Abstract
Heart failure (HF) and permanent atrial fibrillation (AF) interact mutually, exacerbating hemodynamic effects and causing adverse outcomes and increased healthcare costs. Monitoring hemodynamic indicators in patients with these comorbidities is crucial for effective clinical management. Transthoracic impedance cardiography (ICG) has been widely employed in assessing hemodynamic status in clinical settings. Given the limited research on the prognostic significance of ICG parameters in HF with permanent AF, we undertook this study. A total of 66 HF patients with permanent AF were included in this retrospective study, and the primary outcome was rehospitalization due to worsening HF within 180-day post-discharge. Cox regression analysis was performed to explore the connection between ICG-evaluated parameters and the outcome risk. Receiver operating characteristic (ROC) curve analysis determined the optimal cutoff values of risk factors, subsequently applied in plotting Kaplan Meier (KM) survival curves. Multivariate Cox regression analysis revealed that systemic vascular resistance (SVR) both on admission and at discharge independently predicted rehospitalization for worsening HF. ROC analysis established optimal SVR cutoff values: 320.89 (kPa s/L) on admission and 169.94 (kPa s/L) at discharge (sensitivity 70%, specificity 94.4%, area under the curve (AUC) 0.831, respectively, sensitivity 90%, specificity 55.6%, AUC 0.742). KM survival curves analysis showed that patients with SVR > 320.89 (kPa s/L) on admission had an 8.14-fold (P < 0.001) increased risk of the end-point event compared with those with SVR ≤ 320.89 (kPa s/L). Similarly, patients with SVR > 169.94 (kPa s/L) at discharge faced a risk elevated by 6.57 times (P = 0.002) relative to those with SVR ≤ 169.94 (kPa s/L). In HF patients with permanent AF, SVR measured by ICG emerges as an independent risk factor and clinical predictor for HF deterioration-related readmission within 180 days after discharge. Higher SVR levels, both upon admission and at discharge, correlate with an incremental rehospitalization risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) has been a growing public health issue. Despite marked reductions in HF-related mortality rates, rehospitalization owing to recurrent HF deterioration remains prevalent worldwide. Some studies [1, 2] have indicated that approximately 50% of HF patients experienced readmission within 6 months after discharge, with 70% of these cases linked to known HF exacerbation [3]. Atrial fibrillation (AF) has been the most common persistent arrhythmia in HF, impacting around 25% of patients on average, with its incidence rising [4]. HF and AF mutually worsen each other, leading to higher risks of death and readmission after discharge [5, 6], imposing a substantial burden on healthcare systems.
Inflammation, oxidative stress, and neuroendocrine abnormalities related to HF contribute to AF while ongoing AF worsens left ventricular function and HF progression. HF triggers increased left atrial pressure, affecting atrial function and causing hemodynamic imbalance [7, 8], raising hospitalization and all-cause mortality [9,10,11]. Considering that disrupted hemodynamics by HF and AF result in unfavorable results, understanding the hemodynamic state of patients with these comorbidities is essential and holds clinical value.
The Swan–Ganz floating catheter [12] is globally acknowledged as the "Gold Standard" for determining hemodynamic status. However, it involves invasive procedures with demanding technical prerequisites, complication rates ranging between 3 and 5% [13, 14], and high costs. It is clinically used in critically ill patients. As a non-invasive hemodynamic monitoring method, transthoracic impedance cardiography (ICG) can conveniently and comprehensively detect hemodynamic data, understand the immediate hemodynamic changes, and provide objective and quantitative indicators. Relevant studies have verified the accuracy of ICG [15,16,17,18,19,20].
In recent years, ICG has played an essential role in guiding medical therapy for acute and chronic HF [21,22,23], in clinical drug trials [24], in the evaluation of the effectiveness of other treatments for HF [25], in aiding medical care [26], and in monitoring during AF ablation and examining postoperative effects [27, 28]. However, there is a lack of studies on using ICG variables to judge the prognosis of patients with HF and AF, which prompted us to undertake this study.
Methods
Study population
We reviewed the patients with HF and permanent AF admitted to the Cardiology Department of Aoyang Hospital Affiliated to Jiangsu University from January 2021 to December 2022 and ultimately included 66 subjects (Fig. 1). HF and permanent AF criteria meet the European Society of Cardiology guideline definitions [29, 30].
Exclusion criteria: body weight below 40 kg or above 100 kg, inability to cooperate as a result of mental and psychological abnormalities, pacemaker implantation, skin ulceration of chest wall, Second-Degree Type II or Third-Degree atrioventricular block, acute infectious or autoimmune diseases in the acute stage, hyperthyroidism, acute coronary syndrome, hypertrophic obstructive cardiomyopathy, large arteritis, aortic aneurysm, severe peripheral vascular disease, dialysis status, severe valve stenosis or regurgitation, congenital heart disease, severe pulmonary hypertension, acute pulmonary embolism, constrictive pericarditis, massive pleural or pericardial effusion, pneumothorax, malignancy, shock status, severe anemia, cachexia.
Subjects were categorized into the readmission and non-admission groups based on rehospitalization due to worsening HF within a 180-day follow-up period after discharge. Readmission for HF deterioration was the end-point event. All participants included were discharged with clinical improvement, defined as stable vital signs, alleviation of symptoms and signs of circulatory congestion, and no need for intravenous drug management. The criteria for worsening HF: symptoms and signs accompanied by circulatory congestion, New York Heart Association (NYHA) classification of cardiac function ≥ 3, and no improvement in symptoms with oral pharmacotherapy. This study was approved by the Medical Ethics Committee of our institution ((2021) Ethics Approval No. 010), and written informed consent was waived because of the retrospective nature.
Data collection and follow-up
The following data were collected through the hospital database: demographic information, medical history, physical examination, blood tests, including N-terminal pro-B-type natriuretic peptide (NT-pro BNP), high-sensitivity cardiac troponin (HS-cTn), creatinine clearance evaluated by Cockcroft–Gault formula, total bilirubin, serum albumin, hemoglobin, D-Dimer, thyroid-stimulating hormone (TSH), blood sodium, serum total cholesterol, serum triglyceride, low-density lipoprotein. Arterial pressure, rhythm type, mean ventricular rate determined by 24-h ambulatory monitoring, left atrial transverse diameter, left atrial volume index, left ventricular end-diastolic diameter, left ventricular ejection fraction (LVEF) measured by transthoracic echocardiography, and medication prescriptions at discharge were gathered. The post-discharge end-point event was collected through retrospective medical records until December 2022. Data were checked and entered collaboratively by two investigators.
Transthoracic impedance cardiography (ICG)
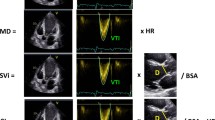
ICG is non-invasive, relying on varying electrical impedance in various tissues and water contents of the human body. In one cardiac cycle, impedance value changes with the blood volume and flow rate in thoracic vessels. Analyzing thoracic impedance shifts help determine hemodynamic parameters of blood movement [31]. Notably, the procedure is simple, requiring four pairs of electrodes on the neck and chest. Two pairs generate signals, while the other two detect them. After thoracic tissue rectification, instant signal changes can be observed (Fig. 2). In this study, nine clinically significant hemodynamic variables obtained by the non-invasive Hemodynamic Monitoring System (CSM3000, Qianfan Medical Co., Ltd, China) were defined as follows (Table 1).
Statistical analysis
Continuous variables with normal distribution were expressed as means ± standard deviation, while non-normally distributed variables as medians and interquartile ranges (IQRs); categorical variables were presented as frequency and percentage (%). Statistical tests included the t test for continuous variables, the Chi-square or Fisher’s exact test for categorical variables, and the Mann–Whitney U test for non-normally distributed variables. Cox regression analysis examined the relationship between the end-point event and parameters. Variables (P < 0.05) were included in multivariate analysis. Receiver-operating characteristic (ROC) curves established cutoff values for rehospitalization prediction. Kaplan–Meier (KM) analysis assessed the prognostic value, and significance was set at P < 0.05. Data were analyzed using SPSS version 25 and plotted with GraphPad Prism version 9.3 and R version 4.2.2.
Results
Baseline characteristics
The study included 66 HF patients with permanent AF. Of these, 30 with worsening HF were readmitted within 180 days (readmission group), while 36 were not (non-admission group, Table 2). No significant differences were found in age, gender, and BMI between the two groups. Mean ventricular rate, systolic, and diastolic blood pressure showed no group variations. Clinical conditions (smoking, alcohol, hypertension, diabetes, stroke history, CAD/MI history, PCI/CABG history, left bundle branch block, and right bundle branch block) were similar. Blood test parameters (NT-pro BNP, HS-cTn, etc.) did not differ significantly. Echocardiography measurements remained without significant differentiation.
Medication administration at discharge
At discharge, the drug prescriptions of β-blocks, loop diuretics, Valsartan/Sacubitril, SGLT2i, ACEI/Sartans, aldosterone antagonists, Nitrates, Digoxin, Statins, antiplatelet drugs, oral anticoagulants, Diltiazem or Verapamil, Propafenone, Amiodarone, between the two groups were no statistical differences (Table 3).
Relationship between ICG parameters and the rehospitalization event
As shown in Table 4, univariate Cox regression analysis showed that the occurrence of rehospitalization due to worsening HF was significantly correlated with SVR, Chronotropy, and CI on admission: SVR (HR: 1.007, 95% CI 1.005–1.010, P < 0.001), Chronotropy (HR: 0.995, 95% CI 0.994–0.997, P < 0.001), CI (HR: 0.180, 95% CI 0.064–0.512, P = 0.001), and multivariate analysis confirmed the unique efficacy of SVR (HR: 1.012, 95% CI 1.006–1.018, P < 0.001). In addition, SVR at discharge was significantly associated with the end-point event (HR: 1.004, 95% CI 1.002–1.006, P < 0.001 and HR: 1.004, 95% CI 1.000–1.008, P = 0.041, respectively), even after adjusting for the significant variable CI in univariate analysis (HR: 0.452, 95% CI 0.251–0.814, P = 0.008).
The value of SVR for predicting the end-point event
ROC analysis of the SVR to predict readmission for HF exacerbation revealed (Fig. 3) that the SVR value 320.89 (kPa s/L) on admission was the best cutoff level for predicting rehospitalization, which gave 70% sensitivity and 94.4% specificity with an area under the curve (AUC) of 0.831 (95% CI 0.72–0.94, P < 0.001). Meanwhile, employing the designated cutoff value to divide subjects into distinct groups, the KM survival analysis was performed using the log-rank test (Fig. 4). Patients with SVR > 320.89 (kPa s/L) on admission had an 8.14-fold increased risk of the end-point event (95% CI 3.66–18.07, P < 0.001) compared with those with SVR ≤ 320.89 (kPa s/L). Interestingly, an SVR value of 169.94 (kPa s/L) at discharge was the optimal cutoff level to predict rehospitalization for worsening HF, presenting 90% sensitivity and 55.6% specificity with an AUC of 0.742 (95% CI 0.62–0.86, P = 0.001). Survival analysis suggested that patients with SVR > 169.94 (kPa s/L) at discharge faced a 6.57-fold increased risk of the end-point event (95% CI 1.99–21.73, P = 0.002) in contrast to those with SVR ≤ 169.94 (kPa s/L).
Discussion
This study found that SVR, both on admission and at discharge, emerged as an independent risk factor and predictor of rehospitalization for worsening HF within 180-day post-discharge in HF patients with permanent AF. Moreover, patients with elevated SVR faced an increased risk of readmission stemming from HF aggravation within this period. To the best of our knowledge, this is the first study demonstrating the utility of ICG parameter SVR as a straightforward marker and predictive risk factor for 180-day rehospitalization in patients with HF and permanent AF.
Clinical predictors of rehospitalization in HF and AF
Reducing rehospitalization for HF and AF patients is vital for better outcomes and cost control. For this purpose, it is essential to identify high-risk groups and implement interventions. Currently, there is an ongoing inquiry into the rehospitalization risk for HF and AF patients. However, compared to the previous studies [32,33,34,35], new findings still emphasize sociodemographic [36, 37], clinical tests [38, 39], comorbidities [40, 41], medical regimens and quality of care [42], and risk models [43,44,45,46]. These outcomes identified risk factors at diffident time intervals (30, 90, 180, or ≥ 365 days). In addition to differences in study durations, distinctions in participant demographics, health status, and research methods result in incomparable data. Moreover, due to an uncertain balance between medical and non-medical factors, the multi-marker prediction models for HF and AF readmission may not be optimally accurate. In addition, the complexity and limited availability of these factors impact their objective evaluation and clinical applicability. Unlike prior studies, our research centers on a crucial pathophysiological mechanism of HF deterioration, hemodynamic imbalance unexplored in other investigations. Of importance, this methodology holds promise because of its convenience, scientific rigor, objectivity, and reproducibility.
Prognostic value of ICG parameters in HF and AF
Limited research has explored the relationship between non-invasive hemodynamic parameters and the prognosis of HF and AF. Some studies have focused on cardiac death as the end-point event. For instance, Andrius et al. reported [47] that chronic HF patients with TFC ≥ 36.91/kΩ had a 4.6-fold higher risk of cardiac death within 36 months of follow-up. Similarly, ICU-admitted acute HF patients with TFC ≥ 34.1/kΩ faced increased 6-month mortality [48]. However, these studies did not account for SVR and specify AF presence. Another investigation [49] noted higher mortality at 1 and 4 years in systolic HF patients with BN P ≥ 450 pg/ml and TFC ≥ 40.1/kΩ, excluding AF patients. Hao‐Chih et al. [50] linked exponential TFC ≥ 0.5/kΩ/m2 to increased HF readmission and all-cause death based on nocturnal impedance measurements. The observation did not include SVR, affecting comparability. A cohort study [51] tied exponential TFC and LVET measured by ICG to HF events within 14 days. Yet, it lacked data beyond this period and was underpowered to assess the predictive value of ICG over 14 days. Notably, participants with no improvements in HF symptoms within 7 days of treatment and those planned for intravenous medications (diuretics, vasodilators, or inotropic agents) were excluded, making comparisons with our study inconclusive. Hence, differences in study participants, parameters, and outcomes prevent direct comparison of prognostic implications of ICG parameters in HF and AF.
Prognosis value of SVR in HF and AF
Dynamic changes in vascular tone are another critical component in worsening HF pathogenesis. Heightened sympathetic activation and vasoconstrictor substances release, common in deteriorating HF, intensify arterial constriction, then increase SVR. Increased afterload triggers a rise in left ventricular pressure, enhancing ventricular wall stress, worsening myocardial ischemia, causing myocardial injury, deteriorating left ventricular pump performance, and elevating the likelihood of severe cardiac events [52,53,54]. Teerlink et al. [55] found novel vasodilator agents stabilize HF patients’ hemodynamic balance by improving SVR, reducing HF deterioration and mortality risk. It is speculated that vasodilators targeting vascular resistance pathways hold promise for treating HF deterioration [56]. In an investigation [57] of HF patients during 1-month outpatient follow-up after improvement and discharge, hemodynamic indices by whole-body impedance measurement examined the rehospitalization risk for HF aggravation. Univariate analysis indicated that higher SVR predicted HF rehospitalization (100% sensitivity, 68.6% specificity, and 0.89 AUC), but the multivariate analysis found no interplay. Conversely, our findings suggested that SVR admission value had better specificity, while discharge value had good sensitivity. This variation comes from research design: their patients were younger, with better cardiac status, lower proportion of AF and baseline SVR than ours, not technology [58]. In addition, a multi-center prospective cohort study [59] analyzed SVR via ultrasound electrocardiogram. It focused on coronary heart disease patients, dividing them into SVR tertiles: < 5.6, 5.6–6.9, and ≥ 6.9. Over a 5-year follow-up, the ≥ 6.9 group had higher cardiovascular risks. This study differs from ours in design, included population, SVR measurement (direct correlation between this approach and invasive one has never been verified), and end-points. Nevertheless, its conclusions and ours endorse the significance of high SVR levels as a crucial risk factor in worsening HF and predicting adverse cardiovascular outcomes.
SVR and underlying cardiac diseases
SVR pertains to the level of hindrance encountered by blood flow within the vessels of the circulatory system. The elevation of SVR stems from a multifaceted interplay of factors, including vasoconstriction, heightened vascular wall thickness, augmented blood viscosity, reduced vascular elasticity, vascular endothelial dysfunction, disturbances in the neuroendocrine system, and inflammatory responses within the vascular wall, among others. These underlying pathophysiological mechanisms may interact to contribute to the escalation of SVR.
Cardiovascular diseases directly or indirectly raise SVR by affecting these mechanisms. For instance, the diminished cardiac function activates the sympathetic nervous and renin–angiotensin–aldosterone systems, causing vasoconstriction, water retention, and increased blood volume, elevating resistance [60]. Hypertension leads to peripheral vasoconstriction and vascular remodeling, narrowing arterial diameter and obstructing blood flow [61].
In our study, the exclusion of various hemodynamically impactful diseases and the presence of comparable baseline data, including blood pressure and relevant resistance-affecting medications, minimized external influences on SVR, enabling a direct evaluation of the link between HF with AF and SVR. Since we did not specify the cardiac etiology of the enrolled population, we cannot ascertain whether different cardiac causes might affect SVR measurement in our study. Further research on this aspect would also hold significant value.
Our conclusions quantified the linkage between SVR and rehospitalization within 180 days for HF patients with permanent AF. This insight may aid clinicians in identifying high-risk readmissions, optimizing treatment plans, strengthening outpatient follow-up, and even ultimately reducing adverse events—an essential contribution of this study.
Limitations and future research directions
This study has limitations: small sample size, all Chinese participants, HF types, some comorbidities excluded, and retrospective design impact generalization. Prospective multi-center research with a large sample is needed to further validate the prognostic value of SVR in different HF and permanent AF cases.
Conclusions
In conclusion, our findings show a strong link between elevated SVR measured by ICG and a 180-day readmission risk for worsening HF in cardiac insufficiency patients with permanent AF.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Shah RU, Tsai V, Klein L, Heidenreich PA (2011) Characteristics and outcomes of very elderly patients after first hospitalization for heart failure. Circ Heart Fail 4(3):301–307
Butler J, Kalogeropoulos A (2008) Worsening heart failure hospitalization epidemic we do not know how to prevent and we do not know how to treat! J Am Coll Cardiol 52(6):435–437
Gheorghiade M, Zannad F, Sopko G, Klein L, Piña IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L (2005) Acute heart failure syndromes: current state and framework for future research. Circulation 112(25):3958–3968
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, Kalani R, Kazi DS, Ko D, Levine DA, Liu J, Ma J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Virani SS, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Martin SS (2023) Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation 147(8):e93–e621
Patel RB, Greene SJ, Xu H, Alhanti B, Peterson P, Yancy CW, Piccini J, Fonarow GC, Vaduganathan M (2023) Intersection of atrial fibrillation and heart failure with mildly reduced and preserved ejection fraction in >400 000 participants in the get with the guidelines-heart failure registry. Eur J Heart Fail 25(1):63–73
Butt JH, Fosbøl EL, Gerds TA, Andersson C, McMurray JJV, Petrie MC, Gustafsson F, Madelaire C, Kristensen SL, Gislason GH, Torp-Pedersen C, Køber L, Schou M (2020) Readmission and death in patients admitted with new-onset versus worsening of chronic heart failure: insights from a nationwide cohort. Eur J Heart Fail 22(10):1777–1785
Prabhu S, Voskoboinik A, Kaye DM, Kistler PM (2017) Atrial fibrillation and heart failure—cause or effect? Heart Lung Circ 26(9):967–974
Carlisle MA, Fudim M, DeVore AD, Piccini JP (2019) Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail 7(6):447–456
Lardizabal JA, Deedwania PC (2012) Atrial fibrillation in heart failure. Med Clin North Am 96(5):987–1000
Middlekauff HR, Stevenson WG, Stevenson LW (1991) Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation 84(1):40–48.
Rivero-Ayerza M, Scholte Op Reimer W, Lenzen M, Theuns DA, Jordaens L, Komajda M, Follath F, Swedberg K, Cleland JG (2008) New-onset atrial fibrillation is an independent predictor of in-hospital mortality in hospitalized heart failure patients: results of the EuroHeart Failure Survey. Eur Heart J 29(13):1618–1624
Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D (1970) Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med 283(9):447–451
Peters SG, Afessa B, Decker PA, Schroeder DR, Offord KP, Scott JP (2003) Increased risk associated with pulmonary artery catheterization in the medical intensive care unit. J Crit Care 18(3):166–171
Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K (2005) Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet 366(9484):472–477
Belardinelli R, Ciampani N, Costantini C, Blandini A, Purcaro A (1996) Comparison of impedance cardiography with thermodilution and direct Fick methods for noninvasive measurement of stroke volume and cardiac output during incremental exercise in patients with ischemic cardiomyopathy. Am J Cardiol 77(15):1293–1301
Drazner MH, Thompson B, Rosenberg PB, Kaiser PA, Boehrer JD, Baldwin BJ, Dries DL, Yancy CW (2002) Comparison of impedance cardiography with invasive hemodynamic measurements in patients with heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol 89(8):993–995
Raaijmakers E, Faes TJ, Scholten RJ, Goovaerts HG, Heethaar RM (1999) A meta-analysis of three decades of validating thoracic impedance cardiography. Crit Care Med 27(6):1203–1213
Albert NM, Hail MD, Li J, Young JB (2004) Equivalence of the bioimpedance and thermodilution methods in measuring cardiac output in hospitalized patients with advanced, decompensated chronic heart failure. Am J Crit Care 13(6):469–479
Malfatto G, Blengino S, Perego GB, Branzi G, Villani A, Facchini M, Parati G (2012) Transthoracic impedance accurately estimates pulmonary wedge pressure in patients with decompensated chronic heart failure. Congest Heart Fail 18(1):25–31
Kim GE, Kim SY, Kim SJ, Yun SY, Jung HH, Kang YS, Koo BN (2019) Accuracy and efficacy of impedance cardiography as a non-invasive cardiac function monitor. Yonsei Med J 60(8):735–741
Siedlecka J, Siedlecki P, Bortkiewicz A (2015) Impedance cardiography—old method, new opportunities. Part I. Clinical applications. Int J Occup Med Environ Health 28(1):27–33.
Lasater M, Von Rueden KT (2003) Outpatient cardiovascular management utilizing impedance cardiography. AACN Clin Issues 14(2):240–250
Ruocco G, Pastorini G, Feola M (2021) Echocardiography and impedance cardiography as determinants of successful slow levosimendan infusion in advanced older heart failure patients. J Geriatr Cardiol 18(12):1058–1062
Van Tassell B, Mihalick V, Thomas G, Marawan A, Talasaz AH, Lu J, Kang L, Ladd A, Damonte JI, Dixon DL, Markley R, Turlington J, Federmann E, Del Buono MG, Biondi-Zoccai G, Canada JM, Arena R, Abbate A (2022) Rationale and design of interleukin-1 blockade in recently decompensated heart failure (REDHART2): a randomized, double blind, placebo controlled, single center, phase 2 study. J Transl Med 20(1):270
Wang Y, Xiao Y, Tang J, Liu Y, Li H, Peng Z, Xu D, Shen L (2022) Effects of early phase 1 cardiac rehabilitation on cardiac function evaluated by impedance cardiography in patients with coronary heart disease and acute heart failure. Front Cardiovasc Med 9:958895
Krzesinski P, Sobotnicki A, Gacek A, Siebert J, Walczak A, Murawski P, Gielerak G (2021) Noninvasive bioimpedance methods from the viewpoint of remote monitoring in heart failure. JMIR Mhealth Uhealth 9(5):e25937
Nakatani Y, Sakamoto T, Yamaguchi Y, Tsujino Y, Kataoka N, Nishida K, Mizumaki K, Kinugawa K (2018) Improvement of hemodynamic parameters in patients with preserved left ventricular systolic function by catheter ablation of atrial fibrillation - a prospective study using impedance cardiography. Circ J 83(1):75–83
Iqbal AM, Li KY, Aznaurov SG, Lugo RM, Venkataraman R, Gautam S (2022) Catheter ablation for atrial fibrillation can be safely performed without invasive hemodynamic monitoring: A multi-center study. J Interv Card Electrophysiol 64(3):743–749
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42(36):3599–3726
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL (2021) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the european heart rhythm association (EHRA) of the ESC. Eur Heart J 42(5):373–498
Bayram M, Yancy CW (2009) Transthoracic impedance cardiography: a noninvasive method of hemodynamic assessment. Heart Fail Clin 5(2):161–168
Barrett TW, Jenkins CA, Self WH (2015) Validation of the risk estimator decision aid for atrial fibrillation (RED-AF) for predicting 30-day adverse events in emergency department patients with atrial fibrillation. Ann Emerg Med 65(1):13-21.e13
Sherer AP, Crane PB, Abel WM, Efird J (2016) Predicting heart failure readmissions. J Cardiovasc Nurs 31(2):114–120
Saito M, Negishi K, Marwick TH (2016) Meta-analysis of risks for short-term readmission in patients with heart failure. Am J Cardiol 117(4):626–632
Pierre-Louis B, Rodriques S, Gorospe V, Guddati AK, Aronow WS, Ahn C, Wright M (2016) Clinical factors associated with early readmission among acutely decompensated heart failure patients. Arch Med Sci 12(3):538–545
Gangu K, Bobba A, Chela HK, Avula S, Basida S, Yadav N (2022) In-hospital mortality rate and predictors of 30-day readmission in patients with heart failure exacerbation and atrial fibrillation: a cross-sectional study. Int J Heart Fail 4(3):145–153
Son YJ, Kim DY, Won MH (2021) Sex differences in the association between atrial fibrillation and 90-day adverse outcomes among older adults with heart failure: A retrospective cohort study. Int J Environ Res Public Health 18(5):2237
Sommerfeld A, Althouse AD, Prince J, Atwood CW, Mulukutla SR, Hickey GW (2017) Obstructive sleep apnea is associated with increased readmission in heart failure patients. Clin Cardiol 40(10):873–878
Oeun B, Nakatani D, Hikoso S, Kojima T, Dohi T, Kitamura T, Okada K, Sunaga A, Kida H, Yamada T, Uematsu M, Yasumura Y, Higuchi Y, Mano T, Nagai Y, Fuji H, Mizuno H, Sakata Y (2020) Factors associated with elevated n-terminal pro B-type natriuretic peptide concentrations at the convalescent stage and 1-year outcomes in patients with heart failure with preserved ejection fraction. Circ Rep 2(8):400–408
Di Mauro M, Petroni R, Clemente D, Foschi M, Tancredi F, Camponetti V, Gallina S, Calafiore AM, Penco M, Romano S (2018) Clinical profile of patients with heart failure can predict rehospitalization and quality of life. J Cardiovasc Med (Hagerstown) 19(3):98–104
Holm H, Bachus E, Jujic A, Nilsson ED, Wadström B, Molvin J, Minthon L, Fedorowski A, Nägga K, Magnusson M (2020) Cognitive test results are associated with mortality and rehospitalization in heart failure: Swedish prospective cohort study. ESC Heart Fail 7(5):2948–2955
Bahiru E, Ziaeian B, Moucheraud C, Agarwal A, Xu H, Matsouaka RA, DeVore AD, Heidenreich PA, Allen LA, Yancy CW, Fonarow GC (2021) Association of dual eligibility for medicare and medicaid with heart failure quality and outcomes among get with the guidelines-heart failure hospitals. JAMA Cardiol 6(7):791–800
Zhuang B, Shen T, Li D, Jiang Y, Li G, Luo Q, Jin Y, Shan Z, Che L, Wang L, Zheng L, Shen Y (2021) A model for the prediction of mortality and hospitalization in Chinese heart failure patients. Front Cardiovasc Med 8:761605
Atzema CL, Dorian P, Fang J, Tu JV, Lee DS, Chong AS, Austin PC (2018) A clinical decision instrument to predict 30-day death and cardiovascular hospitalizations after an emergency department visit for atrial fibrillation: The Atrial Fibrillation in the Emergency Room, Part 2 (AFTER2) study. Am Heart J 203:85–92
Shibata N, Kondo T, Morimoto R, Kazama S, Sawamura A, Nishiyama I, Kato T, Kuwayama T, Hiraiwa H, Umemoto N, Asai T, Okumura T, Murohara T (2022) Clinical value of the HATCH score for predicting adverse outcomes in patients with heart failure. Heart Vessels 37(8):1363–1372
Johnson LSB, Oldgren J, Barrett TW, McNaughton CD, Wong JA, McIntyre WF, Freeman CL, Murphy L, Engström G, Ezekowitz M, Connolly SJ, Xu L, Nakamya J, Conen D, Bangdiwala SI, Yusuf S, Healey JS (2021) LVS-HARMED risk score for incident heart failure in patients with atrial fibrillation who present to the emergency department: data from a world-wide registry. J Am Heart Assoc 10(18):e017735
Ališauskas A, Naudžiūnas A, Sadauskas S, Jankauskienė L, Kalinauskienė E, Vanagaitė G (2022) Single-center study in lithuania to evaluate the role of transthoracic impedance cardiography in the diagnosis and outcome evaluation of 301 patients with chronic heart failure exacerbation. Med Sci Monit 28:e938389
Sadauskas S, Naudžiūnas A, Unikauskas A, Mašanauskienė E, Ališauskas A, Bakšytė G, Macas A (2018) Diagnostic and outcome prediction value of transthoracic impedance cardiography in heart failure patients during heart failure flare-ups. Med Sci Monit 24:6573–6578
Malfatto G, Corticelli A, Villani A, Giglio A, Della Rosa F, Branzi G, Facchini M, Parati G (2013) Transthoracic bioimpedance and brain natriuretic peptide assessment for prognostic stratification of outpatients with chronic systolic heart failure. Clin Cardiol 36(2):103–109
Chang HC, Huang CJ, Cheng HM, Yu WC, Chiang CE, Sung SH, Chen CH (2020) Nocturnal thoracic volume overload and post-discharge outcomes in patients hospitalized for acute heart failure. ESC Heart Fail 7(5):2807–2817
Packer M, Abraham WT, Mehra MR, Yancy CW, Lawless CE, Mitchell JE, Smart FW, Bijou R, O’Connor CM, Massie BM, Pina IL, Greenberg BH, Young JB, Fishbein DP, Hauptman PJ, Bourge RC, Strobeck JE, Murali S, Schocken D, Teerlink JR, Levy WC, Trupp RJ, Silver MA (2006) Utility of impedance cardiography for the identification of short-term risk of clinical decompensation in stable patients with chronic heart failure. J Am Coll Cardiol 47(11):2245–2252
Torre-Amione G, Milo-Cotter O, Kaluski E, Perchenet L, Kobrin I, Frey A, Rund MM, Weatherley BD, Cotter G (2009) Early worsening heart failure in patients admitted for acute heart failure: time course, hemodynamic predictors, and outcome. J Card Fail 15(8):639–644
Njoroge JN, Teerlink JR (2021) Pathophysiology and therapeutic approaches to acute decompensated heart failure. Circ Res 128(10):1468–1486
Hartupee J, Mann DL (2017) Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 14(1):30–38
Teerlink JR, Davison BA, Cotter G, Maggioni AP, Sato N, Chioncel O, Ertl G, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Edwards C, Senger S, Teichman SL, Nielsen OW, Voors AA, Metra M (2020) Effects of serelaxin in patients admitted for acute heart failure: a meta-analysis. Eur J Heart Fail 22(2):315–329
Albert NM (2018) An integrative review of the literature on in-hospital worsening heart failure. Heart Lung 47(5):437–445
Tanino Y, Shite J, Paredes OL, Shinke T, Ogasawara D, Sawada T, Kawamori H, Miyoshi N, Kato H, Yoshino N, Hirata K (2009) Whole body bioimpedance monitoring for outpatient chronic heart failure follow up. Circ J 73(6):1074–1079
Beck R, Milella L, Labellarte C (2018) Continuous non-invasive measurement of stroke volume and cardiac index in infants and children: comparison of Impedance Cardiography NICaS® vs CardioQ® method. Clin Ter 169(3):e110–e113
Lu DY, Fang Q, Bibby D, Arora B, Schiller NB (2022) Association of systemic vascular resistance analog and cardiovascular outcomes: the heart and soul study. J Am Heart Assoc 11(17):e026016
Hirsch AT, Dzau VJ, Creager MA (1987) Baroreceptor function in congestive heart failure: effect on neurohumoral activation and regional vascular resistance. Circulation 75(5 Pt 2):IV36–48.
Julius S (1988) Transition from high cardiac output to elevated vascular resistance in hypertension. Am Heart J 116(2 Pt 2):600–606
Funding
This work was supported by the Science and Technology Project of Zhangjiagang City (ZKS2043), Zhangjiagang City Health Youth Science and Technology Project (ZJGQNKJ202113), and Suzhou Science and Technology Development Plan (SKJYD2021006).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by ZX, ZJ, JZ, and JD. The first draft of the manuscript was written by ZX and ZJ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
Approval was obtained from the Medical Ethics Committee of Aoyang Hospital Affiliated to Jiangsu University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jing, Z., Zhang, J., Ding, J. et al. The prognostic value of systemic vascular resistance in heart failure patients with permanent atrial fibrillation: a retrospective study. Heart Vessels 38, 1431–1441 (2023). https://doi.org/10.1007/s00380-023-02314-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-023-02314-0