Abstract
Purpose
Transplantation of pigmented tissue-engineered human autologous skin substitutes represents a promising procedure to cover skin defects. We have already demonstrated that we can restore the patient’s native light or dark skin color by adding melanocytes to our dermo-epidermal skin analogs. In this long-term study, we investigated if melanocytes in our skin substitutes continue to express markers as BCL2, SOX9, and MITF, known to be involved in survival, differentiation, and function of melanocytes.
Methods
Human epidermal melanocytes and keratinocytes, as well as dermal fibroblasts from light- and dark-pigmented skin biopsies were isolated and cultured. Bovine collagen hydrogels containing fibroblasts were prepared, and melanocytes and keratinocytes were seeded in a 1:5 ratio onto the gels. Pigmented dermo-epidermal skin substitutes were transplanted onto full-thickness wounds of immuno-incompetent rats and analyzed for the expression of melanocyte markers after 15 weeks.
Results
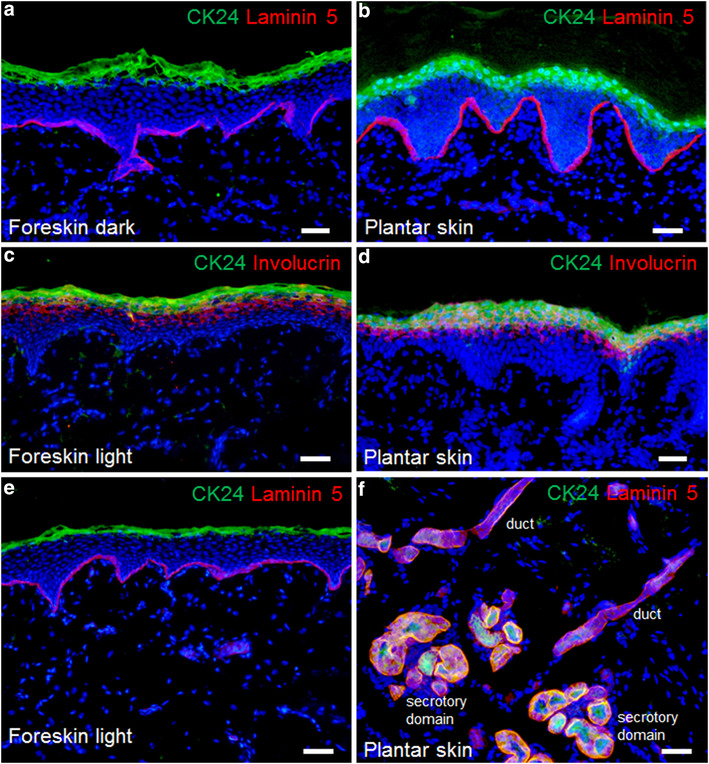
Employing immunofluorescence staining techniques, we observed that our light and dark dermo-epidermal skin substitutes expressed the same typical melanocyte markers including BCL2, SOX9, and MITF 15 weeks after transplantation as normal human light and dark skin.
Conclusions
These data suggest that, even in the long run, our light and dark dermo-epidermal tissue-engineered skin substitutes contain melanocytes that display a characteristic expression pattern as seen in normal pigmented human skin. These findings have crucial clinical implications as such grafts transplanted onto patients should warrant physiological numbers, distribution, and function of melanocytes.




Similar content being viewed by others
References
Berman B, Viera MH, Amini S, Huo R, Jones IS (2008) Prevention and management of hypertrophic scars and keloids after burns in children. J Craniofac Surg 19(4):989–1006
Schiestl C, Stiefel D, Meuli M (2010) Giant naevus, giant excision, eleg(i)ant closure? Reconstructive surgery with Integra Artificial Skin to treat giant congenital melanocytic naevi in children. J Plast Reconstr Aesthet Surg 63(4):610–615
Gallico GG 3rd, O’Connor NE, Compton CC, Kehinde O, Green H (1984) Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 311(7):448–451
Meuli M, Raghunath M (1997) Burns (part 2). Tops and flops using cultured epithelial autografts in children. Pediatr Surg Int 12(7):471–477
Boyce ST (2001) Design principles for composition and performance of cultured skin substitutes. Burns 27(5):523–533
Biedermann T, Boettcher-Haberzeth S, Reichmann E (2013) Tissue engineering of skin for wound coverage. Eur J Pediatr Surg 23(5):375–382
Marino D, Reichmann E, Meuli M (2014) Skingineering. Eur J Pediatr Surg 24(3):205–213
Boyce ST, Kagan RJ, Yakuboff KP, Meyer NA, Rieman MT, Greenhalgh DG, Warden GD (2002) Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann Surg 235(2):269–279
Böttcher-Haberzeth A, Klar AS, Biedermann T, Schiestl C, Meuli-Simmen C, Reichmann E, Meuli M (2013) “Trooping the color”: restoring the original donor skin color by addition of melanocytes to bioengineered skin analogs. Pediatr Surg Int 29(3):239–247
Pontiggia L, Biedermann T, Meuli M, Widmer D, Böttcher-Haberzeth S, Schiestl C, Schneider J, Braziulis E, Montaño I, Meuli-Simmen C, Reichmann E (2009) Markers to evaluate the quality and self-renewing potential of engineered human skin substitutes in vitro and after transplantation. J Invest Dermatol 129(2):480–490
Böttcher-Haberzeth S, Biedermann T, Pontiggia L, Braziulis E, Schiestl C, Hendriks B, Eichhoff OM, Widmer DS, Meuli-Simmen C, Meuli M, Reichmann E (2013) Human eccrine sweat gland cells turn into melanin-uptaking keratinocytes in stratifying dermo-epidermal skin substitutes. J Invest Dermatol 133(2):316–324
Biedermann T, Böttcher-Haberzeth S, Klar AS, Pontiggia L, Schiestl C, Meuli-Simmen C, Reichmann E, Meuli M (2013) Rebuild, restore, reinnervate: do human tissue engineered dermo-epidermal skin analogs attract host nerve fibers for innervation? Pediatr Surg Int 29(1):71–78
Schneider J, Biedermann T, Widmer D, Montano I, Meuli M, Reichmann E, Schiestl C (2009) Matriderm versus integra: a comparative experimental study. Burns 35(1):51–57
Kiowski G, Biedermann T, Widmer DS, Civenni G, Burger C, Dummer R, Sommer L, Reichmann E (2012) Engineering melanoma progression in a humanized environment in vivo. J Invest Dermatol 132(1):144–153
Biedermann T, Pontiggia L, Böttcher-Haberzeth S, Tharakan S, Braziulis E, Schiestl C, Meuli M, Reichmann E (2010) Human eccrine sweat gland cells can reconstitute a stratified epidermis. J Invest Dermatol 130(8):1996–2009
Szabo G (1954) The number of melanocytes in human epidermis. Br Med J 1(4869):1016–1017
Fitzpatrick TB, Breathnach AS (1963) The epidermal melanin unit system. Dermatol Wochenschr 147:481–489
Frenk E, Schellhorn JP (1969) Morphology of the epidermal melanin unit. Dermatologica 139(4):271–277
Anvekar RA, Asciolla JJ, Missert DJ, Chipuk JE (2011) Born to be alive: a role for the BCL-2 family in melanoma tumor cell survival, apoptosis, and treatment. Front Oncol 1(34). doi:10.3389/fonc.2011.00034
Czabotar PE, Lessene G, Strasser A, Adams JM (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15(1):49–63
Klein-Parker HA, Warshawski L, Tron VA (1994) Melanocytes in human skin express bcl-2 protein. J Cutan Pathol 21(4):297–301
Sellheyer K, Krahl D, Ratech H (2001) Distribution of Bcl-2 and Bax in embryonic and fetal human skin: antiapoptotic and proapoptotic proteins are differentially expressed in developing skin. Am J Dermatopathol 23(1):1–7
McGill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, Lin YL, Ramaswamy S, Avery W, Ding HF, Jordan SA, Jackson IJ, Korsmeyer SJ, Golub TR, Fisher DE (2002) Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 109(6):707–718
Stefanato CM, Yaar M, Bhawan J, Phillips TJ, Kosmadaki MG, Botchkarev V, Gilchrest BA (2003) Modulations of nerve growth factor and Bcl-2 in ultraviolet-irradiated human epidermis. J Cutan Pathol 30(6):351–357
Cook AL, Donatien PD, Smith AG, Murphy M, Jones MK, Herlyn M, Bennett DC, Leonard JH, Sturm RA (2003) Human melanoblasts in culture: expression of BRN2 and synergistic regulation by fibroblast growth factor-2, stem cell factor, and endothelin-3. J Invest Dermatol 121(5):1150–1159
Passeron T, Valencia JC, Bertolotto C, Hoashi T, Le Pape E, Takahashi K, Ballotti R, Hearing VJ (2007) SOX9 is a key player in ultraviolet B-induced melanocyte differentiation and pigmentation. Proc Natl Acad Sci USA 104(35):13984–13989
Shi G, Sohn KC, Li Z, Choi DK, Park YM, Kim JH, Fan YM, Nam YH, Kim S, Im M, Lee Y, Seo YJ, Kim CD, Lee JH (2013) Expression and functional role of Sox9 in human epidermal keratinocytes. PLoS One 8(1):e54355
Harris ML, Baxter LL, Loftus SK, Pavan WJ (2010) Sox proteins in melanocyte development and melanoma. Pigment Cell Melanoma Res 23(4):496–513
Harris ML, Buac K, Shakhova O, Hakami RM, Wegner M, Sommer L, Pavan WJ (2013) A dual role for SOX10 in the maintenance of the postnatal melanocyte lineage and the differentiation of melanocyte stem cell progenitors. PLoS Genet 9(7):e1003644
Hasegawa J, Goto Y, Murata H, Takata M, Saida T, Imokawa G (2008) Downregulated melanogenic paracrine cytokine linkages in hypopigmented palmoplantar skin. Pigment Cell Melanoma Res 21:687–699
Wan P, Hu Y, He L (2011) Regulation of melanocyte pivotal transcription factor MITF by some other transcription factors. Mol Cell Biochem 354(1–2):241–246
Steingrímsson E, Copeland NG, Jenkins NA (2004) Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet 38:365–411
Lin JY, Fisher DE (2007) Melanocyte biology and skin pigmentation. Nature 445(7130):843–850
Acknowledgments
We thank Ines Kleiber-Schaaf (Department of Dermatology, University Hospital, Zurich) for precious help in the preparation and analysis of the probes for transmission electron microscopy and histology. This work was financially supported by the EU-FP7 project EuroSkinGraft (FP7/2007-2013: grant agreement no 279024), by the EU-FP7 (MultiTERM, grant agreement no 238551) and the Clinical Research Priority Programs (KFSP: From basic research to the clinic: Novel tissue engineered skin grafts for Zurich) of the Faculty of Medicine of the University of Zurich. We are particularly grateful to the Fondation Gaydoul and the sponsors of “DonaTissue” (Thérèse Meier and Robert Zingg) for their generous financial support and interest in our work.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Biedermann and A. S. Klar contributed equally.
Rights and permissions
About this article
Cite this article
Biedermann, T., Klar, A.S., Böttcher-Haberzeth, S. et al. Long-term expression pattern of melanocyte markers in light- and dark-pigmented dermo-epidermal cultured human skin substitutes. Pediatr Surg Int 31, 69–76 (2015). https://doi.org/10.1007/s00383-014-3622-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-014-3622-7