Abstract
Introduction
The 12-gene recurrence score (RS) is a clinically validated assay which predicts recurrence risk in patients with stage II/III colon cancer. Decisions regarding adjuvant chemotherapy may be guided using this assay or based on the judgement of tumour board.
Aims
To assess the concordance between the RS and MDT decisions regarding adjuvant chemotherapy in colon cancer.
Methods
A systematic review was performed in accordance with PRISMA guidelines. Meta-analyses were performed using the Mantel–Haenszel method using the Review Manager version 5.4 software.
Results
Four studies including 855 patients with a mean age of 68 years (range: 25–90 years) met inclusion criteria. Overall, 79.2% had stage II disease (677/855) and 20.8% had stage III disease (178/855). For the entire cohort, concordant results between the 12-gene assay and MDT were more likely than discordant (odds ratio (OR): 0.38, 95% confidence interval (CI): 0.25–0.56, P < 0.001). Patients were more likely to have chemotherapy omitted than escalated when using the RS (OR: 9.76, 95% CI: 6.72–14.18, P < 0.001). For those with stage II disease, concordant results between the 12-gene assay and MDT were more likely than discordant (OR: 0.30, 95% CI: 0.17–0.53, P < 0.001). In stage II disease, patients were more likely to have chemotherapy omitted than escalated when using the RS (OR: 7.39, 95% CI: 4.85–11.26, P < 0.001).
Conclusions
The use of the 12-gene signature refutes the decision of tumour board in 25% of cases, with 75% of discordant decisions resulting in omission of adjuvant chemotherapy. Therefore, it is possible that a proportion of such patients are being overtreated when relying on tumour board decisions alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adjuvant chemotherapy confers a survival advantage for patients treated for high-risk stage II and all patients with stage III colonic carcinoma [1,2,3,4]. Accordingly, these therapies have become embedded into the clinical guidelines and expert consensus statements [5, 6]. Nevertheless, almost 80% of patients diagnosed with stage II colonic cancers are cured by surgical resection alone, with limited survival advantage conferred by adjuvant chemotherapy [2], questioning therapeutic benefit. Notwithstanding these favourable oncological outcomes, the evidence supporting addition of 5-fluorouracil (5-FU)-based regimens in the adjuvant setting is controversial due to inconsistent results [7, 8]. Recent recommendations from American Society of Clinical Oncology (ASCO) endorse adjuvant chemotherapy use in ‘high risk’ stage II disease, with clinicopathological parameters used to inform tumour board regarding adjuvant chemotherapy [5]. In the setting of stage III disease, there remains debate as to the benefit of oxaliplatin and recommended duration [9,10,11], with adverse clinicopathological features used to guide its use, as in stage II disease [12]. Recently, International Duration Evaluation of Adjuvant Chemotherapy (IDEA) performed a pooled analysis of 6 randomised clinical trials, encompassing 12,835 patients with stage III disease. This illustrated the non-inferiority of shorter course of adjuvant chemotherapies (compared to longer courses), with premise to omit oxaliplatin in certain incidences [9]. Accordingly, these results have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines for managing stage III colon cancer [13].
There is however data highlighting inconsistencies in the reproducibility of several histopathological and molecular parameters to estimate recurrence risk in colon cancer [14, 15], questioning validity of adjuvant chemotherapy regimens. Translation research efforts have focused on identifying novel and reproducible biomarkers which estimate patient-specific risk of disease recurrence and inherent benefit of systemic chemotherapy.
Using formalin-fixed, paraffin-embedded resected specimens from four independent patient cohorts [16,17,18,19], O’Connell et al. designed and prospectively validated a multigene assay capable of estimating prognoses for patients with stage II/III colonic carcinoma [20]. This molecular signature, known as the 12-gene expression assay (commercially available as the OncotypeDX© Recurrence Score (RS) from Genomic Health Inc.©, Redwood City, CA, USA) accurately provides prognostication and estimates derived benefit from chemotherapy [20]. This algorithm uses the reverse transcriptase polymerase chain reaction (RT-PCR) products of 7 cancer-related genes and 5 reference genes, producing a RS which represents the patient-specific risk of recurrence at long-term follow-up [20]. The 12-gene expression assay has subsequently been validated on several occasions [21,22,23,24], however is yet to be endorsed by expert consensus statements, recommendations or guidelines [25].
At present, individualising therapeutic strategies presents a challenge to tumour board [26]. This challenge, coupled with the potential of the 12-gene assay (and the inherent success of other RS assays in other malignancies [27,28,29]), suggests the signature may be of benefit in tailoring chemotherapy regimens to the needs of each patient. Evaluating the rates of concordance between the 12-gene expression assay and colorectal tumour board decisions may be useful for management of prospective patients. Thus, the objective of this study was to perform a systematic review and meta-analysis assessing congruency between the RS assay and tumour board decisions regarding adjuvant chemotherapy in patients with stage II/III colon cancer. The secondary outcome involved the assessment of the impact of discordant decisions the multigene assay and tumour board and whether treatment was omitted or escalated.
Methods
This systematic review and meta-analysis was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and MOOSE guidelines [30, 31]. Local institutional ethical review and approval was not required as this is a review of previously published data. Each author contributed to formulating the study protocol and this was published on the International Prospective Register of Systematic Reviews (PROSPERO).
PICO
Using the PICO framework [32], the aspects the authors wished to address were as follows:
-
Population – Patients with newly diagnosed stage II/III mismatch proficient colonic carcinoma who were aged 18 years or older without distant metastatic disease who underwent 12-gene RS testing (Genomic Health Inc., Redwood City, CA) performed on their resected colonic carcinoma specimen. All included patients had to have prior recommendations from tumour board in relation to whether the patient would benefit from adjuvant chemotherapy prescription.
-
Intervention – Any patient in the selected group found to RS testing results which a concordant with tumour board decision.
-
Comparison – Any patient in the selected group found to RS testing results which a discordant with the tumour board decision.
-
Outcomes – The rate of concordance and discordance of adjuvant chemotherapy decision making based on tumour board decisions and the results of the 12-gene RS assay.
Search strategy
An electronic search was performed of the PubMed, Embase, Cochrane and Science Direct databases for relevant studies. The final search was performed on the 29th of September 2022. This search was performed by two independent reviewers (MGD and MON), using a predetermined search strategy that was designed by the senior authors (AMH and MJ). This search included the search terms: (Oncotype DX) and (colorectal cancer), which was linked by the Boolean operator ‘AND’. Included studies were limited to the English language and were not restricted by year of publication. All duplicate studies were manually removed, before titles were screened, and studies considered appropriate had their abstracts and/or full-text reviewed. Retrieved studies were reviewed to ensure inclusion criteria were met for one primary and secondary outcome at a minimum. In cases of discrepancies of opinion, a third author was asked to arbitrate (EN).
Inclusion and exclusion criteria
Clinical studies comparing patients with stage II/III mismatch repair proficient disease who had previously received a recommendation from the tumour board in relation to whether adjuvant chemotherapy were included. Resected specimens subsequently underwent RS genomic testing. All studies included patients aged 18 years or greater at the time of colonic cancer diagnosis. Outcomes of interest included RS testing, clinicopathological data and the concordance between tumour board recommendation and RS results. Studies including patients with metastatic disease (stage IV colonic carcinoma) were excluded. Published abstracts from conference proceedings were excluded, as were case reports, case series reporting outcomes in five patients or less, and editorial articles. Studies providing data which could not be utilised for meta-analyses were excluded.
Data extraction and quality assessment
The following data was extracted and collated from retrieved studies meeting inclusion criteria: (1) first author name, (2) year of publication, (3) study design, (4) country of origin, (5) number of patients diagnosed with CRC, (6) number of patients with RS testing results, (7) tumour board recommendations, (8) median age (and range) at diagnosis, (9) mean RS, (10) RS categorisation, and (11) clinicopathological data. Risk of bias and methodology quality assessment was performed in accordance to the Newcastle–Ottawa scale [33].
Statistical analysis
Descriptive statistics were used to determine associations between tumour board recommendations and RS categories. Data was expressed as dichotomous or binary outcomes, reported as odds ratios (ORs) were expressed with 95% confidence intervals (CIs) following estimation using the Mantel–Haenszel method. Either fixed or random effects models were applied on the basis of whether significant heterogeneity (I2 > 50%) existed between studies included in the analysis. Statistical heterogeneity was determined using I2 statistics. All tests of significance were two-tailed with P < 0.050 indicating statistical significance. Descriptive statistics were performed using the Statistical Package for Social Sciences (SPSS) version 26 (International Business Machines Corporation, Armonk, New York). Meta-analysis was performed using Review Manager (RevMan), Version 5.4 (Nordic Cochrane Centre, Copenhagen, Denmark). MGD performed each statistical analyses with supervision of the senior author (AMH).
Results
Literature search
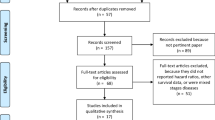
The initial electronic literature search retrieved 559 studies. Overall, 33 duplicate studies were manually extracted. The remaining 526 titles were screened for relevance, before 36 studies had their abstracts, and 20 manuscripts had their full texts reviewed. In total, 4 studies fulfilled the predetermined inclusion criteria and were included in this meta-analysis [34,35,36,37] (Table 1 and Fig. 1).
Study and patient characteristics
Four studies were included in this analysis, which included two prospective and two retrospective cohort studies respectively. Overall, 855 patients were included with a mean age of 68 years (range: 25–90 years). Overall, 79.2% of patients had stage II disease (677/855) and the 87.1% of patients had colonic cancers of the adenocarcinoma histological subtype (425/488). From the 3 studies reporting adjuvant chemotherapy prescription, 32.9% received adjuvant chemotherapy (164/499) and 67.1% underwent surveillance (335/499). Table 1 demonstrates patient demographic data and the risk of bias assessments for the individual studies included in this meta-analysis.
12-gene expression assay results
All 855 included patients underwent RS testing. The mean RS was 28 (range: 7–70). Of note, Brenner et al. was the sole study reporting mean RS results [34]. All studies used the traditional numerical categorisation of RS, which considered RS < 30 as low risk, RS 30–40 as intermediate risk, and RS > 40 as high risk as previously outlined [20]. Of those evaluated compared to the MDT treatment decisions, 71.7% had RS < 30 (556/776), 21.5% had RS 30–40 (167/776), and 6.8% had RS > 40 (53/776) (Table 2).
Overall changes to tumour board recommendations
For the overall patient cohort, 25.8% of recommendations made by tumour board were discordant with the results of 12-gene expression assay testing (200/776) (Table 3). Concordant results between the 12-gene expression assay and tumour board were more likely than discordant results (odds ratio (OR): 0.38, 95% confidence interval (CI): 0.25–0.56, P < 0.001, heterogeneity (I2): 71%) (Fig. 2A). For patients with discordant results between the tumour board and the 12-gene expression assay, these were more likely to have adjuvant chemotherapy omitted (76.0%, 228/300) than treatment escalated to include adjuvant chemotherapy (or the addition of oxaliplatin) (24.0%, 72/300). At meta-analysis, these patients were almost 10 times more likely to have adjuvant chemotherapy omitted than escalated when relying on the 12-gene expression assay (OR: 9.76, 95% CI: 6.72–14.18, P < 0.001, I2 = 47%) (Fig. 2B).
A Forest plot illustrating the likelihood of concordance or discordance between the 12-gene expression assay and the multidisciplinary team for the overall patient cohort. B Forest plot illustrating the likelihood of discordant 12-gene expression assay and the multidisciplinary team results leading to the omission or escalation of adjuvant chemotherapy prescription for the overall patient cohort
Changes to tumour board recommendations for stage II disease
For patients with stage II disease, 20.0% of recommendations made by tumour board were discordant with the results of 12-gene expression assay testing (120/599) (Table 3). Concordant results between the 12-gene expression assay and tumour board were more likely than discordance (OR: 0.30, 95% CI: 0.17–0.53, P < 0.001, I2: 80%) (Fig. 3A). Patients with stage II disease with discordant tumour board and 12-gene expression assay results were more likely to have adjuvant chemotherapy omitted (73.2%, 161/220) than escalated to include adjuvant chemotherapy (26.8%, 59/220). At meta-analysis, patients with stage II disease were more than 7 times more likely to have adjuvant chemotherapy omitted than escalated when relying on the 12-gene expression assay (OR: 7.39, 95% CI: 4.85–11.26, P < 0.001, I2 = 0%) (Fig. 3B).
A Forest plot illustrating the likelihood of concordance or discordance between the 12-gene expression assay and the multidisciplinary team for those with stage II colon cancers. B Forest plot illustrating the likelihood of discordant 12-gene expression assay and the multidisciplinary team results leading to the omission or escalation of adjuvant chemotherapy prescription for those with stage II colon cancers
Changes to tumour board recommendations for stage III disease
Discordance between recommendations made by tumour board and 12-gene expression assay testing results were more likely to occur in stage III disease (44.9%, 80/178) than in stage II disease (20.0%, 120/599) (P < 0.001) (Table 3). As results for stage III disease were only available from the study performed by Oki et al., meta-analysis could not be performed [37].
Discussion
This is the first systematic review and meta-analysis evaluating the importance of using the 12-gene expression assay to guide adjuvant chemotherapy prescription in patients with resected stage II/III colonic carcinoma. The results illustrate that in a quarter of cases, the 12-gene assay provides treatment decisions discordant with that of the MDT, with treatment de-escalation more likely when the RS assay is used. Overall, these results suggest there is a proportion of patients with colonic carcinoma which may be currently overtreated with systemic chemotherapy, which may become more apparent when translating these results from the 855 patients included in this study to a population or global level. Notwithstanding these important findings, further prospective validation of the 12-gene expression assay’s utility is important to ensure oncological outcomes will not be jeopardised by the omission of adjuvant chemotherapy in this cohort, should the assay be used as the sole biomarker used to guide treatment decisions in contemporary colon cancer management.
In this study, an overall discordance rate of 25.8% in treatment decisions between the 12-gene expression assay and tumour board was observed. Discordance between the multigene signature was increasingly prominent in those with stage III disease (44.9%), compared to those with stage II disease (20.0%) (P < 0.001). This is an important finding, as the current management paradigm for colon carcinoma relies on objective assessment of histopathological features, such as lymphovascular invasion, differentiation, and microsatellite instability, to guide therapeutic decision making surrounding adjuvant chemotherapy [7]. Moreover, these results suggest the 12-gene assay captures important predictive biomolecular parameters which are not at the routine discretion of tumour board, when the assay is not available. Accordingly, this suggests the multigene expression assay may correctly identify patients who may be safely spared adjuvant treatment, due to limited long-term benefit of such therapies, and surpasses other risk stratification strategies: Recent data from the IDEA’s ACCENT database highlighted the failure of the IDEA risk classification in successfully predicting the benefit of the addition of oxaliplatin to conventional chemotherapy in stage III colon cancer [38]. Thus, the current analysis highlights the pragmatism of the RS assay to facilitate a personalised approach to oxaliplatin chemotherapy in the setting of stage III colonic carcinoma.
Adjuvant chemotherapy regimens were de-escalated in 75% of cases where 12-gene expression assay refuted decisions of tumour board, with 73% of those with stage II disease having adjuvant chemotherapy omitted completely. Therefore, this suggests the 12-gene signature facilitates judicious use of adjuvant chemotherapy, as for every three patients spared treatment, one patient will require having their treatment escalated. These findings support the recent work of Fu et al.. In their analysis of 58,133 patients with stage II colon cancer, the authors observed inferior oncological outcomes for those in receipt of adjuvant chemotherapy when analysing data from the Surveillance, Epidemiology, and End Results (SEER) database over a 25-year period (1988–2013) [39]. The modern perception is that the omission of adjuvant chemotherapy in the setting of stage II colon cancer may be acceptable for a majority [5]. This could suggest that the utility of molecular signatures (such as RS) in the setting of stage II disease may be questionable, given that a majority could be spared systemic treatment. Nevertheless, there remains a subgroup of patients with RS > 40 who do benefit from adjuvant chemotherapy [20], validating the requirement of such biomarkers in tailoring treatment bespoke. This analysis illustrates the role of the RS in refuting the decisions of four independent tumour boards, across three continents, indicating that RS assay provides a degree of consistency which may not be congruent across the globe [40]. Furthermore, this becomes even more evident when considering those diagnosed with stage III colon cancer, where patient selection using the 12-gene expression assay refuted tumour board decisions in 45% of cases. Therefore, the routine use of multigene signatures may be considered useful in providing reproducible results while individualising patient care in the era where personalised medicine is paramount.
In this study, mean RS was 28 and approximately 70% of patients were classified as being ‘low-risk’, 20% classified as ‘intermediate-risk’ and less than 10% as ‘high-risk’ of recurrence, as stratified by the 12-gene assay. Overall, these results are largely in keeping with previous studies [41, 42], and integrates international data, including patients of various ethnic origins [34,35,36,37]. It is well described that socioeconomic, genetic and racial disparities are factors which may confound results within cancer research and genomic testing [43,44,45]; therefore, consideration for such confounding factors is imperative upon translating these results into clinical practice worldwide.
This study is subject to certain unavoidable limitations. Firstly, while the RS assay has become embedded into contemporary management of breast and prostate cancers, its adoption into the management of colonic carcinoma has been less robust, potentially limiting the impact of these results in clinical practice. Secondly, of the four included studies, just two were of retrospective design, inferring the inherent risks of ascertainment, selection and confounding biases [34, 35]. These factors inevitably and uncontrollably limit the results of this study. Thirdly, the current analysis only included 6.8% of patients classified as ‘high-risk’ (56/776). Although this is consistent with previously published data [41, 42], other analyses have previously illustrated a higher proportion of ‘high-risk’ patients, for which discordance rates with tumour board decisions are not quantified. Fourthly, it is important to note the direct and indirect conflict of interests reported between authors of all included studies and the Genomic Health Inc. who develop the RS assay. This inevitably limits the transparency and robustness of the results yielded in this study. Finally, and most importantly, this analysis fails evaluate the long-term oncological implications of the discordant decisions observed between the RS and tumour board; these results will require thorough interrogation in a prospective fashion prior to implementation as standard of care in conventional colonic carcinoma management. Despite these limitations, the authors wish to reiterate that this analysis is the largest providing real world data comparing outcomes following 12-gene expression assay characterisation and tumour board decisions regarding adjuvant chemotherapy in the setting of stage II/III colonic carcinoma.
In conclusion, this systematic review and meta-analysis of current evidence illustrates the value in the 12-gene expression assay to guide therapeutic decision making when compared to that of international tumour boards. This is evident as using this molecular signature refutes the treatment decisions of the tumour board in 25% of cases, with 75% of these discordant decisions resulting in the omission of adjuvant chemotherapy. Overall, these results suggest there is a proportion of patients with colonic carcinoma which may be currently overtreated with limited oncological benefit. Thus, further prospective validation of 12-gene expression assay in colon cancer is warranted to ensure the individualization of adjuvant chemotherapy decision making.
References
André T, Boni C, Mounedji-Boudiaf L et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350(23):2343–2351
André T, Boni C, Navarro M et al (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27(19):3109–3116
Haller DG, Tabernero J, Maroun J et al (2011) Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 29(11):1465–1471
Sargent D, Sobrero A, Grothey A et al (2009) Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 27(6):872–877
Baxter NN, Kennedy EB, Bergsland E et al (2022) Adjuvant therapy for stage II colon cancer: ASCO guideline update. J Clin Oncol 40(8):892–910
Taieb J, Karoui M, Basile D (2021) How I treat stage II colon cancer patients. ESMO Open 6(4):100184
Dotan E, Cohen SJ (2011) Challenges in the management of stage II colon cancer. Semin Oncol 38(4):511–520
O’Connor ES, Greenblatt DY, LoConte NK et al (2011) Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 29(25):3381–3388
André T, Meyerhardt J, Iveson T et al (2020) Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol 21(12):1620–1629
Grothey A, Sobrero AF, Shields AF et al (2018) Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 378(13):1177–1188
Yoshino T, Oki E, Misumi T et al (2022) Final analysis of 3 versus 6 months of adjuvant oxaliplatin and fluoropyrimidine-based therapy in patients with stage III colon cancer: the randomized phase III ACHIEVE trial. J Clin Oncol 40(29):3419–3429
Schmoll HJ, Van Cutsem E, Stein A et al (2012) ESMO consensus guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 23(10):2479–516
Benson AB, Venook AP, Al-Hawary MM et al (2018) NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw 16(4):359–69
Harris EI, Lewin DN, Wang HL et al (2008) Lymphovascular invasion in colorectal cancer: an interobserver variability study. Am J Surg Pathol 32(12):1816–1821
Roth AD, Tejpar S, Delorenzi M et al (2010) Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol 28(3):466–474
Wolmark N, Fisher B, Rockette H et al (1988) Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst 80(1):30–36
Wolmark N, Rockette H, Wickerham DL et al (1990) Adjuvant therapy of Dukes’ A, B, and C adenocarcinoma of the colon with portal-vein fluorouracil hepatic infusion: preliminary results of National Surgical Adjuvant Breast and Bowel Project Protocol C-02. J Clin Oncol 8(9):1466–1475
Wolmark N, Rockette H, Mamounas E et al (1999) Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 17(11):3553–3559
Lembersky BC, Wieand HS, Petrelli NJ et al (2006) Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol 24(13):2059–2064
O’Connell MJ, Lavery I, Yothers G et al (2010) Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol 28(25):3937–3944
Gray RG, Quirke P, Handley K et al (2011) Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 29(35):4611–4619
Venook AP, Niedzwiecki D, Lopatin M et al (2013) Biologic determinants of tumor recurrence in stage II colon cancer: validation study of the 12-gene recurrence score in cancer and leukemia group B (CALGB) 9581. J Clin Oncol 31(14):1775–1781
Yothers G, O’Connell MJ, Lee M et al (2013) Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin. J Clin Oncol 31(36):4512–4519
Yamanaka T, Oki E, Yamazaki K et al (2016) 12-Gene Recurrence score assay stratifies the recurrence risk in stage II/III colon cancer with surgery alone: the SUNRISE study. J Clin Oncol 34(24):2906–2913
Webber EM, Lin JS, Evelyn PW (2010) Oncotype DX tumor gene expression profiling in stage II colon cancer. Application: prognostic, risk prediction. PLoS Curr 2
Fotheringham S, Mozolowski GA, Murray EMA et al (2019) Challenges and solutions in patient treatment strategies for stage II colon cancer. Gastroenterol Rep (Oxf) 7(3):151–161
Sparano JA, Gray RJ, Makower DF et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121
Kalinsky K, Barlow WE, Gralow JR et al (2021) 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med 385(25):2336–2347
Eggener SE, Rumble RB, Armstrong AJ et al (2020) Molecular biomarkers in localized prostate cancer: ASCO guideline. J Clin Oncol 38(13):1474–1494
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283(15):2008–12
Richardson WS, Wilson MC, Nishikawa J et al (1995) The well-built clinical question: a key to evidence-based decisions. ACP J Club 123(3):A12–A13
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Brenner B, Geva R, Rothney M et al (2016) Impact of the 12-gene colon cancer assay on clinical decision making for adjuvant therapy in stage II colon cancer patients. Value Health 19(1):82–87
Cartwright T, Chao C, Lee M et al (2014) Effect of the 12-gene colon cancer assay results on adjuvant treatment recommendations in patients with stage II colon cancer. Curr Med Res Opin 30(2):321–328
Srivastava G, Renfro LA, Behrens RJ et al (2014) Prospective multicenter study of the impact of oncotype DX colon cancer assay results on treatment recommendations in stage II colon cancer patients. Oncologist 19(5):492–497
Oki E, Watanabe J, Sato T et al (2021) Impact of the 12-gene recurrence score assay on deciding adjuvant chemotherapy for stage II and IIIA/B colon cancer: the SUNRISE-DI study. ESMO Open 6(3):100146
Margalit O, Boursi B, Rakez M et al (2021) Benefit of oxaliplatin in stage III colon cancer according to IDEA risk groups: findings from the ACCENT database of 4934 patients. Clin Colorectal Cancer 20(2):130–136
Fu J, Wu L, Ge C et al (2019) De-escalating chemotherapy for stage II colon cancer? Therap Adv Gastroenterol 12:1756284819867553
Fehervari M, Hamrang-Yousefi S, Fadel MG et al (2021) A systematic review of colorectal multidisciplinary team meetings: an international comparison. BJS Open 5(3)
Jeong DH, Kim WR, Min BS et al (2015) Validation of a quantitative 12-multigene expression assay (Oncotype DX(®) Colon Cancer Assay) in Korean patients with stage II colon cancer: implication of ethnic differences contributing to differences in gene expression. Onco Targets Ther 8:3817–3825
Govindarajan R, Posey J, Chao CY et al (2016) A comparison of 12-gene colon cancer assay gene expression in African American and Caucasian patients with stage II colon cancer. BMC Cancer 16:368
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA: A Canc J Clin 70(1):7–30
Newman LA, Carpten J (2018) Integrating the Genetics of race and ethnicity into cancer research: trailing Jane and John Q. Public JAMA Surg 153(4):299–300
Davey MG, Richard V, Lowery AJ et al (2021) OncotypeDX© Recurrence score in BRCA mutation carriers: a systematic review and meta-analysis. Eur J Cancer 154:209–216
Funding
Open Access funding provided by the IReL Consortium.
Author information
Authors and Affiliations
Contributions
Study conceptualization was performed by Matthew G. Davey. Matthew G. Davey and Maeve O’Neill had access to raw data. Matthew G. Davey, Maeve O’Neill and Babak Meshkat performed statistical analyses. Analysis and interpretation of data were done by Matthew G. Davey, Mark Regan, Myles Joyce., Emmeline Nugent and Aisling Hogan. Matthew G. Davey and Aisling Hogan drafted and wrote the manuscript, while all other authors were consulted with several drafts to appraise the intellectual content of the manuscript. All authors involved in the preparation of this manuscript and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Davey, M.G., O’Neill, M., Regan, M. et al. Impact of the 12-gene recurrence score in influencing adjuvant chemotherapy prescription in mismatch repair proficient stage II/III colonic carcinoma—a systematic review and meta-analysis. Int J Colorectal Dis 38, 71 (2023). https://doi.org/10.1007/s00384-023-04364-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-023-04364-2