Abstract
Fibroblast growth factor 23 (FGF23) is a bone-derived hormone that regulates renal phosphate reabsorption and vitamin D synthesis in renal proximal tubules. High circulating FGF23 levels are associated with increased mortality in patients with chronic kidney disease and those on dialysis. Current data also suggest higher circulating levels of FGF23 are associated with cardiovascular mortality, vascular calcification, and left ventricular hypertrophy; however, evidence on the role of FGF23 in patients on dialysis is incomplete, and some of the data, especially those on cardiovascular disease (CVD), are controversial. This study aimed to evaluate factors associated with FGF23 in hemodialysis patients with or without CVD. Randomly selected 76 patients on maintenance hemodialysis at a single hemodialysis center were enrolled. After the exclusion of eight patients with extremely outlying FGF23 levels, 68 patients, including 48 males and 46 patients with a CVD history, were included in the study. The mean age was 64.4 ± 12.1 years, and the mean dialysis duration was 12.7 ± 7.1 years. Dialysis duration, time-averaged concentration of urea (TAC-urea), ultrafiltration rate (UFR), blood pressure during hemodialysis session, laboratory data, and echocardiographic parameters including interventricular septum thickness (IVST), left ventricular mass indices (LVMI), and ejection fraction were included in univariate and multivariate analyses. The median lgFGF23 levels in the overall cohort and in those with and without CVD were 2.14 (interquartile range, IQR − 0.43 to − 4.23), 2.01 (− 0.52 to 4.12), and 2.59 (0.07 to 4.32), respectively, and there was no difference between the patients with and without CVD (p = 0.14). The univariate analysis revealed that FGF23 was significantly associated with age (r = − 0.12, p < 0.01), duration of hemodialysis (r = − 0.11, p < 0.01), TAC-urea (r = 0.29, p = 0.01), UFR (r = 0.26, p = 0.04), alkaline phosphatase (ALP; r = − 0.27, p = 0.03), corrected serum calcium (cCa; r = 0.32, p < 0.01), serum phosphate (iP, r = 0.57, p < 0.01), intact parathyroid hormone (iPTH; r = 0.38, p < 0.01), IVST (r = 0.30, p = 0.01), and LVMI (r = 0.26, p = 0.04). In multivariate regression analysis, FGF23 was significantly associated with cCa (F = 25.6, p < 0.01), iP (F = 22.5, p < 0.01), iPTH (F = 19.2, p < 0.01), ALP (F = 5.34, p = 0.03), and UFR (F = 3.94, p = 0.05). In addition, the univariate analysis after the categorization of patients according to CVD indicated that FGF23 was significantly associated with cCa (r = 0.34, p = 0.02), iP (r = 0.41, p < 0.01), iPTH (r = 0.39, p = 0.01), and TAC-urea (r = 0.45, p < 0.01) in patients with CVD, whereas only IVST (r = 0.53, p = 0.04) was associated with FGF23 in those without CVD. FGF23 levels in hemodialysis patients were extremely high and associated not only with mineral bone disease-related factors but also with UFR. Additionally, dialysis efficacy might be associated with lower FGF23 levels in patients with CVD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibroblast growth factor 23 (FGF23) is a bone-derived hormone that regulates renal phosphate reabsorption and vitamin D synthesis in renal proximal tubules [1]. Importantly, FGF23 levels start increasing early in the course of chronic kidney disease (CKD) and reach extremely high levels in patients on dialysis [2,3,4,5]. Serum phosphate (iP), calcium, and intact parathyroid hormone (iPTH) are proposed to regulate FGF23 levels in uremic patients on maintenance hemodialysis [6], and current data suggest higher circulating levels of FGF23 are associated with mortality [7,8,9,10,11], especially cardiovascular mortality [10, 12,13,14,15] due to vascular calcification [16,17,18,19] or left ventricular hypertrophy (LVH) [20,21,22,23]. These results indicate that reducing serum FGF23 levels might improve prognosis in patients on dialysis. However, evidence on the role of FGF23 in patients on dialysis is incomplete, and some of the data, especially those on cardiovascular disease (CVD), are controversial. Therefore, we aimed to determine the factors associated with elevated serum FGF23 levels in patients on hemodialysis and to assess whether there were differences between patients with and without CVD.
Materials and methods
Study design and population
This cross-sectional study enrolled 76 randomly selected patients undergoing maintenance hemodialysis performed at a single hemodialysis center (Heisei Hidaka Clinic, Gunma, Japan) in April 2010. All study participants provided informed consent, and the study design was approved by the Ethical Committee on Human Research at Heisei Hidaka Clinic (No. 43). The dialysis prescriptions were hemodialysis and hemodiafiltration in 61 and 15 patients, respectively, which were administered in 4-h sessions three times weekly using a polysulfone hollow-fiber dialyzer (APS or ABH; Asahi Kasei Medical, Tokyo, Japan). Blood and dialysate flows were 120–250 mL/min and 500 mL/min, respectively, with a constant ultrafiltration rate (UFR). The dialysate bath comprised 140 mmol/L sodium, 2.0 mmol/L potassium, 2.5 mmol/L calcium, 1.0 mmol/L magnesium, 8.0 mmol/L acetate, 25.0 mmol/L bicarbonate, and 150 mg/dL glucose (Kindaly 3D; Fuso, Osaka, Japan). Blood samples were collected at the start of dialysis at the end of the longest interdialytic interval, and echocardiographic parameters were measured at the end of a regular 4-h hemodialysis session on the last session of the week.
Clinical and laboratory measurements
All blood samples were collected immediately before the hemodialysis session, as previously described, quickly mixed with 5 mg edetic acid, and centrifuged at 3000 rpm for 5 min to separate plasma. Plasma samples were stored at − 20 °C until analysis. Serum intact FGF23 concentrations were measured with a two-step FGF23 enzyme immunoassay (ELISA) kit (Kainos Laboratories Inc., Tokyo, Japan). Additionally, associated laboratory data, including levels of C-reactive protein, alkaline phosphatase (ALP), corrected serum calcium (cCa), iP, and iPTH, were measured by routine laboratory tests. Clinical data, including age, sex, dialysis duration, time-averaged concentration of urea (TAC-urea), UFR, blood pressure during hemodialysis session, and echocardiographic parameters, including interventricular septum thickness (IVST), left ventricular mass indices (LVMI), and ejection fraction, were also collected.
Statistical analysis
Based on the measurement of intact FGF23 levels, patients who were extreme outliers (n = 8) were excluded, and 68 patients were included in the final analysis. Among these patients, 46 patients had a history of CVD. All statistical analyses were performed using JMP Pro version 12 for Mac (SAS Institute Japan, Tokyo, Japan). Data were reported as means ± standard deviation for normally distributed data and numbers with percentages for nominal data. A two-sided p value of < 0.05 was considered to indicate statistical significance. Univariable and multivariable linear regression analyses were performed to determine factors associated with natural log-transformed (Ln) serum FGF23 levels in patients on hemodialysis. Additional analyses were performed to determine factors associated with Ln serum FGF23 based on the presence of CVD.
Results
The baseline patient characteristics are presented in Table 1. The median levels of FGF23 in the overall cohort and those with and without CVD were 2.14 (interquartile range, IQR − 0.43 to − 4.23), 2.01 (− 0.52 to 4.12), and 2.59 (0.07 to 4.32), respectively, with no significant difference observed between the patients with and without CVD (p = 0.14). We analyzed the relationship between serum FGF23 and the clinical parameters. In univariate analysis, age (r = − 0.12, p < 0.01), duration of hemodialysis (r = − 0.11, p < 0.01), TAC-urea (r = 0.29, p = 0.01), UFR (r = 0.26, p = 0.04), alkaline phosphatase (ALP; r = − 0.27, p = 0.03), corrected serum calcium (cCa; r = 0.32, p < 0.01), serum phosphate (iP, r = 0.57, p < 0.01), intact parathyroid hormone (iPTH; r = 0.38, p < 0.01), IVST (r = 0.30, p = 0.01), and LVMI (r = 0.26, p = 0.04) were associated with FGF23, as shown in Table 2. Multiple regression analysis to determine the association of these factors with serum FGF23 revealed that cCa (F = 25.6, p < 0.01), iP (F = 22.5, p < 0.01), iPTH (F = 19.2, p < 0.01), ALP (F = 5.34, p = 0.03), and UFR (F = 3.94, p = 0.05) were associated with FGF23 (Fig. 1). We divided the study group into two subgroups by median ultrafiltration volume, and it was associated with serum FGF23 level (p = 0.04). In addition, univariate analysis performed after the classification of patients according to the presence of CVD revealed that cCa (r = 0.34, p = 0.02), iP (r = 0.41, p < 0.01), iPTH (r = 0.39, p = 0.01), and TAC-urea (r = 0.45, p < 0.01) were associated with serum FGF23 in patients with CVD, whereas only IVST (r = 0.53, p = 0.04) was associated with serum FGF23 in patients without CVD (Table 3; Fig. 2).
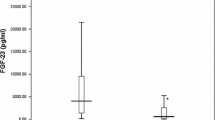
Relationship between serum FGF23 and associated factors by multiple regression analysis. In multiple regression analysis, corrected serum calcium (a), serum phosphate (b), intact parathyroid hormone (c), alkaline phosphate (d), and ultrafiltration rate (e) were associated with serum FGF23. Higher ultrafiltration volume is associated with higher serum FGF23 level by subgroup analysis (p = 0.04) (f). FGF23 fibroblast growth factor 23
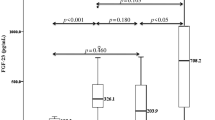
Relationship between serum FGF23 and basic patients’ characteristics with or without CVD. In patients with CVD (left), corrected serum calcium (a), serum phosphate (b), intact parathyroid hormone (c), and TAC-urea (d) were associated with FGF23, whereas only IVST (e) was associated with serum FGF23 in patients without CVD (right). CVD cardiovascular disease; FGF23 fibroblast growth factor 23, IVST interventricular septum thicknesses, TAC-urea time-averaged concentration of urea
Discussion
Serum levels of FGF23, a phosphaturic hormone that suppresses 1,25(OH)2-vitamin D3 production in kidneys [24], increase early in the course of CKD and reach levels that are several hundred times the normal range in patients with advanced CKD and end-stage renal disease (ESRD) [2]. Additionally, high levels of FGF23 have recently emerged as one of the strongest predictors of adverse outcomes in patients with CKD and ESRD [25]. The mean FGF23 level of the overall cohort (17,039 ± 19,178 pg/mL) was significantly higher than the reported normal range. Conversely, the mean serum levels of FGF23 did not differ significantly between patients with and without CVD. Despite an ever-expanding pool of observational data suggesting the potential contribution of FGF23 to CVD and mortality, current reviews and meta-analyses [26,27,28] suggest that the current evidence does not support a causal relationship between FGF23 and adverse events in patients with ESRD or in those with normal kidney function. The results of the current study also failed to find an association between serum FGF23 level and CVD.
Our univariate analysis also revealed that age, duration of hemodialysis, TAC-urea, UFR, cCa, iP, ALP, iPTH, IVST, and LVMI were associated with serum FGF23 in patients on maintenance hemodialysis. We also found that cCa, iP, iPTH, ALP, and UFR were associated with serum FGF23 in multiple regression analysis. As a phosphaturic hormone, FGF23 is stimulated by decreased renal phosphate excretion and subsequent hyperphosphatemia due to a declining glomerular filtration rate in patients with CKD [25]; therefore, it is reasonable that these markers related to iP homeostasis (cCa, iP, ALP, and iPTH) were associated with serum FGF23. IVST and LVMI were also associated with serum FGF23; this finding might reflect LVH in these patients on dialysis. Several studies have reported the association between FGF23 and LVH [20,21,22,23]. Intramyocardial or intravenous injection of FGF23 in wild-type mice was shown to lead to LVH [29], whereas FGF23-induced LVH in patients with CKD might be independent of blood pressure, indicating differences in the cause of LVH between patients with cardiorenal syndrome and those without primary kidney damage [30]. Additionally, LVH was reported to contribute independently to elevations in FGF23 levels [31]. In the setting of experimental myocardial infarction in mice, Andhrukova et al. [32] found that circulating FGF23 levels were increased with a concomitant reduction in the level of 1,25(OH)2-vitamin D3 and that myocardial FGF23 mRNA and protein levels were increased, suggesting that the observed increase in circulating FGF23 levels after myocardial infarction was at least partially derived from the myocardium itself [31]. In fact, a recent review on FGF23 [33] has concluded hypothesis on mono-directional effect that external factors stimulate bone FGF23 release, which in turn induces myocardial damage is challenging. Furthermore, Grabner et al. [34] revealed that specific FGF receptor 4 (FGFR4) inhibition attenuated the established LVH in the rat 5/6 nephrectomy model of CKD and demonstrated that aging mice lacking FGFR4 were protected from LVH. These lines of evidence suggest that FGF23-induced cardiac hypertrophy is reversible in vitro and in vivo, following the removal of hypertrophic stimulus, and indicate that pharmacological interference with cardiac FGF23/FGFR4 signaling might provide protection from CKD- and age-related LVH [35]. Indeed, Leifheit-Nestler et al. [36] reported that vitamin D treatment attenuated cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. However, a reduction of FGF23 as a therapeutic option in kidney or cardiac disease is currently not appealing since the potential chronic effects of FGF23 inhibition are unpredictable in conditions with reactive, secondary FGF23 elevation [33].
The current study also revealed a relationship between serum FGF23 level and hemodialysis efficacy. The results of the limited number of studies to date have evaluated that the relationship between dialysis efficacy and FGF23 supports the current study findings. Zaritsky et al. [37] reported that short daily hemodialysis sessions were associated with lower plasma FGF23 levels compared with conventional hemodialysis, whereas Hacıhamdioglu et al. [38] reported that FGF23 levels were associated with effective dialysis in children on peritoneal dialysis. Together with our findings, these data suggest that more effective dialysis might lower FGF23. Additionally, we have revealed a relationship between high UFR levels and high FGF23 levels. Recent studies suggest a role for FGF23 in volume regulation. Andrukhova et al. [39] showed that FGF23 increased the membrane abundance and phosphorylation of Na+–Cl− cotransporter and induced Na+ uptake in distal renal tubules in vivo and in vitro using recombinant FGF23-administered mice; the authors concluded that FGF23 was a key regulator of renal Na+ reabsorption and plasma volume. One study [40] found that elevated FGF23 correlated with hypervolemia in patients on hemodialysis, although another study [41] reported that FGF23, albeit correlating with volume status in patients on hemodialysis, was not reduced by the hemodialysis.
We performed additional univariate analysis to compare parameters associated with FGF23 based on the presence of CVD and found that cCa, iP, iPTH, and TAC-urea were associated with FGF23 in patients with CVD but not in those without CVD. One potential reason for these observed differences might be the variations in baseline patient characteristics between the two groups. Specifically, TAC-urea levels were significantly lower, and serum iP levels were significantly higher in patients with CVD in the present study. Vascular calcification in patients on dialysis was reported to be associated with FGF23 [16,17,18,19], whereas FGF23 was shown to decline after 3 h of hemodialysis [42]; these findings suggest that lowering circulating FGF23 levels by increasing dialysis efficacy might prevent or lower CVD events in patients on dialysis. Conversely, several studies [43,44,45] reported that there was no evident association between vascular calcification and FGF23, and there is currently no direct evidence supporting a causal relationship between FGF23 and cardiovascular events [26,27,28]. Therefore, the current study findings do not conclusively show that increasing dialysis efficacy and restricting the control of CKD-mineral bone disease might reduce circulating FGF23 levels in patients with CVD. As studies on dialysis patients are few today, further investigation is warranted.
Conclusions
FGF23 levels, which were extremely higher than the normal range in patients on hemodialysis, were associated not only with mineral bone disease-associated factors but also with UFR. Additionally, increased dialysis efficacy might be associated with lower FGF23 in patients with CVD.
References
Liu S, Quarles LD (2007) How fibroblast growth factor 23 works. J Am Soc Nephrol 18:1637–1647
Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79:1370–1378
Hu MC, Shiizaki K, Kuro-o M, Moe OW (2013) Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75:503–533
Anandh U, Mandavkar P, Das B, Rao S (2017) Fibroblast growth factor-23 levels in maintenance hemodialysis patients in India. Indian J Nephrol 27(1):9–12
Bi S, Liang Y, Cheng L, Wang Y, Han Q, Zhang A (2017) Hemodialysis is associated with higher serum FGF23 level when compared with peritoneal dialysis. Int Urol Nephrol 49:1653–1659
Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y (2004) FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int 65:1943–1946
Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M (2008) Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359(6):584–592
Artunc F, Nowak A, Müller C, Peter A, Heyne N, Häring HU, Friedrich B (2014) Mortality prediction using modern peptide biomarkers in hemodialysis patients—a comparative analysis. Kidney Blood Press Res 39:563–572
Scialla JJ, Parekh RS, Eustace JA, Astor BC, Plantinga L, Jaar BG, Shafi T, Coresh J, Powe NR, Melamed ML (2015) Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol 42:25–34
Deo R, Katz R, de Boer IH, Sotoodehnia N, Kestenbaum B, Mukamal KJ, Chonchol M, Sarnak MJ, Siscovick D, Shlipak MG, Ix JH (2015) Fibroblast growth factor 23 and sudden versus non-sudden cardiac death: the cardiovascular health study. Am J Kidney Dis 66(1):40–46
Yang H, Luo H, Tang X, Zeng X, Yu Y, Ma L, Fu P (2016) Prognostic value of FGF23 among patients with end-stage renal disease: a systematic review and meta-analysis. Biomark Med 10(5):547–556
Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, Investigators HOST (2011) FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22:1913–1922
Moldovan D, Moldovan I, Rusu C, Kacso I, Patiu IM, Gherman-Caprioara M (2014) FGF-23, vascular calcification, and cardiovascular diseases in chronic hemodialysis patients. Int Urol Nephrol 46:121–128
Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK (2016) Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol 27:227–237
Kim HJ, Park M, Park HC, Jeong JC, Kim DK, Joo KW, Hwang YH, Yang J, Ahn C, Oh KH (2016) Baseline FGF23 is associated with cardiovascular outcome in incident PD patients. Perit Dial Int 36(1):26–32
Ozkok A, Kekik C, Karahan GE, Sakaci T, Ozel A, Unsal A, Yildiz A (2013) FGF-23 associated with the progression of coronary artery calcification in hemodialysis patients. BMC Nephrol 14:241
Fu X, Cui QQ, Ning JP, Fu SS, Liao XH (2015) High-flux hemodialysis benefits hemodialysis patients by reducing serum FGF-23 levels and reducing vascular calcification. Med Sci Monit 21:3467–3473
Lee YT, Ng HY, Chiu TT, Li LC, Pei SN, Kuo WH, Lee CT (2016) Association of bone-derived biomarkers with vascular calcification in chronic hemodialysis patients. Clin Chim Acta 452:38–43
El Baz TZ, Khamis OA, Ahmed Gheith OA, Abd Ellateif SS, Abdallah AM, Abd El Aal HC (2017) Relation of fibroblast growth factor-23 and cardiovascular calcification in end-stage kidney disease patients on regular hemodialysis. Saudi J Kidney Dis Transpl 28(1):51–60
Hsu HJ, Wu MS (2009) Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci 337(2):116–122
Kirkpantur A, Balci M, Gurbuz OA, Afsar B, Canbakan B, Akdemir R, Ayli MD (2011) Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant 26:1346–1354
Leifheit-Nestler M, Große Siemer R, Flasbart K, Richter B, Kirchhoff F, Ziegler WH, Klintschar M, Becker JU, Erbersdobler A, Aufricht C, Seeman T, Fischer DC, Faul C, Haffner D (2016) Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant 31:1088–1099
Sarmento-Dias M, Santos-Araújo C, Poínhos R, Oliveira B, Silva IS, Silva LS, Sousa MJ, Correia F, Pestana M (2016) Fibroblast growth factor 23 is associated with left ventricular hypertrophy, not with uremic vasculopathy in peritoneal dialysis patients. Clin Nephrol 85(3):135–141
Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87(11):4957–4960
Kovesdy CP, Quarles LD (2016) FGF23 from bench to bedside. Am J Physiol Renal Physiol 310:F1168–F1174
Stubbs JR, Egwuonwu S (2012) Is fibroblast growth factor 23 a harbinger of mortality in CKD? Pediatr Nephrol 27:697–703
Marthi A, Donovan K, Haynes R, Wheeler DC, Baigent C, Rooney CM, Landray MJ, Moe SM, Yang J, Holland L, di Giuseppe R, Bouma-de Krijger A, Mihaylova B, Herrington WG (2018) Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: a meta-analysis. J Am Soc Nephrol 29:2015–2027
Yamashita K, Mizuiri S, Nishizawa Y, Shigemoto K, Doi S, Masaki T (2017) Addition of novel biomarkers for predicting all-cause and cardiovascular mortality in prevalent hemodialysis patients. Ther Apher Dial 22:31–39
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121:4393–4408
Faul C (2012) Fibroblast growth factor 23 and the heart. Curr Opin Nephrol Hypertens 21:369–375
Slavic S, Ford K, Modert M, Becirovic A, Handschuh S, Baierl A, Katica N, Zeitz U, Erben RG, Andrukhova O (2017) Genetic ablation of Fgf23 or klotho does not modulate experimental heart hypertrophy induced by pressure overload. Sci Rep 7(1):11298
Andrukhova O, Slavic S, Odorfer KI, Erben RG (2015) Experimental myocardial infarction upregulates circulating fibroblast growth factor-23. J BoneMiner Res 30:1831–1839
Robert Stöhr R, Schuh A, Heine GH, Brandenburg V (2018) FGF23 in cardiovascular disease: innocent bystander or active mediator? Front Endocrinol (Lausane) 9:351
Grabner A, Schramm K, Silswal N, Hendrix M, Yanucil C, Czaya B, Singh S, Wolf M, Hermann S, Stypmann J, Di Marco GS, Brand M, Wacker MJ, Faul C (2017) FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci Rep 7(1):1993
Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstädt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C (2015) Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 22:1020–1032
Leifheit-Nestler M, Grabner A, Hermann L, Richter B, Schmitz K, Fischer DC, Yanucil C, Faul C, Haffner D (2017) Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol Dial Transplant 32:1493–1503
Zaritsky J, Rastogi A, Fischmann G, Yan J, Kleinman K, Chow G, Gales B, Salusky IB, Wesseling-Perry K (2014) Short daily hemodialysis is associated with lower plasma FGF23 levels when compared with conventional hemodialysis. Nephrol Dial Transplant 29:437–441
Hacıhamdioğlu DÖ, Düzova A, Alehan D, Oğuz B, Beşbaş N (2015) Circulating fibroblast growth factor 23 in children on peritoneal dialysis is associated with effective dialysis. Turk J Pediatr 57(1):9–16
Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG (2014) FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 6:744–759
Unver S, Kavlak E, Gümüsel HK, Celikbilek F, Esertas K, Muftuoglu T, Kirilmaz A (2015) Correlation between hypervolemia, left ventricular hypertrophy and fibroblast growth factor 23 in hemodialysis patients. Ren Fail 37(6):951–956
Humalda JK, Riphagen IJ, Assa S, Hummel YM, Westerhuis R, Vervloet MG, Voors AA, Navis G, Franssen CF, de Borst MH, NIGRAM Consortium (2016) Fibroblast growth factor 23 correlates with volume status in haemodialysis patients and is not reduced by haemodialysis. Nephrol Dial Transplant 31(9):1494–1501
Bielesz BO, Hecking M, Plischke M, Cejka D, Kieweg H, Haas M, Marculescu R, Hörl WH, Bieglmayer C, Sunder-Plassmann G (2014) Correlations and time course of FGF23 and markers of bone metabolism in maintenance hemodialysis patients. Clin Biochem 47:1316–1319
Pencak P, Czerwieńska B, Ficek R, Wyskida K, Kujawa-Szewieczek A, Olszanecka-Glinianowicz M, Więcek A, Chudek J (2013) Calcification of coronary arteries and abdominal aorta in relation to traditional and novel risk factors of atherosclerosis in hemodialysis patients. BMC Nephrol 14:10
Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators (2014) Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25:349–360
Ramirez-Sandoval JC, Casanova I, Villar A, Gomez FE, Cruz C, Correa-Rotter R (2016) Biomarkers associated with vascular calcification in peritoneal dialysis. Perit Dial Int 36(3):262–268
Acknowledgements
We thank the staff of dialysis center in Heisei-Hidaka Clinic for collecting medical records.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nishizawa, Y., Hosoda, Y., Horimoto, A. et al. Fibroblast growth factor 23 (FGF23) level is associated with ultrafiltration rate in patients on hemodialysis. Heart Vessels 36, 414–423 (2021). https://doi.org/10.1007/s00380-020-01704-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-020-01704-y