Abstract
Purpose
Colorectal cancer (CRC) can be classified according to the chromosomal-instability pathway (a microsatellite-stable (MSS) pathway) and the microsatellite-instability (MSI) pathway. Adjuvant therapy after surgery in advanced CRC is usually based on fluoropyrimidine 5-fluorouracil (5-FU) alone or combined with other agents. Controversy however remains on the use of 5-FU-based regimens in treating MSI-related tumours.
Aims
To systematically investigate the relationship between tumour microsatellite profile and 5-year overall survival in patients with CRC treated with 5-FU.
Methods
A systematic literature review of PubMed and Embase databases was conducted. Pre-specified criteria determined study inclusion/exclusion. The PRISMA and QUADAS-2 criteria were used to assess study suitability and quality respectively. Patients were categorised as having either MSI or MSS CRC. Overall 5-year survival was estimated from Kaplan–Meier curves. Publication bias was assessed using funnel-plots and Egger’s test.
Results
1807 studies were identified, with meta-analysis performed using nine studies. 5-FU treated individuals with CRC who died at 5 years were found to be 0.31 times less likely to have MSI than those who were alive, although this was not statistically significant. There was an insufficient number of studies to enable subgroup analysis by stage.
Conclusions
In this meta-analysis, MSI status does not alter 5-year survival of patients with CRC patients treated with adjuvant 5-FU, however there is significant heterogeneity in the design of individual studies in the data synthesis. More studies are necessary to clarify whether CRC patients with MSI CRC, in particular early stage, should be offered 5-FU based adjuvant chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) may be classified according to the molecular pathways behind its pathogenesis and progression [1]. The molecular architecture of colorectal cancer has been largely described as two predominant pathways: the chromosomal-instability pathway accounting for approximately 85% of cases and the microsatellite-instability (MSI) pathway in the remaining 15% of cases. The MSI pathway is related to defective DNA mismatch-repair (MMR). The DNA MMR repair system controls the accuracy of DNA replication by repairing errors such as base substitutions and insertion-deletion occurring during DNA replication.
In the context of defective MMR (dMMR), repetitive DNA sequences are naturally prone to replicative errors (microsatellites). Loss of MMR protein expression may be demonstrated in tumoral tissue by immunohistochemistry. On the other hand MSI is a PCR-based assay which demonstrates the downstream effect of defective repair of DNA mismatches in cancer. Samples are usually tested for MSI by PCR using a panel of microsatellite markers. Non-neoplastic tissue is used as normal control.
Adjuvant therapy is commonly offered to patients after surgery for CRC on the grounds that it may improve the 5-year survival. Alone or combined with other agents, 5-FU has been the mainstay of adjuvant therapy for CRC and has been investigated extensively in terms of potential factors to influence its response or resistance, including MMR status.
Patients with MSI tumours have a more favourable prognosis than their MSS counterparts. This is in contrast to variable reports on the effect of 5-FU on MSI tumours. Early small non-randomized studies show a benefit, but subsequent studies showed no benefit or even a detrimental effect [2]. A seminal study published in 2003 showed that 5-FU -based adjuvant chemotherapy is not associated with a significant increase in overall and disease-free survival of patients with MSI-related CRC, and may decrease their survival [3]. There is, however, on-going controversy about the available evidence on the use fluorouracil-based regimens in treating MSI-related tumours, particularly in relation to the effect of patient’s age and in combination with other drugs.
The aim of this study was therefore to investigate, by performing a systematic review and meta-analysis, the relationship between MSI and response to 5-FU based adjuvant chemotherapy in terms of 5 year overall survival, in CRC patients.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and checklist were followed to carry out the literature review and meta-analysis of this study [4]. The article quality rating was carried out as described by Webber et al. based on the QUADAS-2 (Table 1) [5, 6].
Literature search
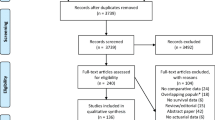
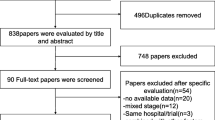
The literature search (Fig. 1) was carried out using the PubMed and Embase databases from conception until June 2021. Potentially informative papers were identified by reading the title of each article, and subsequently the abstract. The literature search was carried out using a combined approach because an initial searching strategy based on combinations of commonly used terms relevant to this study resulted in a number of studies too large or too small. The combination search was based on the following terms: (((colon neoplasm) AND fluorouracil)) AND microsatellite instability; (((rectum neoplasm) AND fluorouracil)) AND microsatellite instability; (((colon neoplasm) AND fluorouracil)) AND DNA mismatch repair; (((rectum neoplasm) AND fluorouracil)) AND DNA mismatch repair.
The final search was carried out by reviewing the titles of papers obtained from the combined search of "microsatellite instability"[MeSH Terms] AND "colorectal neoplasms"[MeSH Terms]” to ensure that no potentially informative papers were omitted for the initial screening process. Two independent reviewers (N.A and A.Q.) assessed the search strategy results. A strict inclusion/exclusion criteria was used to assess study eligibility (Table 2). No additional papers were identified after searching Embase.
If multiple studies included the same cohort of cases or controls, the study with the largest sample size was used. In the case of any uncertainty regarding study inclusion, another investigator (K.M.) was consulted to assess eligibility.
A total of 17 papers was identified from the screening process. The full text of these 17 selected studies was accessed and retrieved for the next stage of the review. The bibliographies of relevant studies were inspected for further eligible studies.
The number of 5-FU treated patients who showed overall survival at 5 years, both in the MSI and MSS was clearly provided in three papers [3, 7, 8]. The corresponding authors of the 14 remaining papers were emailed, requesting raw data of the number of 5-FU treated MSI and MSS patients alive at five years.
A second review of the 17 eligible papers showed that 9 papers included the overall survival in the Kaplan–Meier curve and also specified the initial number of patients, for both MSI and MSS groups. The survival data were extracted using the digitizing software, digitizeIt. Briefly, survival curves from each paper where copied from the PDF document using the snapshot tool, pasted into a power point document, saved as JPEG files and opened with digitezeIt. Using the digitezeIt toolbar x and y buttons, two points on the x-axis and two points on the y-axis of each figure were marked with the cursor and their corresponding x- and y-values as indicated in each survival curve were entered. The next step involved a combination of the three digitizeIt tools, namely the point and click tool, the automatic line-selecting tool or the automatic symbol finder. The advantage of the automatic line-selecting tool is that it allows, by clicking on any point of a survival curve line, the selection of the entire line. The software returns the list of numerous line points each ordered according to its position on the line and listing each point coordinates according to the values allocated to x- and y-axis. Scrolling down the list of retrieved points identifies the 5-year mark. Its corresponding y-value indicates the survival percentage point. As the papers included the number of patients in each MSI and MSS group at the beginning of the study, the proportion of patients obtained from the Kaplan–Meier curve was used to calculate the number of patients alive at 5 years.
Data Extraction
The following data was obtained from the papers selected for the study: study title, first author, publication year, total number of patients with CRC treated with 5-FU and of these the number of patients with MSI tumours and the number of patients with MSS tumours, and tumour stage at the time of diagnosis and treatment.
Statistical analysis
Given that (A) cases and (A) controls correspond to the number of 5-FU treated MSI patients dead at 5 years and alive at 5 years and (B) cases and (B) controls correspond to the number of 5-FU treated MSS patients dead at 5 years and alive at 5 years, the pooled Odds Ratios (OR) were calculated as follows:
\(O.R.=\frac{\left({\displaystyle\frac{n\left(A\right)\;cases}{n\left(A\right)\;controls}}\right)}{\left({\displaystyle\frac{\left(n\left(B\right)\;cases\right)}{\left(n\left(B\right)\;controls\right)}}\right)}\)
The related 95% confidence intervals (CI) were also calculated and considered to be statistically significant if they did not intersect with 1.
The heterogeneity between studies was investigated by Cochrane’s Q statistic [9]. The I2 test was also used to determine whether the variation between studies was due to chance [10]. According to the I2 test, values range from 0 (indicating homogeneity) to 100% (indicating hetrogeneity). Heterogeneity can be graded as low (25%), medium (50%) and high (75%) according to Higgins et al. [10].
The DerSimonian and Laird random effects method was applied to generate pooled ORs if the I2 values were between 50 and 100% [11, 12]. The Mantel–Haenszel fixed effects test was used for I2 values between 0 and 50% [11, 13].
Each individual study was also removed and the analysis performed on the rest of the studies, in order to establish bias by a single study on the overall results (sensitivity analysis).
Funnel plots were used to assess publication bias. An asymmetrical funnel plot would indicate publication bias, which would be quantified using Egger’s test, taking into consideration the recommendations by Sterne et al. [14, 15].
Subgroup analyses according to cancer stage depended on a sufficiently informative number of studies with distinct stage grouping (at least 3 studies per subgroup).
The Metafor package in R (Version 3.2.4) was used for statistical analysis [16].
Results
The selected nine papers (Table 3) included a total of 3051 patients, 2614 of whom had MSS tumours (85.7%) and 437 had MSI tumours (14.3%). Their quality and risk of bias were considered fair. Most patients were male but the ethnicity of the patient cohort was not specified in most studies. Patient’s age was variable and ranged from 20 to 85 years.
Most studies referred to the previously published National Cancer Institute panel for the definition of MSI as follows: ‘high frequency MSI as instability at 30% or more of the screened loci, low frequency instability as less than 30% of the loci screened and microsatellite stability as stability at all loci screened’ [17]. Tumours with low frequency microsatellite instability were generally considered to be biologically similar to those with microsatellite stability and were grouped together.
In five studies the tumours were located in the colon [3, 8, 18,19,20]. Three studies included a combination of colonic and rectal tumours [21,22,23]. In one study the precise location of the tumours investigated was not specified [24]. There was no specific mention in most of these papers whether any of the patients had Lynch syndrome.
The results of the 5-year survival analysis are shown in Table 4. In all studies the number of MSS patients was higher than MSI patients in keeping with the known predominance of chromosomal instability tumours in the general population.
As the I2 value was 64.87%, indicating moderate to high heterogeneity, the random effects model was used to generate the forest plot.
The forest plot for displayed pooled ORs with 95% CI as well as study weightings Is shown in Fig. 2. According to the meta-analysis result, 5-FU treated individuals who died at 5 years were 0.69 times as likely to have MSI, rather than MSS, compared to those who were alive, although this did not reach statistical significance.
In terms of publication bias, the Funnel plot was symmetrical confirming that there was no publication bias (Fig. 3) and this is supported by the Eggers test, which was not significant (t = -1.3784, df = 7, p = 0.2105). Subgroup analyses according to cancer stage were not conducted because there were an insufficient number of informative studies with distinct stage grouping.
Discussion
The aim of this study was to perform a systematic review and meta-analysis to investigate whether microsatellite instability influences 5-year survival in CRC patients treated with 5-FU. The seminal paper by Ribic et al. showed a better survival rate in MSI CRC patients not treated by adjuvant 5-FU therapy; a better survival rate in MSS patients treated by adjuvant 5-FU therapy; and no benefit in providing adjuvant 5-FU therapy to MSI patients [3]. The result of this meta-analysis shows that 5-FU treated CRC patients who died at 5 years were 0.31 times less likely to have MSI tumours, rather than MSS tumours, compared to those who were alive, although this difference did not reach statistical significance. This result broadly confirms two previous meta-analysis studies [2, 5].
Highly conserved from prokaryotes to eukaryotes, the DNA mismatch repair machinery is based on the assembly of the MSH2, MSH3 and MSH6 proteins to make the two heterodimers MutSa (MSH2/MSH6) and MutSb (MSH2/MSH3). MutSa or MutSb form a ternary complex with MutLa (composed of the two other proteins MLH1 and PMS2), and together with other proteins such as PCNA and RPA, repair replication errors [25].
In most MSI CRCs, dMMR is due to epigenetic hypermethylation of CpC islands located in the promoter region of the MLH1 gene and diffuse hypermethylation of CpG dinucleotides in the promoters regions of TP16 and CDH1 tumour suppressor genes. These hypermethylation events are called high frequency CpG island methylator phenotype (CIMP-H). Almost all MLH1 hyper methylated CRCs have CIMP-H [26].
5-FU metabolites are active and have two main actions: a) inhibition of the enzyme nucleotide synthetic enzyme thymidylate synthase (TS); b) incorporation of fluoronucleotides into RNA and DNA. TS catalyses the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), which is essential for DNA replication and repair. Many factors have been shown to correlate with the effect of 5-FU based chemotherapy. Not surprisingly, low TS expression in tumour cells is associated with higher response to 5-FU, which may also depend on TS promoter variants [2]. Deficient hepatocytes can result in 5-FU toxicity and p53 overexpression has been shown to be associated with resistance to 5-FU [18]. There is in vitro evidence that MSI cell lines are resistant to 5-FU, and that biallelic hypermethylation of hMLH1 eliminates 5-FU resistance. Resistance by MSI tumour cells to 5-FU could also be due to a direct interaction between MMR proteins and 5-FU and its metabolites and the effect of other factors such a p53 [2].
We recognise our study has limitations. Tumour staging was variable in the selected studies. It was not possible to perform a subgroup analysis according to tumour stage due to the limited information available and the limited number of informative studies. For example, some studies did not clarify which staging system had been used, and could not be compared to others. Staging remains a relevant criterion to guide adjuvant 5-FU based chemotherapy in CRC patients.
There was insufficient information overall on tumour histology. More aggressive subtypes (e.g. mucinous, undifferentiated) can influence prognosis, although this effect may be overrun by MSI status. Histological criteria for the diagnosis, and staging of CRC have evolved over the years, and therefore data acquired at an interval of 10 to 15 years may not be fully comparable.
As noted in previous meta-analysis studies, the limited number of MSI patients results in larger confidence intervals and a reduced statistical power when compared to data obtained from MSS patients [2, 5]. Of the MSI CRCs, approximately 10–13% are sporadic, and 2–5% occur in the context of a Lynch syndrome. Patients with Lynch syndrome are at increased risk of CRC as well as cancers at other sites including endometrial, ovarian, gastric, and hepato-biliary pancreatic cancers. There was no mention of Lynch syndrome in most papers selected for this meta-analysis.
Most studies adhered to the Bethesda panel for the assessment of microsatellite instability [26]. The methods used however were variable. The number of microsatellite loci investigated ranged from 2 to 11, probably in line with changes in criteria over the years. The core panel recommended by the National Cancer Institute workshop in 1997 consisted of two mononucleotide repeats (BAT25, BAT26) and three dinucleotide repeats (D5S346, D2S123, D17S250) [27]. The revised Bethesda panel in 2002 included additional mononucleotide markers because the use in the original panel of three dinucleotide repeats could underestimate the number of MSI tumours whereas the use of two mononucleotide repeats could overestimate the number of MSI-L tumours (where < 40% of microsatellites demonstrate instability in the panel) [28].
The proportion of tumour cells present in the samples used for MSI testing was mentioned in four studies only, and was at least 50% in two and 60% in the other two. The College of American Pathologists (CAP) MSI proficiency survey of 104 US laboratories showed that an insufficient tumour content in samples used for MSI testing could have been responsible for some misclassified cases when results of different laboratories were compared [29]. Use of microdissection resulted in the reduced rate of misclassified cases, and there was a significant difference in the rate of MSI tumours between the laboratories that used and those that did not use microdissection. This survey also highlighted the lack of consensus on the minimum amount of tumour cellularity necessary for reliable MSI testing, the reported requirement ranging from 11 to 40%. Approximately 10% of tumour cells was the minimum requirement for identifying MSI in a study based on serial dilutions of a microdissected specimen [30]. Laboratories testing for MSI should therefore mention the risk of a false negative result when using suboptimal samples. The interobserver variability in assessing the percentage of neoplastic cells in tissue samples is, however, significant. There is a tendency to overestimate when an overall estimate is compared with a cell counting method. Overestimating tumour cellularity carries the risk of increasing the number of false negatives. A small proportion of the surveyed laboratories used laser-capture microdissection to isolate tumour cells for DNA extraction.
The studies selected in this meta-analysis provided 5-year survival data as Kaplan–Meier curves for 5-FU treated MSI and MSS patients. Survival at different time points can be extracted from survival curves, as noted by Duchateau and colleagues [31]. The approach used in this study is a compromise between the complex techniques of both Guyot et al. and Liu et al. and the more traditional ‘pencil and ruler’ approach to ‘read off survival probabilities’ [32, 33]. The main limitation of this approach, however, is that it does not consider censoring. In our series, we considered the estimated percentage of patients alive at 5 years relatively high ranging from 66 to 98% (average 79%) in the MSI patients and from 59 to 90% (average 72%) in the MSS patients. We also made the assumption that by using the same approach in all the nine selected papers, as opposed to combining data extracted from Kaplan–Meier curve with raw data provided in table format, the effect of censoring would be minimised and data would be comparable. It is for this reason that we decided not to include, in this series, the OR generated by the raw data available in the paper by Sargent et al. and to extract the data from the Kaplan–Meier curves in the two papers by Ribic et al. and Klingbiel et al., despite the raw data being available in table format [3, 7, 8].
Conclusion
This meta-analysis shows there is no significant difference in the overall survival of patients with MSI CRC and MSS CRC treated with adjuvant 5-FU. Further studies are necessary to clarify whether patients with MSI CRC, and in particular those at a relatively early stage, should be offered 5-FU based adjuvant chemotherapy. Additional investigations in the molecular pathways involved in the metabolism and function of 5-FU and its metabolites could help in identifying patients more or less responsive to 5-FU, and monitor its effects, in the context of precision medicine and pharmacogenomics.
References
Guinney J, Dienstmann R, Wang X, De Reyniès A, Schlicker A, Soneson C et al (2015) The consensus molecular subtypes of colorectal cancer. Nature Med. Nature Publishing Group 21(11):1350
Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E (2010) Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. European J Cancer (Oxford, England : 1990). [Online] England 46(15):2788–2798. Available from: https://doi.org/10.1016/j.ejca.2010.05.009
Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM et al (2003) Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. New England J Med Mass Med Soc 349(3):247–257
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Sys Rev BioMed Central 4(1):1
Webber EM, Kauffman TL, O’Connor E, Goddard KAB (2015) Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer BioMed Central 15(1):156
Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Intern Med Am Coll Phys 155(8):529–536
Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR et al (2010) Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol Am Soc of Clin Oncol 28(20):3219
Klingbiel D, Saridaki Z, Roth AD, Bosman FT, Delorenzi M, Tejpar S (2014) Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Annals of Oncol European Soc Med Oncol 26(1):126–132
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J (2006) Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological methods. Am Psychol Assoc 11(2):193
Higgins JPT, Thompson SG (2003) Altman DG. Measuring inconsistency in meta-analyses. British Med J (BMJ)
Kontopantelis E, Springate DA, Reeves D (2013) A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PloS One. Public Library of Science 8(7):e69930
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled clinical trials. Elsevier 7(3):177–188
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. Oxford University Press 22(4):719–748
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Bmj. British Med J Pub Group 315(7109):629–634
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. British Med J Pub Group 343: d4002
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw. UCLA Statistics 36(3):1–48
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW et al (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. United States 58(22):5248–5257
Kim GP, Colangelo LH, Wieand HS, Paik S, Kirsch IR, Wolmark N et al (2007) Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol : Official Journal of the American Society of Clinical Oncology. [Online] United States 25(7):767–772. Available from: https://doi.org/10.1200/JCO.2006.05.8172
Carethers JM, Smith EJ, Behling CA, Nguyen L, Tajima A, Doctolero RT et al (2004) Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterol. Elsevier 126(2):394–401
Bertagnolli MM, Niedzwiecki D, Compton CC, Hahn HP, Hall M, Damas B et al (2009) Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol. Am Soc of Clin Oncol 27(11):1814
Hong SP, Min BS, Kim T Il, Cheon JH, Kim NK, Kim H et al (2012) The differential impact of microsatellite instability as a marker of prognosis and tumour response between colon cancer and rectal cancer. European J Cancer (Oxford, England : 1990). [Online] England 48(8):1235–1243. Available from: https://doi.org/10.1016/j.ejca.2011.10.005
Öhrling K, Edler D, Hallström M, Ragnhammar P (2010) Mismatch repair protein expression is an independent prognostic factor in sporadic colorectal cancer. Acta Oncologica. Taylor & Francis 49(6):797–804
Jensen SA, Vainer B, Kruhoffer M, Sorensen JB (2009) Microsatellite instability in colorectal cancer and association with thymidylate synthase and dihydropyrimidine dehydrogenase expression. BMC Cancer. [Online] England 9:25. Available from: https://doi.org/10.1186/1471-2407-9-25
Jover R, Zapater P, Castells A, Llor X, Andreu M, Cubiella J et al (2009) The efficacy of adjuvant chemotherapy with 5-fluorouracil in colorectal cancer depends on the mismatch repair status. European J Cancer. Elsevier 45(3):365–373
Sharma M, Predeus A V, Kovacs N, Feig M (2014) Differential mismatch recognition specificities of eukaryotic MutS homologs, MutSα and MutSβ. Biophys J. Elsevier 106(11):2483–2492
Boland CR, Goel A (2010) Microsatellite instability in colorectal cancer. Gastroenterology. [Online] 138(6):2073–2087.e3. Available from: https://doi.org/10.1053/j.gastro.2009.12.064
Xicola RM, Llor X, Pons E, Castells A, Alenda C, Piñol V et al (2007) Performance of different microsatellite marker panels for detection of mismatch repair–deficient colorectal tumors. J Nat Cancer Institute. Oxford University Press 99(3):244–252
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J et al (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. [Online] J Nat Cancer Institute. United States, pp 261–268. Available from: https://doi.org/10.1093/jnci/djh034
Boyle TA, Bridge JA, Sabatini LM, Nowak JA, Vasalos P, Jennings LJ et al (2014) Summary of microsatellite instability test results from laboratories participating in proficiency surveys: proficiency survey results from 2005 to 2012. Arch Pathol Lab Med. [Online] United States 138(3):363–370. Available from: https://doi.org/10.5858/arpa.2013-0159-CP
Trusky CL, Sepulveda AR, Hunt JL (2006) Assessment of microsatellite instability in very small microdissected samples and in tumor samples that are contaminated with normal DNA. Diagn Mol Pathol: Am J Surg Pathol, part B. United States 15(2):63–69
Duchateau L, Collette L, Sylvester R, Pignon J (2000) Estimating Number of Events from the Kaplan–Meier Curve for Incorporation in a Literature‐Based Meta‐Analysis: What You Don’t See You Can’t Get! Biometrics. Wiley Online Library 56(3):886–892
Guyot P, Ades AE, Ouwens MJNM, Welton NJ (2012) Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. BioMed Central 12(1):9
Liu Z, Rich B, Hanley JA (2014) Recovering the raw data behind a non-parametric survival curve. Syst Rev. BioMed Central 3(1):151
Acknowledgements
Alberto Quaglia is supported by the National Institute for Health Research (NIHR) UCLH/UCL Biomedical Research Centre (BRC).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Nikhil Aggarwal and Alberto Quaglia. The first draft of the manuscript was written by Nikhil Aggarwal and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aggarwal, N., Quaglia, A., McPhail, M.J.W. et al. Systematic review and meta-analysis of tumour microsatellite-instability status as a predictor of response to fluorouracil-based adjuvant chemotherapy in colorectal cancer. Int J Colorectal Dis 37, 35–46 (2022). https://doi.org/10.1007/s00384-021-04046-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-021-04046-x