Abstract
Purpose
Rasmussen encephalitis (RE) is a very rare chronic neurological disorder of unilateral inflammation of the cerebral cortex. Hemispherotomy provides the best chance at achieving seizure freedom in RE patients, but with significant risks and variable long-term outcomes. The goal of this study is to utilize our multicenter pediatric cohort to characterize if differences in pathology and/or imaging characterization of RE may provide a window into post-operative seizure outcomes, which in turn could guide decision-making for parents and healthcare providers.
Methods
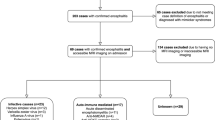
This multi-institutional retrospective review of medical record, imaging, and pathology samples was approved by each individual institution’s review board. Data was collected from all known pediatric cases of peri-insular functional hemispherotomy from the earliest available electronic medical records. Mean follow-up time was 4.9 years. Clinical outcomes were measured by last follow-up visit using both Engel and ILAE scoring systems. Relationships between categorical and continuous variables were analyzed with Pearson correlation values.
Results
Twenty-seven patients met study criteria. No statistically significant correlations existed between patient imaging and pathology data. Pathology stage, MRI brain imaging stages, and a combined assessment of pathology and imaging stages showed no statistically significant correlation to post-operative seizure freedom rates. Hemispherectomy Outcome Prediction Scale scoring demonstrated seizure freedom in only 71% of patients receiving a score of 1 and 36% of patients receiving a score of 2 which were substantially lower than predicted.
Conclusions
Our analysis did not find evidence for either independent or combined analysis of imaging and pathology staging being predictive for post peri-insular hemispherotomy seizure outcomes, prompting the need for other biomarkers to be explored. Our data stands in contrast to the recently proposed Hemispherectomy Outcome Prediction Scale and does not externally validate this metric for an RE cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rasmussen’s encephalitis (RE) is a very rare immune-mediated chronic neurological disorder of unilateral inflammation of the cerebral cortex [1], impacting 1.7–2.4 per 10 million children under the age of 18 [2,3,4]. The exact pathophysiologic mechanism is unknown, but previous research has indicated the disease is likely driven by a T-cell response with a potential contribution from autoantibodies [1, 5]. Typical initial symptomatology for RE includes seizures originating from one cerebral hemisphere, with 40% developing hemiparesis within a year of symptom onset [6]. Epilepsia partialis continua (EPC), prolonged repetitive arrhythmic focal (typically motor) seizures, is also commonly observed (50–81%) [7, 8]. Early MRI features have also been noted, such as focal cortical atrophy, white matter hyperintensity, and caudate head nucleus atrophy. These typically occur within approximately 4 months of seizure onset [9].
Many treatment options have been attempted for those with RE. These include anti-seizure medications, as well as antiviral and immunosuppressive therapies [10,11,12,13]. Unfortunately, the epilepsy becomes refractory to medicinal interventions within months of onset [9]. Disconnective surgical hemispherotomy provides the best chance at achieving seizure freedom in RE patients [1, 14], with seizure freedom rates ranging from 63–80% [15,16,17,18,19,20]. However, there are numerous risks and variable long-term outcomes to hemispherotomy, the most likely being hemiplegia and homonymous hemianopia, contralateral loss of fine motor movement, and loss of language function with dominant hemisphere involvement [1]. Some patients may require additional surgeries or develop hydrocephalus as a complication. Dominant hemispheric surgery or surgery in older children with decreased neuroplasticity present special challenges with regards to weighing risks and benefits.
There are few articles in the literature exploring the impact of preoperative characteristics on long-term post-operative seizure outcomes [18, 21]. Recently, this issue was examined on a large scale via the Hemispherectomy Outcome Prediction Scale (HOPS) study using a combination of simple demographic, semiology, imaging, etiology, and prior surgical data [22]. An initial external validation trial of HOPS was unable to support its inceptive predictive value [23], and since all variables but the age at seizure onset in the scale are likely to remain constant among RE patients, it may not suffice for this population. Studies have analyzed trends of radiographic disease evolution, but biomarkers predictive of medical or surgical treatment success have not been identified [1, 7, 24]. Features, such as unihemispheric focal atrophy, peri-sylvian atrophy, and caudate involvement, are regularly seen in patients with RE, with later disease states typically demonstrating slowing of atrophic changes [1, 7]. Pathology, usually obtained during hemispherotomy, may also provide an estimate of disease severity post-surgery [25]. Based on our review of the literature, no other study has investigated preoperative imaging and post-operative pathology results in a combined way to help predict surgical outcomes. The goal of this study is to utilize our multicenter cohort to characterize if differences in pathology and/or imaging characterization of RE may provide a window into post-hemispherotomy seizure outcomes, which in turn could guide decision-making for parents and healthcare providers.
Methods
Study design and data collection
This multi-institutional retrospective review of medical record, imaging, and pathology samples was approved by each individual institution’s review board. The institutions that participated in contributing data were Cincinnati Children’s Hospital, Seattle Children’s Hospital, and Washington University St. Louis Children’s Hospital. Data was collected from all known cases of peri-insular functional hemispherectomy for RE from the earliest available electronic medical records through January 2015. All patients were diagnosed with RE following the 2005 European consensus statement [14] as well as biopsy. A standardized database template was used to guide data collection from medical records. For Engel and ILAE classification status, the most recent available record describing the patient’s seizure burden was used.
All available historical cranial imaging for each patient was uploaded to a centralized PACS database and analyzed using a modified Bien staging system by a single neuroradiologist (JL) [26]. MRI analysis under the Bien staging system included assessment of unihemispheric cortical atrophy, gray, or white matter T2/FLAIR hyperintensities, and signal change or atrophy of the ipsilateral caudate head. Detailed commentary was also provided by retrospective review of all 3D imaging by the same neuroradiologist [7, 27]. Separate staging scores were administered for basal ganglia structures and cortical structures. Regional differences were noted in many cases such that a range of Bien scores could exist for each patient. Where preoperative positron emission tomography (PET) scans were available, these were also reviewed by the same radiologist and analyzed specifically for hypometabolism on a lobar basis including analysis of the cerebellar hemispheres.
Pathology slides and remaining block specimens were all sent for review by a single pathologist (CF) for staging using the system described by Robitaille and modified by Pardo et al. in 2004 [25, 28]. This staging system assesses histopathologic changes in a four-category progression that represent early, mid-, late-, and end-stage hemispheric destruction regionally [14, 25]. Pathology analysis was carried out separately in each available brain region from the provided specimens. Additionally, a single Robitaille/Pardo stage was assigned to each patient based on the predominate pathologic stage found within all available tissue for that patient.
Statistical analysis
Relationships between categorical and continuous variables were analyzed with Microsoft Excel to determine Pearson correlation values and statistical significance was set at an alpha of < 0.05. P values were calculated with the data analysis regression tool in Excel.
Results
Demographics
Demographic data is highlighted in Table 1. A total of 27 patients were reviewed between the three hospitals. The participant population consisted of 12 male patients (44%) and 15 female patients. All patients had undergone hemispherotomy. Fourteen patients (52%) had surgery on the left hemisphere and 13 underwent surgery on the right hemisphere (48%). Twenty-six patients had follow-up data available for review. The mean follow-up time was 5 years (range: 1 month to 24 years). Preoperatively, ten patients (37%) had documented history of EPC. The mean age at surgery was 8.9 years (SD 4.0), and the average first seizure to surgery interval was 4.3 years (SD 3.9). Fourteen patients had reported post-operative seizure freedom (54%) after hemispherotomy at last follow-up.
MRI and PET
The earliest available preoperative MRI datasets were used for analysis. Preoperative MRI imaging data were available in 19 patients (Table 2) looking at characteristics of both cortical and basal ganglia edema, signal changes, signal enhancement, diffusion restriction, and atrophy.
Twelve patients (68%) showed evidence cortical thickening/edema, most commonly in the frontal lobes (50%). The temporal lobe was the second most common location of cortical edema (33%). Basal ganglia (BG) edema was observed in three patients (16%).Increased cortical T2/FLAIR signal was a common finding in the sample population with 74% of patients showing some cortical signal change, occurring more frequently in the frontal lobes as well (71%). The insular (43%) region was also a common location of signal changes. BG signal changes were observed in six patients, 67% of which had caudate involvement. In contrasted MRI studies, there was no evidence of cortical enhancement. For diffusion analysis, three patients demonstrated restricted diffusion which was associated with various regions. Of the patients with observed diffusion restriction, all had evidence of cortical edema.
Seven patients with MRI data exhibited cortical atrophy (37%), comprising both the frontal (57%) and insular regions (43%). Four patients (21%) with MRI data showed signs of BG atrophy. Of the BG atrophic patients, all demonstrated preferential caudate involvement.
The highest recorded imaging staging for both the cortical and BG regions was recorded. Five percent of patients were cortical stage 1, 11% were stage 4, and 84% were grades 2–3. For BG staging, 37% of patients had no basal ganglia findings, 21% were stage 4, and 42% were stages 2–3. Furthermore, regression analysis was performed to determine if seizure duration (determined from first ictus to first recorded MRI) was associated with severity seen on MRI staging. There was a positive association between epilepsy duration and highest documented cortical severity (r = 0.46, p = 0.04).
Positive emission tomography (PET) data prior to surgical intervention was available for 12 patients (Table 2). One patient’s PET scan exhibited normal findings. Of the PET positive patients, all showed unihemispheric involvement concordant with the structural MRI. Despite diffuse hemispheric hypometabolism, two patients showed predominance within the frontal lobe (22%). Two patients (17%) demonstrated ipsilateral cerebellar hypometabolism and three (25%) had contralateral cerebellar hypometabolism Table 3.
Pathology findings
Pathology slides were analyzed in 27 RE patients, all of which were obtained from peri-insular hemispherotomy. The pathology data is referenced in Table 4. Seventeen patients had positive temporal lobe findings (62.9%), ten of these patients were characterized as Robitaille/Pardo stage 1 (58.8%). Insular (22%) and hippocampal (22%) involvement was noted in six patients, with 83.3% and 66.6% favoring stage 1, respectively. Hippocampal sclerosis was seen in six patients (22.2%). Eight patients (29.6%) had only one pathology stage present through their available specimens. Ten patients (37%) demonstrated deviation of pathological severity greater than one stage across their available specimens.
Imaging and pathology correlation studies
Imaging staging from the cortex and BG were compared with pathology stages and post-operative seizure outcome data, which was organized per individual participant (Table 4). Correlation analysis of the pathology and imaging data can be seen in Table 5. It was hypothesized that pathology Pardo staging data might correlate with Bien imaging staging. This analysis determined there was no statistically significant connection between general pathology staging to either highest or lowest cortical or basal ganglia imaging staging between patients. Of note, the strongest correlation showed a positive association between highest pathological staging to highest MRI staging (Pearson r = 0.43). However, this association was still not statistically significant (p = 0.07).
Seizure outcomes
Seizure outcomes were compared to multiple preoperative and intra-operative measures. A comprehensive list can be viewed in Table 6. Comparing the time interval of first ictus to surgery did not predict seizure outcomes (p = 0.42 ILAE). Similarly, the patient’s age at time of surgery had no correlation to seizure outcomes (ILAE p = 0.87). General pathological stage also failed to predict seizure outcomes (ILAE p = 0.55). Comparing seizure outcomes to MRI staging (cortical or BG) data yielded no significant predictive power.
HOPS scores were obtainable in 25 patients. Fourteen patients demonstrated a HOPS score of 1 (> 3.5 years old at seizure onset). No patients met the criteria for a HOPS score of 3 (< 3 months at seizure onset). All other patients were given a score of 2 (n = 11). Of the 14 participants with documented seizure freedom, ten exhibited a HOPS score of 1 (71%), the remaining demonstrated a HOPS score of 2 (29%).
Additive analysis combining both pathology and MRI imaging staging compared to seizure outcomes was performed as well. Our sample size precluded us from performing a multivariate analysis. Instead, pathologic staging, cortical imaging, and BG imaging staging were analyzed through a combined score analysis. Both the summated highest and lowest values of these categories were considered. A combination score was created by adding either the highest or lowest MRI scores from both basal ganglia and cortex in addition to highest or lowest regional pathology score for each patient (Table 5). Both the lowest and highest pathology plus MRI combined scores suggest that, as with pathology scores alone, more severe total scores trend toward better outcomes (r = − 0.30 to − 0.38). These combined scores trended toward slightly stronger correlations and lower p values than independent comparisons of pathology or imaging to outcomes. However, these findings were also not statistically significant.
Discussion
RE remains an incompletely understood and devastating neurologic disorder demanding the most aggressive of neurosurgical techniques to treat. Fortunately, many patients can achieve a state of prolonged or permanent seizure freedom while maintaining good functional neurologic performance with functional hemispherotomy. The seizure freedom data from this study was approximately 54%. A previous meta-analysis has reported seizure outcomes for Rasmussen’s treated with hemispherotomy ranging from 17 to 100% with the 5-year mean at 65% [29]. Given the morbidity of the hemispherotomy procedure, it would be very helpful to determine which RE patients are more likely to have robust seizure freedom outcomes for surgical selection and prognostication. Currently, clinical and radiographic features have not been particularly useful tools for predicting seizure freedom rates on an individual patient basis. A recent effort to address this issue has been made for all hemispherectomy patients in the form of the HOPS scale, but this currently lacks external validation and largely boils down to the single variable of age at seizure onset for RE patients. Applying the HOPS to our multicenter RE patient cohort, we noted lower seizure freedom percentages than study reference rates (71% vs. 96% for a score of 1, 45% vs. 92% for a score of 2) [22]. The trend in outcome prediction correlates, but the tool does not appear calibrated to make accurate predictions in the RE subset of hemispherotomy patients. We sought to combine retrospective data with a focus on radiographic and pathologic features to determine if criteria from these data could assist with predicting seizure outcomes. Specifically, we investigated if there might be predictive value in combining both radiographic findings and pathology findings. If a useful biomarker was found, this could potentially justify pre-hemispherotomy biopsies for prognostic purposes. Ultimately, our analysis did not find evidence for combined analysis of imaging and pathology staging being predictive for seizure outcomes. However, there were independent trends in both imaging and pathology findings that are noteworthy.
Radiographic findings
With a focus on examining the earliest available MRI scans by a single neuroradiologist to detect potential early prognostic features, there was abundant variability in the scans. Nonetheless, some descriptive themes arose from this analysis. The images showed a predilection for abnormalities in the superior frontal or medial frontal gyri. The imaging at the time of presentation typically showed the most acute radiographic appearance consistent with early immune-mediated inflammatory response [30, 31]. The hippocampus on the affected side was typically abnormal in appearance, and PET imaging was abnormal on the affected hemisphere. Caudate signal change or caudate atrophy was not always present in this pediatric dataset (42%) but was on par with other studies (38–89%) [7, 9, 31, 32]. The putamen was about half as likely to show atrophy compared to the caudate and much less likely to signal changes. The frequency of putaminal signal changes exhibited in this cohort are consistent with prior studies, but putaminal atrophy findings (11%) were less common than previously described (25–79%) [7, 32]. No cases showed enhancement and diffusion restriction was rare. Diffusion restriction is not a typical feature of RE [7, 33, 34] but has been reported with early-stage edema [35]. The three cases observed with diffusion restriction all demonstrated cortical edema as well.
Pathology findings
The widespread variability of pathologic changes within individual patients makes single point biopsy potentially misleading with regard to the overall severity of the cellular impact of the RE disease process. Our review of multi-institutional pathological specimens by a single neuropathologist confirmed widespread variability of Robitaille/Pardo staging within and across patients [25]. While pathology has been shown to provide clues to the duration or progression of illness based on stage and location, it does not appear to hold significant predictive power with regards to the long-term success of disconnective surgical intervention [25]. A prior study has shown that younger age and shorter time between diagnosis and surgical treatment are linked to greater durations of seizure freedom [29]. These are likely proxies of decreased pathological burden. With a mean post-operative follow-up assessment of 5 years, we did not see a correlation between either age at surgery or pathological burden and seizure freedom rate.
Limitations of this study include the retrospective nature of data collection, variability in available follow-up data, unavailability of pathology and/or imaging data for all patients, inconsistent pathology sampling at the time of surgeries, and a prolonged period of data collection.
For many patients with Rasmussen’s disease, the only option for seizure control is hemispheric disconnection surgery and many pediatric patients achieve complete seizure freedom with good functional outcomes. However, long-term surgical seizure outcomes have only modestly improved in recent decades and improvements in patient selection for these surgeries could aid surgical decision-making. While combining currently available MRI imaging findings and tissue pathology data does not appear to help with surgical outcome prognostication, other biomarkers should still be explored. RNA expression levels of interferon genes [36] or CSF protein markers [37] might be better variables to combine with imaging findings for multivariate prediction of hemispherotomy seizure outcomes. Since the underlying etiology of RE is incompletely understood, it is possible that the observed phenotype may have different variants in mechanisms that drive the hemispheric encephalitis. It is also possible that the disease process lies on a spectrum. Here, we have described some of the more commonly observed radiographic and pathologic findings in our multi-institutional cohort. We did observe a trend toward the cases that were at the more severe end of the radiographic and pathologic spectrum having the best response to hemispherotomy. Neither the patient age at surgery nor the duration between seizure onset and surgical intervention affected the seizure outcomes. Given the current lack of reliable indicators for long-term outcomes, we currently advocate for disconnective surgery at an early age such that additional treatment routes are not delayed if seizures are refractory and the greatest opportunity for functional plasticity is present.
Conclusion
Existing techniques for preoperative predictions of seizure freedom for hemispherotomy have not been shown to be effective in the setting of RE. Our analysis did not find evidence for combined analysis of imaging and pathology staging being predictive for seizure outcomes, prompting the need for other biomarkers to be explored.
Data availability
Data is available upon request by contacting the corresponding author.
References
Varadkar SBC, Kruse C et al (2014) Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol 13:195–205
Bien CG, Tiemeier H, Sassen R, Kuczaty S, Urbach H, von Lehe M, Becker AJ, Bast T, Herkenrath P, Karenfort M, Kruse B, Kurlemann G, Rona S, Schubert-Bast S, Vieker S, Vlaho S, Wilken B, Elger CE (2013) Rasmussen encephalitis: incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia 54:543–550
Lamb K, Scott W, Mensah A, Robinson R, Varadkar S, Cross J (2013) Prevalence and clinical outcome of Rasmussen encephalitis in children from the United Kingdom. Dev Med Child Neurol 55:14
Oguni H, Andermann F, Rasmussen TB (1992) The syndrome of chronic encephalitis and epilepsy. A study based on the MNI series of 48 cases. Adv Neurol 57:419–433
Rogers SW, Andrews PI, Gahring LC, Whisenand T, Cauley K, Crain B, Hughes TE, Heinemann SF, McNamara JO (1994) Autoantibodies to glutamate receptor GluR3 in Rasmussen’s encephalitis. Science 265:648–651
Hart Y (2004) Rasmussen’s encephalitis. Epileptic Disord 6:133–144
Pradeep K, Sinha S, Saini J, Mahadevan A et al (2014) Evolution of MRI changes in Rasmussen’s encephalitis. Acta Neurol Scand 130(4):253–259
Thomas JE, Reagan TJ, Klass DW (1977) Epilepsia partialis continua. A review of 32 cases. Arch Neurol 34:266–275
Granata TGG et al (2003) Rasmussen’s encephalitis: early characteristics allow diagnosis. Neurology 60:422–425
Hart YM, Cortez M, Andermann F, Hwang P, Fish DR, Dulac O, Silver K, Fejerman N, Cross H, Sherwin A et al (1994) Medical treatment of Rasmussen’s syndrome (chronic encephalitis and epilepsy): effect of high-dose steroids or immunoglobulins in 19 patients. Neurology 44:1030–1036
McLachlan RS, Levin S, Blume WT (1996) Treatment of Rasmussen’s syndrome with ganciclovir. Neurology 47:925–928
Andrews PI, Dichter MA, Berkovic SF, Newton MR, McNamara JO (2001) Plasmapheresis in Rasmussen’s encephalitis. 1996. Neurology 57:S37-41
Antozzi C, Granata T, Aurisano N, Zardini G, Confalonieri P, Airaghi G, Mantegazza R, Spreafico R (1998) Long-term selective IgG immuno-adsorption improves Rasmussen’s encephalitis. Neurology 51:302–305
Bien CG, Granata T, Antozzi C, Cross JH, Dulac O, Kurthen M, Lassmann H, Mantegazza R, Villemure JG, Spreafico R, Elger CE (2005) Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 128:454–471
Bien CG, Schramm J (2009) Treatment of Rasmussen encephalitis half a century after its initial description: promising prospects and a dilemma. Epilepsy Res 86:101–112
Guan Y, Chen S, Liu C, Du X, Zhang Y, Chen S, Wang J, Li T, Luan G (2017) Timing and type of hemispherectomy for Rasmussen’s encephalitis: analysis of 45 patients. Epilepsy Res 132:109–115
Guan Y, Zhou J, Luan G, Liu X (2014) Surgical treatment of patients with Rasmussen encephalitis. Stereotact Funct Neurosurg 92:86–93
Jonas R, Nguyen S, Hu B, Asarnow RF, LoPresti C, Curtiss S, de Bode S, Yudovin S, Shields WD, Vinters HV, Mathern GW (2004) Cerebral hemispherectomy: hospital course, seizure, developmental, language, and motor outcomes. Neurology 62:1712–1721
Kossoff EH, Vining EP, Pillas DJ, Pyzik PL, Avellino AM, Carson BS, Freeman JM (2003) Hemispherectomy for intractable unihemispheric epilepsy etiology vs outcome. Neurology 61:887–890
Vining EP, Freeman JM, Pillas DJ, Uematsu S, Carson BS, Brandt J, Boatman D, Pulsifer MB, Zuckerberg A (1997) Why would you remove half a brain? The outcome of 58 children after hemispherectomy-the Johns Hopkins experience: 1968 to 1996. Pediatrics 100:163–171
Berkovic SF, McIntosh AM, Kalnins RM, Jackson GD, Fabinyi GC, Brazenor GA, Bladin PF, Hopper JL (1995) Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology 45:1358–1363
Weil AG, Lewis EC, Ibrahim GM, Kola O, Tseng CH, Zhou X, Lin KM, Cai LX, Liu QZ, Lin JL (2021) Hemispherectomy Outcome Prediction Scale: development and validation of a seizure freedom prediction tool. Epilepsia 62:1064–1073
Hale AT, Estevez-Ordonez D, Badrani J, Sha W, Arynchyna-Smith A, Goyal M, Mohamed I, Kankirawatana P, Rozzelle CJ, Blount JP (2023) Hemispherectomy Outcome Prediction Scale: a validity study. J Neurosurg Pediatr 32:488–496
Wagner J, Schoene-Bake JC, Bien CG, Urbach H, Elger CE, Weber B (2012) Automated 3D MRI volumetry reveals regional atrophy differences in Rasmussen encephalitis. Epilepsia 53:613–621
Pardo CA, Vining EP, Guo L, Skolasky RL, Carson BS, Freeman JM (2004) The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia 45:516–526
Olson HE, Lechpammer M, Prabhu SP, Ciarlini PDSC, Poduri A, Gooty VD, Anjum MW, Gorman MP, Loddenkemper T (2013) Clinical application and evaluation of the Bien diagnostic criteria for Rasmussen encephalitis. Epilepsia 54:1753–1760
Bien CG, Urbach H, Deckert M, Schramm J, Wiestler OD, Lassmann H, Elger CE (2002) Diagnosis and staging of Rasmussen’s encephalitis by serial MRI and histopathology. Neurology 58:250–257
Robitaille Y (1991) Neuropathologic aspects of chronic encephalitis. Chronic Encephalitis and Epilepsy: Rasmussen Syndrome 79–110
Harris WB, Phillips HW, Chen JS, Weil AG, Ibrahim GM, Fallah A (2019) Seizure outcomes in children with Rasmussen’s encephalitis undergoing resective or hemispheric epilepsy surgery: an individual participant data meta-analysis. J Neurosurg Pediatr 6:1–10
Bien CG, Widman G, Urbach H, Sassen R, Kuczaty S, Wiestler OD, Schramm J, Elger CE (2002) The natural history of Rasmussen’s encephalitis. Brain 125:1751–1759
Chiapparini LGT et al (2003) Diagnostic imaging in 13 cases of Rasmussen’s encephalitis: can early MRI suggest the diagnosis? Neuroradiology 45:171–183
Ramesha KN, Rajesh B, Ashalatha R, Kesavadas C, Abraham M, Radhakrishnan VV, Sarma PS, Radhakrishnan K (2009) Rasmussen’s encephalitis: experience from a developing country based on a group of medically and surgically treated patients. Seizure 18:567–572
Sener RN (2000) Rasmussen’s encephalitis: proton MR spectroscopy and diffusion MR findings. J Neuroradiol 27:179–184
Sener RN (2003) Diffusion MRI and spectroscopy in Rasmussen’s encephalitis. Eur Radiol 13:2186–2191
Furruqh F, Thirunavukarasu S, Biswas A, Vivekandan R (2015) Complete right cerebral hemispheric diffusion restriction and its follow-up in a case of Rasmussen’s encephalitis. BMJ Case Rep 2015:bcr2015212256
Owens GC, Huynh MN, Chang JW, McArthur DL, Hickey MJ, Vinters HV, Mathern GW, Kruse CA (2013) Differential expression of interferon-γ and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response. J Neuroinflammation 10:56
Walker LE, Griffiths MJ, McGill F, Lewthwaite P, Sills GJ, Jorgensen A, Antoine DJ, Solomon T, Marson AG, Pirmohamed M (2017) A comparison of HMGB1 concentrations between cerebrospinal fluid and blood in patients with neurological disease. Biomarkers 22:635–642
Author information
Authors and Affiliations
Contributions
Conceptualization: Jesse Skoch, Francesco Mangano; Methodology: Francesco Mangano, Jesse Skoch, Christine Fuller, James Leach, Kathleen Knudson; formal analysis and investigation: Alexander Doherty, Kathleen Knudson, Christine Fuller, James Leach, Anthony Wang, Neena Marupudi, Rowland Han; writing—original draft preparation: Alexander Doherty, Kathleen Knudson, Jesse Skoch; writing—review and editing: Alexander Doherty, Rowland Han, Suart Tomoko, Jeff Ojemann, Matthew D. Smyth, Francesco Mangano, Jesse Skoch; funding acquisition: Jesse Skoch; resources: Rowland Han, Neena Marupudi, Jeff Ojemann, Matthew D. Smyth; supervision: Jesse Skoch, Francesco Mangano, Matthew D. Smyth, Jeff Ojemann.
Corresponding author
Ethics declarations
Ethics approval
This multi-institutional retrospective review of medical record, imaging, and pathology samples was approved by each individual institution’s review board (CCHMC IRB ID 2018–6625).
Conflict of interest
The authors declare no conflict of interest.
Statement of exclusivity
This manuscript is a unique submission and is not being considered for publication, in part or in full, with any other source in any medium.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doherty, A., Knudson, K., Fuller, C. et al. MRI and pathology comparisons in Rasmussen’s encephalitis: a multi-institutional examination of hemispherotomy outcomes relative to imaging and histological severity. Childs Nerv Syst 40, 1799–1806 (2024). https://doi.org/10.1007/s00381-024-06353-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-024-06353-4