Abstract
Purpose
The clinical application of autologous tissue-engineered skin analogs is an important strategy to cover large skin defects. Investigating biological dynamics, such as reinnervation after transplantation, is essential to improve the quality of such skin analogs. Previously, we have examined that our skin substitutes are reinnervated by host peripheral nerve fibers as early as 8 weeks after transplantation. Here, we wanted to investigate the presence and possible differences regarding myelinated and unmyelinated host nerve fibers 15 weeks after the transplantation of light and dark human tissue-engineered skin analogs.
Methods
Human epidermal keratinocytes, melanocytes, and dermal fibroblasts were isolated from human light and dark skin biopsies. Keratinocytes and melanocytes were seeded on fibroblast-containing collagen type I hydrogels after expansion in culture. After additional culturing, the tissue-engineered dermo-epidermal skin analogs were transplanted onto full-thickness skin wounds created on the back of immuno-incompetent rats. Skin substitutes were excised and analyzed 15 weeks after transplantation. Histological sections were examined with regard to the ingrowth pattern of myelinated and unmyelinated nerve fibers into the skin analogs using markers, such as Substance P, NF200, and S100-Beta.
Results
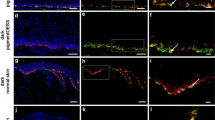
We found myelinated and unmyelinated peripheral host nerve fibers 15 weeks after transplantation in the dermal part of our human skin substitutes. In particular, we identified large-diameter-myelinated Aβ- and Aδ-fibers, and small-diameter C-fibers. Furthermore, we observed myelinated nerves in close proximity to CD31-positive blood capillaries. In the long run, both types of ingrown host fibers showed an identical pattern in both light and dark skin analogs.
Conclusion
Our data suggest that myelinated and unmyelinated peripheral nerves reinnervate human skin substitutes in a long-term in vivo transplantation assay. Our tissue-engineered skin analogs attract A- and C-fibers to supply both light and dark skin analogs. Potentially, this process restores skin sensitivity and has, therefore, a significant relevance with regard to future application of autologous pigmented dermo-epidermal skin substitutes onto patients.






Similar content being viewed by others
References
Biedermann T, Boettcher-Haberzeth S, Reichmann E (2013) Tissue engineering of skin for wound coverage. Eur J Pediatr Surg 23(5):375–382
Hermanson A, Dalsgaard CJ (1987) Sensory reinnervation and sensibility in skin transplants. Med Biol 65(1):49–52
Altun V, Hakvoort TE, van Zuijlen PP, van der Kwast TH, Prens EP (2001) Nerve outgrowth and neuropeptide expression during the remodeling of human burn wound scars. A 7-month follow-up study of 22 patients. Burns 27(7):717–722
Ward RS, Tucket RP, English KB, Johansson O, Saffle JR (2003) Substance P axons and sensory threshold increase in burn-graft human skin. J Surg Res 118:154–160
Nedelec B, Hou Q, Sohbi I, Choiniüre M, Beauregard G, Dykes RW (2005) Sensory perception and neuroanatomical structures in normal and grafted skin of burn survivors. Burns 31:817–830
Anderson JR, Zorbas JS, Phillips JK, Harrison JL, Dawson LF, Bolt SE, Rea SM, Klatte JE, Paus R, Zhu B, Giles NL, Drummond PD, Wood FM, Fear MW (2010) Systemic decreases in cutaneous innervation after burn injury. J Invest Dermatol 130:1948–1951
Hamed K, Giles N, Anderson J, Phillips JK, Dawson LF, Drummond P, Wallace H, Wood FM, Rea SM, Fear MW (2011) Changes in cutaneous innervation in patients with chronic pain after burns. Burns 37(4):631–637
Anderson JR, Fear MW, Phillips JK, Dawson LF, Wallace H, Wood FM, Rea SM (2011) A preliminary investigation of the reinnervation and return of sensory function in burn patients treated with INTEGRA®. Burns 37(7):1101–1108
Lim JY, Lum CH, Tan AJ, Jackson T, Burrows S, Edgar DW, Wood FM (2014) Long term sensory function after minor partial thickness burn: a pilot study to determine if recovery is complete or incomplete. Burns 40(8):1538–1543
Yang YS, Cho SI, Choi MG, Choi YH, Kwak IS, Park CW, Kim HO (2015) Increased expression of three types of transient receptor potential channels (TRPA1, TRPV4 and TRPV3) in burn scars with post-burn pruritus. Acta Derm Venereol 95(1):20–24
Biedermann T, Böttcher-Haberzeth S, Klar AS, Pontiggia L, Schiestl C, Meuli-Simmen C, Reichmann E, Meuli M (2013) Rebuild, restore, reinnervate: do human tissue engineered dermo-epidermal skin analogs attract host nerve fibers for innervation? Pediatr Surg Int 29(1):71–78
Biedermann T, Klar AS, Böttcher-Haberzeth S, Schiestl C, Reichmann E, Meuli M (2014) Tissue-engineered dermo-epidermal skin analogs exhibit de novo formation of a near natural neurovascular link 10 weeks after transplantation. Pediatr Surg Int 30(2):165–172
Böttcher-Haberzeth S, Biedermann T, Klar AS, Widmer DS, Neuhaus K, Schiestl C, Meuli M, Reichmann E (2015) Characterization of pigmented dermo-epidermal skin substitutes in a long-term in vivo assay. Exp Dermatol 24(1):16–21
Biedermann T, Klar AS, Böttcher-Haberzeth S, Michalczyk T, Schiestl C, Reichmann E, Meuli M (2015) Long-term expression pattern of melanocyte markers in light- and dark-pigmented dermo-epidermal cultured human skin substitutes. Pediatr Surg Int 31(1):69–76
Böttcher-Haberzeth S, Klar AS, Biedermann T, Schiestl C, Meuli-Simmen C, Reichmann E, Meuli M (2013) “Trooping the color”: restoring the original donor skin color by addition of melanocytes to bioengineered skin analogs. Pediatr Surg Int 29(3):239–247
Biedermann T, Böttcher-Haberzeth S, Klar AS, Widmer DS, Pontiggia L, Weber AD, Weber DM, Schiestl C, Meuli M, Reichmann E (2015) The influence of stromal cells on the pigmentation of tissue-engineered dermo-epidermal skin grafts. Tissue Eng Part A 21(5–6):960–969
Klar AS, Güven S, Biedermann T, Luginbühl J, Böttcher-Haberzeth S, Meuli-Simmen C, Meuli M, Martin I, Scherberich A, Reichmann E (2014) Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 35(19):5065–5078
Devor M, Schonfeld D, Seltzer Z, Wall PD (1979) Two modes of cutaneous reinnervation following peripheral nerve injury. J Comp Neurol 185(1):211–220
Jancsó G, Király E (1983) Cutaneous nerve regeneration in the rat: reinnervation of the denervated skin by regenerative but not by collateral sprouting. Neurosci Lett 36(2):133–137
Brenan A, Jones L, Owain NR (1988) The demonstration of the cutaneous distribution of saphenous nerve C-fibres using a plasma extravasation technique in the normal rat and following nerve injury. J Anat 157:57–66
Mellgren SI, Nolano M, Sommer C (2013) The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol 115:171–188
Trojanowski JQ, Walkenstein N, Lee VM (1986) Expression of neurofilament subunits in neurons of the central and peripheral nervous system: an immunohistochemical study with monoclonal antibodies. J Neurosci 6(3):650–660
Shen H, Barry DM, Garcia ML (2010) Distal to proximal development of peripheral nerves requires the expression of neurofilament heavy. Neuroscience 170(1):16–21
Elder GA, Friedrich VL Jr, Kang C, Bosco P, Gourov A, Tu PH, Zhang B, Lee VM, Lazzarini RA (1998) Requirement of heavy neurofilament subunit in the development of axons with large calibers. J Cell Biol 143(1):195–205
Lawson SN, Waddell PJ (1991) Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435:41–63
Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkühler J (2007) Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. J Comp Neurol 502(2):325–336
Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413(6852):203–210
Hunt SP, Mantyh PW (2001) The molecular dynamics of pain control. Nat Rev Neurosci 2(2):83–91
Lisney SJ (1989) Regeneration of unmyelinated axons after injury of mammalian peripheral nerve. Q J Exp Physiol 74(6):757–784
Jenq CB, Coggeshall RE (1985) Numbers of regenerating axons in parent and tributary peripheral nerves in the rat. Brain Res 326(1):27–40
Carter DA, Lisney SJ (1987) The numbers of unmyelinated and myelinated axons in normal and regenerated rat saphenous nerves. J Neurol Sci 80(2–3):163–171
Blais M, Parenteau-Bareil R, Cadau S, Berthod F (2013) Concise review: tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med 2(7):545–551
Blais M, Grenier M, Berthod F (2009) Improvement of nerve regeneration in tissue-engineered skin enriched with Schwann cells. J Investig Dermatol 129(12):2895–2900
Gagnon V, Larouche D, Parenteau-Bareil R, Gingras M, Germain L, Berthod F (2011) Hair follicles guide nerve migration in vitro and in vivo in tissue-engineered skin. J Investig Dermatol 131(6):1375–1378
Lundborg G (2005) Nerve injury and repair: regeneration, reconstruction, and cortical remodeling, 2nd edn. Elsevier, London, ISBN 9780443067112
Bae JY, Kim JH, Cho YS, Mah W, Bae YC (2015) Quantitative analysis of afferents expressing substance P, calcitonin gene-related peptide, isolectin B4, neurofilament 200, and Peripherin in the sensory root of the rat trigeminal ganglion. J Comp Neurol 523(1):126–138
McCarthy PW, Lawson SN (1989) Cell type and conduction velocity of rat primary sensory neurons with substance P-like immunoreactivity. Neuroscience 28(3):745–753
McCarthy PW, Lawson SN (1997) Differing action potential shapes in rat dorsal root ganglion neurones related to their substance P and calcitonin gene-related peptide immunoreactivity. J Comp Neurol 388(4):541–549
Dirajlal S, Pauers LE, Stucky CL (2003) Differential response properties of IB(4)-positive and -negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol 89(1):513–524
Coggeshall RE, Dougherty PM, Pover CM, Carlton SM (1993) Is large myelinated fiber loss associated with hyperalgesia in a model of experimental peripheral neuropathy in the rat? Pain 52(2):233–242
Bhatheja K, Field J (2006) Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol 38(12):1995–1999
Sorci G, Riuzzi F, Arcuri C, Tubaro C, Bianchi R, Giambanco I, Donato R (2013) S100B protein in tissue development, repair and regeneration. World J Biol Chem 4(1):1–12
Sbai O, Devi TS, Melone MA, Feron F, Khrestchatisky M, Singh LP, Perrone L (2010) RAGE-TXNIP axis is required for S100B-promoted Schwann cell migration, fibronectin expression and cytokine secretion. J Cell Sci 123(Pt 24):4332–4339
Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC (2015) Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell 162(5):1127–1139
Carmeliet P (2003) Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet 4(9):710–720
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436(7048):193–200
Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I (2009) S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 1793(6):1008–1022
Steiner J, Marquardt N, Pauls I, Schiltz K, Rahmoune H, Bahn S, Bogerts B, Schmidt RE, Jacobs R (2011) Human CD8+ T cells and NK cells express and secrete S100B upon stimulation. Brain Behav Immun 25(6):1233–1241
Lumpkin EA, Caterina MJ (2007) Mechanisms of sensory transduction in the skin. Nature 445(7130):858–865
Acknowledgments
This work was financially supported by the EU-FP7 project EuroSkinGraft (FP7/2007-2013: Grant Agreement No 279024), by the EU-FP7 (MultiTERM, Grant Agreement No 238551), and the Clinical Research Priority Programs (KFSP: From basic research to the clinic: Novel tissue-engineered skin grafts for Zurich) of the Faculty of Medicine of the University of Zurich. We are particularly grateful to the Fondation Gaydoul and the sponsors of “DonaTissue” (Thérèse Meier and Robert Zingg) for their generous financial support and interest in our work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Biedermann, T., Klar, A.S., Böttcher-Haberzeth, S. et al. Myelinated and unmyelinated nerve fibers reinnervate tissue-engineered dermo-epidermal human skin analogs in an in vivo model. Pediatr Surg Int 32, 1183–1191 (2016). https://doi.org/10.1007/s00383-016-3978-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-016-3978-y