Abstract
Clinical observations have demonstrated that microsatellite instability-high (MSI-H) and/or deficient MMR (dMMR) status are associated with favorable prognosis and no benefit from 5-Fluorouracil (5-FU)-based adjuvant chemotherapy in patients with resected stage II colorectal cancer (CRC). This study represents a systematic review and meta-analysis exploring the predictive role of MSI-H status in stage III CRC undergoing or not adjuvant chemotherapy. Published articles that evaluated the role of adjuvant chemotherapy in resected stage III CRC from inception to September 2020 were identified by searching the PubMed, EMBASE, and Cochrane Library databases. The random-effects model was conducted to estimate the pooled effect size of OS and DFS. The primary outcome of interest was OS. 21,590 patients with MSI-H/dMMR stage III CRC, from n = 17 retrospective studies, were analyzed. Overall, OS was improved with any adjuvant chemotherapy vs. any control arm (single-agent 5-FU or surgery alone): HR 0.42, 95% CI 0.26–0.66; P < 0.01. Conversely, DFS was not significantly improved (HR 0.7, 95% CI 0.45–1.09; P = 0.11). In patients with stage III MSI-H/dMMR CRC, adjuvant chemotherapy is associated with a significant OS improvement. Thus, MSI-H/dMMR status does represent a predictive factor for postoperative chemotherapy benefit in stage III CRC beyond its prognostic role.
Similar content being viewed by others
Introduction
A large amount of evidence is currently available on the less beneficial, or even potentially detrimental effect of adjuvant, single-agent, fluoropyrimidine-based chemotherapy in patients with microsatellite high (MSI-H) or deficient mismatch repair (dMMR) stage II colorectal cancer (CRC)1. Much less clear is the impact of MSI-H/dMMR status in radically resected, node-positive CRCs. Updated results of the "MOSAIC" trial with 10-year median follow up, showed important disease-free (DFS) and overall survival (OS) improvements (hazard ratios [HRs] 0.48 [95% CI 0.20 to 1.12] and 0.41 [95% CI 0.16 to 1.07], respectively), in 9.4% of patients with stage II to III dMMR by the addition of oxaliplatin to 5-FU chemotherapy backbone2. However, the study resulted underpowered to show statistically significant differences due to the retrospective nature of the analysis and the small sample size. Further studies evaluating the effect of FOLFOX in patients with nonmetastatic MSI-H colorectal adenocarcinoma produced opposite results failing to find any correlation between MSI-H status and survival3,4. Therefore, we performed a systematic review and meta-analysis to explore the predictive role of MSI-H/dMMR status in stage III CRC patients undergoing or not adjuvant chemotherapy.
Material and methods
Search strategy and inclusion criteria
Three authors (GT, BG and FP) searched the databases PubMed, Cochrane Central Library, and Embase for potential articles published before September 2020. Search terms were: (((("microsatellite instability"[All Fields] OR "MSI"[All Fields]) OR "dMMR"[All Fields]) AND (((((((((((((((("adjuvancy"[All Fields] OR "adjuvanted"[All Fields]) OR "adjuvanting"[All Fields]) OR "adjuvants"[All Fields]) OR "adjuvants pharmaceutic"[Pharmacological Action]) OR "adjuvants immunologic"[Pharmacological Action]) OR "adjuvants, pharmaceutic"[MeSH Terms]) OR ("adjuvants"[All Fields] AND "pharmaceutic"[All Fields])) OR "pharmaceutic adjuvants"[All Fields]) OR "adjuvant"[All Fields]) OR "adjuvants, immunologic"[MeSH Terms]) OR ("adjuvants"[All Fields] AND "immunologic"[All Fields])) OR "immunologic adjuvants"[All Fields]) OR "adjuvated"[All Fields]) OR "adjuvation"[All Fields]) OR "adjuvent"[All Fields]) AND (((((("chemotherapy s"[All Fields] OR "drug therapy"[MeSH Terms]) OR ("drug"[All Fields] AND "therapy"[All Fields])) OR "drug therapy"[All Fields]) OR "chemotherapies"[All Fields]) OR "drug therapy"[MeSH Subheading]) OR "chemotherapy"[All Fields]))) AND (((((("stage"[All Fields] OR "staged"[All Fields]) OR "stages"[All Fields]) OR "staging"[All Fields]) OR "stagings"[All Fields]) AND "III"[All Fields]) OR "Dukes C"[All Fields])) AND (((((((("colon"[MeSH Terms] OR "colon"[All Fields]) OR "colonic"[All Fields]) OR "colons"[All Fields]) OR "colon s"[All Fields]) OR "colonal"[All Fields]) OR "colonically"[All Fields]) OR "colonitis"[All Fields]) OR "colorectal"[All Fields]).
Inclusion criteria were: (1) original articles (prospective or retrospective), with outcome data available, that compared the survival among adjuvant and no adjuvant chemotherapy arms in stage III MSI-H CRC, (2) comparison of single-agent 5-FU/capecitabine or combination chemotherapy with observation, (3) available hazard ratio (HR) for OS, disease-free survival (DFS), that compared experimental and control arms, or that may be calculated from survival curves. Papers were excluded if the number of patients in the MSI-H group was less than 10. We also did not include letters, review articles, and case reports. Data extraction was performed by two authors separately (FP and MG). If multiple articles were used to investigate the patients in the same clinical trial or medical institution, the latest or largest one would be included to prevent overlapping in case of disagreement, a third reviewer (FG) made the decision. The included studies included both randomized trials and retrospective series, therefore, both Cochrane and Newcastle–Ottawa scales were used to assess the methodological quality.
Statistical analysis
The adjusted hazard ratios (HRs) calculated through a multivariate analysis were used to estimate the predictive value of MSI-H in stage III CRC. The HRs were extracted from the Cox proportional hazards regression model provided in the relevant articles. When the HR between experimental and control arms was not provided but the Kaplan–Meier survival curves were available, it was calculated with the method described by Tierney et al.1. Meta-analysis comparing treated and not treated patients aimed to explore the relationship between MSI-H status and benefit of adjuvant chemotherapy. RevMan 5.3 software was used for calculation. Heterogeneity was described using the I2 statistic. The random-effects model was used to estimate the effect size of OS and DFS. Funnel plots were provided in OS analysis to examine publication bias. Sensitivity analysis was also performed in OS analysis to describe heterogeneity. The RCT studies were assessed according to the Cochrane protocol5. The non-RCT studies were assessed using the Newcastle–Ottawa scale6, which considers participant selection, comparability, and outcome with a maximum total score of 9. A score higher than 7 was considered to be of good quality for the individual study.
Results
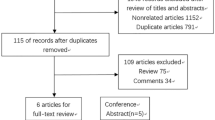
The flow of article selection process is depicted in Fig. 1. After screening 307 records, 17 studies finally met the predefined criteria and were considered eligible for inclusion in the systematic review7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23. Descriptive characteristics of the eligible studies are summarized in Table 1. There were n = 10 retrospective single-center series; n = 5 were analysis of n = 31 historical randomized trials, and 2 were cohort studies (n = 21,590 patients analysed). Patients were analyzed from 2004 to 2020. The median rate of MSI-H CRC was 13.7% (n = 2958). It was evaluated with immunohistochemistry biomarkers (n = 4), with MSI loci (n = 4) or both methods (n = 6). In 3 studies, method was not reported. Studies showed a prevalence of right sided CRC and of female patients. In 5 publications, experimental arms were combination chemotherapies (5-FU plus irinotecan or oxaliplatin or 5-FU + oxaliplatin + cetuximab). The quality of trials, was moderate (median NOS score 6.5) in retrospective studies with a low-moderate risk of bias in randomized trials.
Overall survival was improved with any comparison of any adjuvant chemotherapy vs any control arm (HR 0.42, 95% CI 0.26–0.66; P < 0.01; Fig. 2). Conversely, DFS was not significantly improved (HR 0.7, 95% CI 0.45–1.09; P = 0.11; Fig. 3). In studies where any type of adjuvant chemotherapy (combination chemotherapy or single agents) was compared to surgery alone, survival was even more improved (HR for OS 0.34, 95% CI 0.22–0.54; P < 0.01). In studies where polychemotherapy was the experimental arm the benefit was of similar magnitude (HR 0.37, 95% CI 0.19–0.74). The results were significant in retrospective series (HR for OS 0.32, 95% CI 0.20–0.51; P < 0.01) but not in n = 3 randomized studies (HR 0.98, 95% CI 0.55–1.75; P = 0.94). However, this last analysis included only 204 patients.
To explore if clinical risk classification (defined high risk when tumor had pT4 and/or pN2 status) may influence benefit of adjuvant chemotherapy we performed a meta-regression analysis according to low-risk population. This analysis was possible only for DFS analysis for lack of sufficient data in OS population and confirmed a significant trend for reduced DFS benefit for adjuvant chemotherapy in low risk subgroup (P = 0.04).
Begg's and Egger's tests were not significant for publication bias (P = 0.31 and P = 0.08; Fig. 4)24.
Discussion
Stage II colorectal tumors harboring microsatellite instability or dMMR status are commonly associated with a more favorable prognosis as compared to their MSS counterpart. Patients in the same stage without high-risk features are also not likely to derive significant benefit from adjuvant fluoropyrimidine-based therapy. While the negative impact of adjuvant chemotherapy in stage II has been established, less clear is the role of postoperative treatments for MSI-H/dMMR stage III CRC. Unfortunately, little evidence is currently available for this patient subgroup, coming almost exclusively from retrospective analyses of large randomized controlled trials or single-center case series. Most early studies failed in demonstrating any survival benefit associated with fluoropyrimidine chemotherapy. However, a recently published large multicenter AGEO study indicated opposite results20. Specifically, this retrospective trial included 433 dMMR stage II/III CRC patients who received surgery alone (n = 263) or surgery plus adjuvant chemotherapy consisting of fluoropyrimidine with (n = 119) or without (n = 51) oxaliplatin. Interestingly, at multivariate analysis, a statistically significant DFS advantage (HR 0.41, 95% CI 0.19–0.87, P = 0.02) in favor of stage III patients receiving adjuvant fluoropyrimidine plus oxaliplatin chemotherapy (n = 89) compared with those treated with surgery alone (n = 58) was shown, while chemotherapy with fluoropyrimidine alone (n = 40) was not associated with a longer DFS compared with surgery alone (HR 0.66, 95% CI 0.29–1.50, P = 0.32).
Despite the clear limitation of the nonrandomized and retrospective design of all studies included, this meta-analysis provides evidence that adjuvant chemotherapy is beneficial in patients with stage III MSI-H/dMMR colorectal cancer. Specifically, in patients receiving any adjuvant chemotherapy, the risk of death is reduced by 58% compared with surgery alone or 5-FU single-agent. More interestingly, compared with only observation following surgery, mono- or combination chemotherapy was associated with a more considerable OS benefit corresponding to an impressive death risk reduction by 66%.
Although a DFS difference between any adjuvant treatment and surgery was evident, it did not reach statistical significance. This finding may be explained with the fact that some large cohort or registry-based series collected only survival data, with DFS available in only half of studies. Also, MSI-H/dMMR are low rate of relapse events, in particular for N1 sub stage, and a prolonged post recurrence survival as showed in the ACCENT analysis previously published by Tajeb et al.25.
Our analysis has some intrinsic limitations. Firstly, despite no obvious publication bias, we observed notable heterogeneity due to retrospective nature of the study, and the inclusion of relatively different populations. We took this into account with a random effect model analysis and with subgroup analysis. In addition, a significant difference was observed for the type of MMR evaluation (biomarker vs. IHC analysis) and the quality/size of publications. Secondly, this meta-analysis was based on published data instead of individual patient data. Thirdly, analysis was not performed according to side (right vs. left CRC), nodal stage (high vs. low-risk stage III disease), and for colon vs. rectal cancers. Fourth, we did not consider the prognostic effect of MSI status, but this was already known from previous reviews and meta-analysis of randomized studies that showed a similar prognosis of MSS, stage III tumors. However, this represents the more comprehensive and updated meta-analysis exploring the predictive effect of MSI status in stage III CRC.
Our results align with those very recently published from an ACCENT pooled analysis of twelve adjuvant trials26, which evaluated the effect of fluoropyrimidine with or without oxaliplatin on DFS and OS on a large cohort of patients with MSI stage III CRC. This study showed that the combination of fluoropyrimidine plus oxaliplatin significantly improves OS of patients with MSI/dMMR stage III CRC (HR, 0.52; 95% CI, 0.28 to 0.93) from the two randomized trials testing fluoropyrimidines with or without oxaliplatin. Moreover, authors investigated the prognostic value of MSI among the 4,250 patients treated with fluoropyrimidines plus oxaliplatin, and found that MSI was associated with better OS in the N1 group compared with MSS (adjusted HR 0.66; 95% CI 0.46–0.95) but similar survival in the N2 population (adjusted HR 1.13; 95% CI 0.86–1.48; P interaction = 0.029). Our analysis provide a more expanded population of MSI CRC patients including also n = 31 randomized studies other than observational series (n = 2958), and showed a trend for better DFS with adjuvant chemotherapy in high-risk stage III CRCs. Also, unlike ACCENT analysis where no significant benefit was registered with 5FU alone, in the present cohort we found a similar benefit of adjuvant chemotherapy independent of treatment schedule.
In conclusion, based on the findings of our meta-analysis, MSI-H/dMMR condition should be regarded as an important predictive factor for adjuvant chemotherapy benefit in node-positive radically resected CRC patients, in particular with high risk features (pT4 and/or pN2 disease). According to age and performance status, when feasible, and in the absence of specific medical contraindications to chemotherapy, at least single-agent 5-FU or combination treatments should be recommended. Studies exploring the addition of immune-checkpoint inhibitors to chemotherapy in this specific setting are currently ongoing. Hopefully, positive results will further improve and expand the available therapeutic options.
References
Battaglin, F., Naseem, M., Lenz, H. J. & Salem, M. E. Microsatellite instability in colorectal cancer: Overview of its clinical significance and novel perspectives. Clin. Adv. Hematol. Oncol. 16(11), 735–745 (2018).
AndrÈ, T. et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: Updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J. Clin. Oncol. 33(35), 4176–4187. https://doi.org/10.1200/JCO.2015.63.4238 (2015).
Gavin, P. G. et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: An assessment of their prognostic and oxaliplatin predictive value. Clin. Cancer Res. 18(23), 6531–6541. https://doi.org/10.1158/1078-0432.CCR-12-0605 (2012).
Kim, J. E. et al. Microsatellite instability was not associated with survival in stage III colon cancer treated with adjuvant chemotherapy of oxaliplatin and infusional 5-fluorouracil and leucovorin (FOLFOX). Ann. Surg. Oncol. 24(5), 1289–1294. https://doi.org/10.1245/s10434-016-5682-5 (2017).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. https://doi.org/10.1136/bmj.d5928 (2011).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25(9), 603–605. https://doi.org/10.1007/s10654-010-9491-z (2010).
Alwers, E. et al. Microsatellite instability and survival after adjuvant chemotherapy among stage II and III colon cancer patients: results from a population-based study. Mol. Oncol. 14(2), 363–372. https://doi.org/10.1002/1878-0261.12611 (2020).
Chouhan, H., Sammour, T., Thomas, M. L. & Moore, J. W. The interaction between BRAF mutation and microsatellite instability (MSI) status in determining survival outcomes after adjuvant 5FU based chemotherapy in stage III colon cancer. J. Surg. Oncol. 118(8), 1311–1317. https://doi.org/10.1002/jso.25275 (2018).
de Vos tot Nederveen Cappel, W. H. et al. Survival after adjuvant 5-FU treatment for stage III colon cancer in hereditary nonpolyposis colorectal cancer. Int. J. Cancer. 109(3), 468–471. https://doi.org/10.1002/ijc.11712 (2004).
Elsaleh, H. & Iacopetta, B. Microsatellite instability is a predictive marker for survival benefit from adjuvant chemotherapy in a population-based series of stage III colorectal carcinoma. Clin. Colorect. Cancer 1(2), 104–109. https://doi.org/10.3816/CCC.2001.n.010 (2001).
Klingbiel, D. et al. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: Results of the PETACC-3 trial. Ann. Oncol. 26(1), 126–132. https://doi.org/10.1093/annonc/mdu499 (2015).
Lanza, G. et al. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J. Clin. Oncol. 24(15), 2359–2367. https://doi.org/10.1200/JCO.2005.03.2433 (2006).
Ogino, S. et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: Results from intergroup trial CALGB 89803. Clin. Cancer Res. 18(3), 890–900. https://doi.org/10.1158/1078-0432.CCR-11-2246 (2012).
Ooki, A. et al. Combined microsatellite instability and BRAF gene status as biomarkers for adjuvant chemotherapy in stage III colorectal cancer. J. Surg Oncol. 110(8), 982–988. https://doi.org/10.1002/jso.23755 (2014).
Sasaki, Y. et al. Prognostic and predictive value of extended RAS mutation and mismatch repair status in stage III colorectal cancer. Cancer Sci. 107(7), 1006–1012. https://doi.org/10.1111/cas.12950 (2016).
Shaib, W. L. et al. Survival outcome of adjuvant chemotherapy in deficient mismatch repair stage III colon cancer. Cancer https://doi.org/10.1002/cncr.33049 (2020).
Sinicrope, F. A. et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J. Natl. Cancer Inst. 103(11), 863–875. https://doi.org/10.1093/jnci/djr153 (2011).
Tan, W. J. et al. Evaluation of long-term outcomes of microsatellite instability status in an asian cohort of sporadic colorectal cancers. J. Gastrointest. Cancer. 49(3), 311–318. https://doi.org/10.1007/s12029-017-9953-6 (2018).
Thomas, M. L., Hewett, P. J., Ruszkiewicz, A. R. & Moore, J. W. Clinicopathological predictors of benefit from adjuvant chemotherapy for stage C colorectal cancer: Microsatellite unstable cases benefit. Asia Pac. J. Clin. Oncol. 11(4), 343–351. https://doi.org/10.1111/ajco.12411 (2015).
Tougeron, D. et al. Efficacy of adjuvant chemotherapy in colon cancer with microsatellite instability: A large multicenter AGEO study. J. Natl. Cancer Inst. https://doi.org/10.1093/jnci/djv438 (2016).
Wang, S. M. et al. Clinical significance of MLH1/MSH2 for stage II/III sporadic colorectal cancer. World J. Gastrointest. Oncol. 11(11), 1065–1080. https://doi.org/10.4251/wjgo.v11.i11.1065 (2019).
Zaanan, A. et al. Impact of p53 expression and microsatellite instability on stage III colon cancer disease-free survival in patients treated by 5-fluorouracil and leucovorin with or without oxaliplatin. Ann. Oncol. 21(4), 772–780. https://doi.org/10.1093/annonc/mdp383 (2010).
Zaanan, A. et al. Clinical outcomes in patients with colon cancer with microsatellite instability of sporadic or familial origin treated with adjuvant FOLFOX with or without cetuximab: A pooled analysis of the PETACC8 and N0147 trials. JCO Precis. Oncol. https://doi.org/10.1200/PO.19.00237 (2020).
Lin, L. & Chu, H. Quantifying publication bias in meta-analysis. Biometrics 74(3), 785–794. https://doi.org/10.1111/biom.12817 (2018).
Taieb, J. et al. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: Results of an ACCENT pooled analysis of seven studies. Ann. Oncol. 30(9), 1466–1471 (2019).
Cohen, R. et al. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: An ACCENT pooled analysis of 12 adjuvant trials. J. Clin. Oncol. 39(6), 642 (2021).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tomasello, G., Ghidini, M., Galassi, B. et al. Survival benefit with adjuvant chemotherapy in stage III microsatellite-high/deficient mismatch repair colon cancer: a systematic review and meta-analysis. Sci Rep 12, 1055 (2022). https://doi.org/10.1038/s41598-022-05065-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05065-6
- Springer Nature Limited