury and 30-Day Mortality Among Patients Undergoing Noncardiac Surgery. JAMA 317:1642–1651. https://doi.org/10.1001/jama.2017.4360
Devereaux PJ, Szczeklik W (2020) Myocardial injury after non-cardiac surgery: diagnosis and management. Eur Heart J 41:3083–3091. https://doi.org/10.1093/eurheartj/ehz301
Zahid JA, Orhan A, Ekeloef S, Gögenur I (2021) Myocardial Injury After Colorectal Cancer Surgery and Postoperative 90-Day Mortality and Morbidity: A Retrospective Cohort Study. Dis Colon Rectum 64:1531–1541. https://doi.org/10.1097/DCR.0000000000002061
Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators, Devereaux PJ, Chan MTV et al (2012) Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 307:2295–2304. https://doi.org/10.1001/jama.2012.5502
Aoyama T, Oba K, Honda M et al (2017) Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients’ data from three large phase III randomized trials. Cancer Med 6:1573–1580. https://doi.org/10.1002/cam4.1126
Paterson DI, Wiebe N, Cheung WY et al (2022) Incident Cardiovascular Disease Among Adults With Cancer. JACC CardioOncology 4:85–94. https://doi.org/10.1016/j.jaccao.2022.01.100
van Kruijsdijk RCM, van der Graaf Y, Peeters PHM et al (2013) Cancer risk in patients with manifest vascular disease: effects of smoking, obesity, and metabolic syndrome. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 22:1267–1277. https://doi.org/10.1158/1055-9965.EPI-13-0090
Devereaux PJ, Duceppe E, Guyatt G et al (2018) Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. The Lancet 391:2325–2334. https://doi.org/10.1016/S0140-6736(18)30832-8
Gustafsson UO, Scott MJ, Hubner M et al (2019) Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 43:659–695. https://doi.org/10.1007/s00268-018-4844-y
Munk-Madsen P, Eriksen JR, Kehlet H, Gögenur I (2019) Why still in hospital after laparoscopic colorectal surgery within an enhanced recovery programme? Colorectal Dis 21:1438–1444. https://doi.org/10.1111/codi.14762
Dohrn N, Yikilmaz H, Laursen M et al (2022) Intracorporeal Versus Extracorporeal Anastomosis in Robotic Right Colectomy: A Multicenter, Triple-blind, Randomized Clinical Trial. Ann Surg 276:e294. https://doi.org/10.1097/SLA.0000000000005254
Crone V, Hasselager RP, Fransgaard T, Gögenur I (2020) Anaesthetic technique and outcomes after colorectal cancer surgery. Dan Med J 67:A04190255
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Lee TH, Marcantonio ER, Mangione CM et al (1999) Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 100:1043–1049
Le Manach Y, Collins G, Rodseth R et al (2016) Preoperative Score to Predict Postoperative Mortality (POSPOM): Derivation and Validation. Anesthesiology 124:570–579. https://doi.org/10.1097/ALN.0000000000000972
Amsterdam EA, Wenger NK, Brindis RG et al (2014) 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes. Circulation 130:e344–e426. https://doi.org/10.1161/CIR.0000000000000134
Concato J, Peduzzi P, Holford TR, Feinstein AR (1995) Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin Epidemiol 48:1495–1501. https://doi.org/10.1016/0895-4356(95)00510-2
Peduzzi P, Concato J, Feinstein AR, Holford TR (1995) Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48:1503–1510. https://doi.org/10.1016/0895-4356(95)00048-8
Fine JP, Gray RJ (1999) A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 94:496–509. https://doi.org/10.1080/01621459.1999.10474144
Roshanov PS, Sheth T, Duceppe E et al (2019) Relationship between Perioperative Hypotension and Perioperative Cardiovascular Events in Patients with Coronary Artery Disease Undergoing Major Noncardiac Surgery. Anesthesiology 130:756–766. https://doi.org/10.1097/ALN.0000000000002654
Gill NP, Wright B, Reilly CS (1992) Relationship between hypoxaemic and cardiac ischaemic events in the perioperative period. Br J Anaesth 68:471–473. https://doi.org/10.1093/bja/68.5.471
Bojesen RD, Fitzgerald P, Munk-Madsen P et al (2019) Hypoxaemia during recovery after surgery for colorectal cancer: a prospective observational study. Anaesthesia 74:1009–1017. https://doi.org/10.1111/anae.14691
Ekeloef S, Larsen MHH, Schou-Pedersen AMV et al (2017) Endothelial dysfunction in the early postoperative period after major colon cancer surgery. Br J Anaesth 118:200–206. https://doi.org/10.1093/bja/aew410
Ekeloef S, Godthaab C, Schou-Pedersen AMV et al (2019) Peri-operative endothelial dysfunction in patients undergoing minor abdominal surgery: An observational study. Eur J Anaesthesiol 36:130–134. https://doi.org/10.1097/EJA.0000000000000935
Ekeloef S, Oreskov JO, Falkenberg A et al (2020) Endothelial dysfunction and myocardial injury after major emergency abdominal surgery: a prospective cohort study. BMC Anesthesiol 20:67. https://doi.org/10.1186/s12871-020-00977-0
Van’t Klooster CC, Ridker PM, Hjortnaes J et al (2019) The relation between systemic inflammation and incident cancer in patients with stable cardiovascular disease: a cohort study. Eur Heart J 40:3901–3909. https://doi.org/10.1093/eurheartj/ehz587
Oh AR, Park J, Lee S-H et al (2021) Elevated high-sensitivity C-reactive protein concentrations may be associated with increased postdischarge mortality in patients with myocardial injury after noncardiac surgery: A retrospective observational study. Eur J Anaesthesiol 38:S33–S40. https://doi.org/10.1097/EJA.0000000000001409
Hu W, Chen Y, Zhao K et al (2021) Association of Perioperative Myocardial Injury with 30-Day and Long-Term Mortality in Older Adult Patients Undergoing Orthopedic Surgery in China. Med Sci Monit Int Med J Exp Clin Res 27:e932036. https://doi.org/10.12659/MSM.932036
Yu J, Lim B, Lee Y et al (2020) Risk factors and outcomes of myocardial injury after non-cardiac surgery in high-risk patients who underwent radical cystectomy. Medicine (Baltimore) 99:e22893. https://doi.org/10.1097/MD.0000000000022893
Watt DG, Horgan PG, McMillan DC (2015) Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery 157:362–380. https://doi.org/10.1016/j.surg.2014.09.009
Veenhof A, a. FA, Vlug MS, van der Pas MHGM et al (2012) Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg 255:216–221. https://doi.org/10.1097/SLA.0b013e31824336e2
Foss NB, Kehlet H (2020) Challenges in optimising recovery after emergency laparotomy. Anaesthesia 75:e83–e89. https://doi.org/10.1111/anae.14902
Lee IK, Jalloun H (2018) ERAS protocol influence on postoperative inflammation and short-term postoperative recovery outcomes in colorectal cancer surgery. Clin Nutr ESPEN 25:184. https://doi.org/10.1016/j.clnesp.2018.03.057
Gorgun E, Lan BY, Aydinli HH et al (2016) Troponin Elevation After Colorectal Surgery: Significance and Management. Ann Surg 264:605–611. https://doi.org/10.1097/SLA.0000000000001854
Ekeloef S, Alamili M, Devereaux PJ, Gögenur I (2016) Troponin elevations after non-cardiac, non-vascular surgery are predictive of major adverse cardiac events and mortality: a systematic review and meta-analysis. Br J Anaesth 117:559–568. https://doi.org/10.1093/bja/aew321
Hua G, Levin M, Zhang H et al (2023) Post-operative survival in head and neck cancer patients with elevated troponins. Clin Otolaryngol Off J ENT-UK Off J Neth Soc Oto-Rhino-Laryngol Cervico-Facial Surg 48:200–205. https://doi.org/10.1111/coa.14009
Halvorsen S, Mehilli J, Cassese S et al (2022) 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery: Developed by the task force for cardiovascular assessment and management of patients undergoing non-cardiac surgery of the European Society of Cardiology (ESC) Endorsed by the European Society of Anaesthesiology and Intensive Care (ESAIC). Eur Heart J 43:3826–3924. https://doi.org/10.1093/eurheartj/ehac270
Funding
Open access funding provided by Royal Library, Copenhagen University Library
Author information
Authors and Affiliations
Contributions
Jawad Ahmad Zahid, Adile Orhan, Sarah Ekeloef, and Ismail Gögenur contributed to the study conception and design. Jawad Ahmad Zahid, Adile Orhan, and Noor Al-Huda Hadi carried out the data collection. Jawad Ahmad Zahid performed statistical analyses supervised by Sarah Ekeloef and with Ismail Gögenur contributed to the interpretation of the results. Jawad Ahmad Zahid took the lead in writing the manuscript. All authors provided critical feedback and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests and Funding
This article did not receive any funding for research or publication and the authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zahid, J.A., Orhan, A., Hadi, N.AH. et al. Myocardial injury and long-term oncological outcomes in patients undergoing surgery for colorectal cancer. Int J Colorectal Dis 38, 234 (2023). https://doi.org/10.1007/s00384-023-04528-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-023-04528-0