Abstract
Cobalamin (Vitamin B12) is a cofactor for many enzymes, including those in bacteria, archaea, algae, and mammals. In humans, cobalamin deficiency can lead to pernicious anaemia as well as gastrointestinal and neurological disorders. In contrast to marine ecosystems, there is a great paucity of information on the role of soils and terrestrial plants in the supply of cobalt and cobalamin to microorganisms and animals. The content of cobalt cations in most soils is usually sufficient to maintain growth, and the density of cobalamin-producing soil prokaryotes is high in comparison to water bodies. The cobalt content of most soils is usually sufficient in comparison with water, and the density of cobalamin-producing soil prokaryotes is high. Therefore, terrestrial plants are an important cobalt source for cobalamin-producing rumen and gut prokaryotes. The major source of cobalamin for most other animals is the meat of ruminants as well as other animal-derived products, bacteria in insects, and coprophagy, e.g., by rodents. In addition, faecal deposits, and fertilizers as well as soil bacteria add to the cobalamin supply. However, those archaea and bacteria that do not produce cobalamin obtain this coenzyme or its analogues from the environment. Therefore, presence or absence of cobalamin-producing species in soil affects the whole soil microbiome. However, our knowledge concerning microbial producers and consumers of cobalamin in soils is still limited, despite some recent advances. The main reasons are a low cobalamin content in soils and challenging methods of determination. In this regard, advanced analytical knowledge and technical equipment are required, which are usually unavailable in soil laboratories. This review provides relevant methodological information on sample homogenization, extraction, concentration, and purification as well as analysis of cobalamin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cobalamin (vitamin B12) is a cofactor for many enzymes, having a critical function in some microorganisms and all animals (Roth et al. 1993). In humans, cobalamin deficiency can result in, e.g., pernicious anaemia, gastrointestinal, and neurological disorders (Rowley and Kendall 2019). Pure vegetarian and in particular vegan diets may cause cobalamin deficiency, due to lack of its intake (Donaldson 2000; Watanabe et al. 2014). In addition, the elderly and persons with gastrointestinal diseases are among the risk groups, as they have limited production of specific transporting proteins, which mediate the intestinal absorption of cobalamin and its delivery to the tissues (Rowley and Kendall 2019).
Cobalamin belongs to a group of similar water-soluble coordination complexes of cobalt cations (usually Co3+ and Co2+), which occupies the centre of a heterocyclic corrin ring (Fig. 1). These complexes are similar to the tetrapyrrole chlorin ring of chlorophyl (photosynthesis) and the porphyrin ring of haem (oxygen transport). In bacteria, all tetrapyrrole compounds derive from δ-amino-levulinate and exhibit complex inter-relationships in numerous species (Yin and Bauer 2013). The cobalamin corrin complex is further bound to a benzimidazole lower ligand and an upper group, which can be represented by a 5'-deoxyadenosyl-methyl and hydroxy group in nature or by a cyano group in manufacturing processes (Roth et al. 1993; Fang et al. 2017). The different upper ligands do not affect cobalamin functioning. In contrast, the replacement of the lower ligand dimethyl-benzimidazole (DMB) by other ligands results in cobalamin analogues, called cobamides or corrinoids (Hallberg et al. 2022). These analogues are inactive in animals but serve as coenzymes in different microorganisms. The de novo biosynthesis of cobalamin alternatively occurs in aerobic or anaerobic pathways, solely carried out by bacteria and archaea. Some prokaryotes can also synthesize cobalamin by absorbing and remodelling other corrinoids via a salvage pathway (Fang et al. 2017).
General form of cobalamin analogues according to Heal et al. (2017); shown are a schematic of the conserved corrin ring with various upper (β) and DMB as lower (α) ligand for cobalamin and adenine as examples for pseudocobalamin
In marine ecosystems intensive research has been carried out over the last decades to investigate the symbiosis of algae and bacteria (Bunbury et al. 2022; Helliwell et al. 2016), e.g., for elucidating the cobalamin content of fish (Watanabe et al. 2013; Watanabe and Bito 2018). In contrast, there is a great paucity of information on the role of soils and terrestrial plants in the supply of cobalt and cobalamin to microorganisms and animals. This is astonishing, considering (1) that the Co content of most soils is often sufficient (Srivastava et al. 2022), particularly in comparison with water, (2) that the density of cobalamin-producing soil prokaryotes is high (Hallberg et al. 2022), and (3) that terrestrial plants are an important source of Co for cobalamin-producing rumen and gut prokaryotes (Morton 1986; Paterson et al. 1991).
It is certainly possible to supply cobalamin as a pharmaceutical dietary supplement to humans in many countries, but the majority still rely on cobalamin uptake from their food. Main cobalamin sources for most carnivores and omnivores (including humans) are meat and other animal-derived products, e.g., eggs, milk, and other dairy products (Combs and McClung 2022). The richest sources are liver and kidney. Consequently, it is still important to have information on the dietary cobalamin supply to avoid deficiency due to a low meat or vegan diet (Donaldson 2000; Watanabe et al. 2014), sometimes called “hidden hunger” on a global scale (Titcomb and Tanumihardjo 2019). The central objective of the current review is to highlight the importance of Co compounds and microorganisms in soil for cobalamin turnover in terrestrial ecosystems. The diversity of cobalamin compounds and its analogues leads to considerable analytical challenges, highlighting the importance of strengthening cooperation between food chemistry, soil biochemistry, and soil microbiology.
Cobalt and cobalamin in soil
Co is a ferromagnetic transition metal with the atomic number 27 and a high density of 8.9 g cm−3. The only stable isotope is 59Co and a variety of radioisotopes exist, 60Co being the most important, which is used as a tracer and source of high-energy γ-rays. In the biochemical context, the salts of Co2+ and Co3+ are much more relevant than oxides, although the oxidation grades of Co can range from Co3− to Co5+. The Co content of environments has been considerably increased over the last decades, due to the growing industrial demand (Kosiorek and Wyszkowski 2019; Srivastava et al. 2022), accompanied by emissions from coal and oil burning (Biswas et al. 2013; Srivastava et al. 2022). Co is primarily used in rechargeable lithium-ion accumulators and in magnetic, wear-resistant, and high-strength alloys. Traditionally, Co aluminate gives a distinctive deep blue colour to many products, e.g., paints, glasses, and ceramics.
The Co content of soils is often low but, especially in loamy soils, it is usually high enough to supply sufficient quantities of Co cations to arable crops and grassland vegetation (Linhares et al. 2019; Srivastava et al. 2022). Consequently, Co has not been regularly measured in investigations on trace metal effects on soil microorganisms (Chander et al. 2001). For this reason, a great paucity of information exists on Co in soils (Srivastava et al. 2022), despite their importance as a primary source of Co cations for most cobalamin-producing bacteria and archaea.
Soils with Co contents < 5 µg g−1 usually provide a grassland vegetation with Co concentrations < 0.1 µg g−1 DW, which might be the reason for Co insufficiency in the gastro-intestinal microbiome of herbivores (Linhares et al. 2019). Soils low in Co contents are mainly developed from acidic igneous rocks (Table 1), e.g., granite and rhyolite, from sedimentary rocks, e.g., sandstone, or from metamorphic rocks, e.g., quartzite and gneiss (McGrath and Fleming 2007; Srivastava et al. 2022; Tyler 2004). Peat soils are also inherently low in cobalt (Stepanova et al. 2015). The same is true for organic forest floor layers, with a median of 1.1 µg g−1 soil in Norway (Nygård et al. 2012), due to the low Co uptake of trees. In addition, coniferous trees cause podsolization, which transfers Co by leaching from the A horizon to the iron-rich B horizon. Many sandy and acidic soils, already inherently low in cobalt, are further depleted by podsolization (McGrath and Fleming 2007).
Many soils have high Co contents but low availability to pasture plants (McGrath and Fleming 2007). High soil pH in combination with high contents of carbonate (McGrath and Fleming 2007; Srivastava et al. 2022) and Mn oxides (Li et al. 2004; McGrath and Fleming 2007) lower the soil Co availability to plants (Collins and Kinsela 2011). Different soil fractions, namely the soluble and exchangeable, the organically bound, the oxide bound pools, etc., dynamically regulate soil Co availability (Srivastava et al. 2022). Co2+ dominates in soils and exhibits a higher solubility and stability in soil as well as a higher bioavailability to microorganisms and plants than Co3+, which is formed by surface oxidation of Co2+ on oxy-hydroxide minerals (Medyńska-Juraszek et al. 2020; Srivastava et al. 2022; Wendling et al. 2009). The background Co content of soils can increase into toxic ranges of > 40 µg g−1 soil by dust deposition (Lison 2015), especially in mining areas (Narendrula et al. 2012), by sewage sludge application (Zupančič and Skobe 2014), by rock phosphate addition (Saaltink et al. 2014), and by using Co containing pesticides (Defarge et al. 2018). However, no published data exist for Co monitoring schemes to validate the effects of these numerous anthropogenic sources.
The information on soil Co contents is usually limited (Srivastava et al. 2022), especially in comparison with other trace metals. This is even more true for cobalamin, although the majority of cobalamin-producing bacterial and archaeal species live in soil. In one field experiment on a silt loam, Mozafar (1994) measured a mean cobalamin content of 9.5 ng g−1 soil in 4 treatments, ranging from 5 to 14 ng g−1 soil. In 40 soil samples from different environments, Lu et al. (2020) found an average of 1.6 ng cobalamin g−1 soil, ranging from 0.08 to 9.3 ng g−1 soil. Approximately 10% of total cobalamin have been found to be water leachable (Lu et al. 2020), i.e., bound to soil organic matter. In contrast, alumina, kaolinite, and sand caused only a low retardation factor, which is the ratio of groundwater velocity to solute velocity, in column studies with addition of free cobalamin (Hashsham and Freedman 2003). Overall, there is a serious lack in knowledge on soil cobalamin.
Cobalt and cobalamin in plants
Plant Co concentrations vary from virtually zero if grown in Co deficient soils, and up to 10.2 mg Co g−1 DW in native plant species, grown on Cu and Co mining dumps in the Katanga Province, Congo (Li et al. 2004). Despite a long research history, plant uptake of Co from soils is far from being resolved (Arif et al. 2016; Banerjee and Bhattacharya 2021). Several soil characteristics are important for the Co concentration in plants, for example pH, clay minerals, soil organic matter, rhizosphere microbiome, especially mycorrhiza, and redox conditions, e.g., in the presence of Mn or Fe oxides (Collins and Kinsela 2011; Srivastava et al. 2022; Wendling et al. 2009). Antagonistic relationships exist between Co and Mn (Li et al. 2004), Co and Fe (Gad et al. 2013), Co and Zn (Huwait et al. 2015) as well as Co and Ni (He et al. 2015). Co is primarily accumulated in plant roots, before being translocated and distributed to other plant parts (Bakkaus et al. 2005; Ilunga Kabeya et al. 2018; Young 1979). Plants might control their Co uptake by roots to a certain degree, but the specific accumulation and translocation mechanisms as well as transporter systems of cobalt inside plants are still largely unknown (Banerjee and Bhattacharya 2021).
Co regulates various developmental and metabolic aspects of plants, namely stress management and enzyme activation as well as N2 fixation in legumes (Banerjee and Bhattacharya 2021; Hu et al. 2021). Co helps for example to regulate coleoptile elongation, leaf expansion, and bud development (Banerjee and Bhattacharya 2021; Kandil 2007). Co seems also to activate various enzymes and co-enzymes in the synthesis of photosynthetic pigments, amino acids, and alkaloids (Banerjee and Bhattacharya 2021; Basu 2011; Minz et al. 2018). Consequently, application of Co fertilizers up to 50 µg g−1 soil significantly improved yield parameters of crops (Jaleel et al. 2009; Gad et al. 2013). However, Co has still not been classified as an essential nutrient element (Banerjee and Bhattacharya 2021; Iram et al. 2017; Lwalaba et al. 2020).
In contrast to animals, plants do not require cobalamin, because their biochemical reactions, such as methionine synthesis, use cobalamin-independent enzymes. However, plants can take up cobalamin from concentrated nutrient solutions (Bito et al. 2013; Mozafar and Oertli 1992; Oh et al. 2021). Under hydroponic greenhouse conditions, cobalamin uptake by soybean (Glycine max (L.) Merr.) roots and xylem transfer to leaves was a linear function over an extremely high range from 10 to 3200 nmol mL−1 in the nutrient solution (Mozafar and Oertli 1992). Their results were confirmed by Bito et al. (2013), who observed that lettuce (Lactuca sativa L.) leaves, grown in hydroponic culture with various concentrations of cyanocobalamin, increased its concentration from non-detectable to 165 ± 75 ng g−1 fresh weight. Cobalamin was not only found in the vegetative parts of horticultural crops, but also in generative parts such as soybean and barley seeds (Mozafar 1994). However, it remains unclear to what extent plants can take up cobalamin from the soil, because its usual concentration is by several orders of magnitude lower (Mozafar 1994). In addition, the ligand is most likely bound to microbial proteins, which might constrain its uptake. Yet, there are indications that some cobalamin is transferred from soil to plants (Mozafar 1994). However, it remains unknown whether cobalamin is accumulated in its free form or due to a microbial invasion into the plants.
Keshavarz and Moghadam (2017) detected that priming of common been seeds with cobalamin provided significant protection against salinity stress in comparison with non-treated plants. They suggested that cobalamin might stimulate the antioxidant system of plants and increase their resistance to salinity. This result indicates that cobalamin apparently might have further unknown physiological functions in plant cells. The transfer of cobalamin into the plant cells, e.g., by a membrane shifting transport in micro-vesicles as observed for bacterial extracellular enzymes, is still unknow (Kikuchi et al. 2022). It has been reported that edible plants, grown on fermented poultry manure organic fertilizer products, were enriched with cobalamin (Katsura et al. 2021). Consequently, crops grown on fields amended with organic fertilizers might contain higher cobalamin concentrations than those supplied with inorganic fertilizers.
Cobalt and Cobalamin in algae
Mean Co concentrations of marine water vary between 10 and 30 pg Co mL−1 water in different areas of the Atlantic and Pacific Ocean (Robertson et al. 1970), whereas coastal regions often contained slightly higher concentrations. Co assimilation by phytoplankton and marine organisms cannot explain the observed variations, although micro-algae have a strong ability to accumulate Co in their cells (Coleman et al. 1971). A typical Co concentration of marine algae was 100 ng g−1 dry weight (Robertson et al. 1970). Cobalt is a limiting micronutrient for algae not only in saline marine water but also in freshwater (Bertrand et al. 2015; Bundy et al. 2020; Noble et al. 2017). Photosynthetic algae often provide many heterotrophic bacteria with Co and assimilates in their phycosphere, a region closely connected to the algae cell surface, which seems to be analogous to the rhizosphere (Bunbury et al. 2022; Kimbrel et al. 2019; Seymour et al. 2017).
In contrast to plants, cobalamin is needed by over 50% of all micro-algae species as an external source for growth (Croft et al. 2005). However, low Co concentrations in water limit cobalamin formation, leading to co-limitation of algae living in symbiosis with prokaryotes (Watanabe and Bito 2018). Examples for this symbiosis are the unicellular green micro-algae Lobomonas rostrata (Helliwell et al. 2018) or Chlamydomonas reinhardtii (Bunbury et al. 2022), which are often used as model organisms in laboratory studies to investigate algae-prokaryote interactions. High concentrations of cobalamin, its analogues, or both groups of corrinoids were found in marine red macro-algae Porphyra sp. and in sweet-water green micro-algae Chlorella sp. (Watanabe and Bito 2018), but particularly in cyanobacterial (blue-green algae) Spirulina sp. (Watanabe et al. 1999). Consequently, feeding on these algae is the basis for the potentially high cobalamin content of fish and shellfish (Watanabe and Bito 2018).
Micro-algae species that do not obligatory require cobalamin for growth possess alternative, cobalamin-independent enzymes (Helliwell et al. 2018). However, even in these organisms, cobalamin accumulates in their cells and is used as a cofactor of cobalamin-dependent methionine synthase (Watanabe and Bito 2018).
Cobalt and cobalamin in animal rumen, gut, and faeces
High Co concentrations in air, water, and soil are toxic for animals, especially mammals and humans (Lison 2015). Less is known on the Co requirements or Co toxicity of invertebrates (Bouguerra et al. 2019; Gál et al. 2008; He et al. 2015). As bacteria of the insect digestive system can be expected as a source of cobalamin and particularly cobalamin analogues (Okamoto et al. 2021; Schmidt et al. 2019), the leftovers of insects and other soil invertebrates are natural fertilizers that would require more consideration.
Small amounts of Co-feed intake by animals support the formation of cobalt proteins that bind Co cations directly (Kobayashi and Shimizu 1999). However, most animals (except for herbivores) have not only to ingest further Co salts but also cobalamin, as their metabolism is unable to form this vitamin. This is a special problem for mammals because cobalamin-producing bacteria and archaea live solely in the colon of most species. In contrast, cobalamin is absorbed earlier in the ileum, the last part of the small intestine, due to a cobalamin-binding protein as an intrinsic factor (IF) produced in the stomach (Alpers and Russell-Jones 2013). In ruminants, the microbiological synthesis of cobalamin occurs in the forestomaches, whereupon the cobalamin-containing mass proceeds to the ileum, where the protein-carriers are digested, and the ligand absorbed (Wei et al. 2021). However, herbivore ruminants require a sufficient Co supply by the pasture vegetation for cobalamin production (Smith 1990; Waterman et al. 2017) to avoid fatal Co deficiency (Klessa et al. 1989).
In particular, the colon but also the caecum of mammals contains a large microbiome able to produce cobalamin (Danchin and Braham 2017), so that faeces contain considerable cobalamin concentrations (Hallberg et al. 2022). For this reason, coprophagy is an important behaviour to supply cobalamin (Rosenberg and Zilber-Rosenberg 2016). Especially rodents and lagomorphs, such as rabbit pups, exhibit extensive coprophagy, called caecotrophy, based on special faecal pellets formed during the night in the caecum (Combes et al. 2014). Coprophagy is also performed to a certain extent by piglets, foals, and dogs (Danchin and Braham 2017). Coprophagy of non-human primates in the zoo has been often thought to be an abnormal behaviour (Jacobson et al. 2016), although it is normal in the wild (Sakamaki 2010). Parasite propagation (Walsh et al. 2013) is an important reason for the coprophobic behaviour of humans, who can receive sufficient cobalamin by consuming meat and other animal-derived products (Combs and McClung 2022; Danchin and Braham 2017).
As faeces of some animals contains reasonably high concentrations of cobalamin (Mozafar 1994), faecal fertilizers, such as cow dung (Mozafar 1994) or poultry manure (Katsura et al. 2021) as well as sewage sludge (Hoover et al. 1951) supply cobalamin to the soil, which can be partly taken up by crops (Mozafar 1994). Aerosols created above faecal contaminated land surfaces might contribute to the cobalamin supply of animals and humans to an unknown extent (Grzyb and Pawlak 2021; Islam et al. 2019). However, soil eating did not improve the cobalamin status of humans in a rural area with strong anaemia prevalence (Karaoglu et al. 2010), although often recommended as a cobalamin source on the internet.
Production of cobalamin by soil and rhizosphere bacteria
Soil bacteria are usually used in biotechnology to produce cobalamin as a dietary supplement (Fang et al. 2017; Stahmann 2019). Important bacterial species for this purpose are Pseudomonas denitrificans (Gram-negative, γ-Proteobacteria), Proprionibacterium shermanii, also known as P. freudenreichii (Gram-positive, Actinobacteria, Swiss cheese production), and Sinorhizobium meliloti (Gram-negative, α-Proteobacteria, symbiotic N2 fixation), also known as Rhizobium meliloti or Ensifer meliloti (Balabanova et al. 2021; Bunbury et al. 2022; Fang et al. 2017). In contrast to these soil bacteria, the abundant marine cyanobacterium Synechococcus synthesizes only pseudo-cobalamin (Helliwell et al. 2016).
Soils harbour a large microbial biomass (Khan et al. 2016; Wardle 1998) and a highly diverse microbial community (Bastida et al. 2021; Hartmann et al. 2015). Most soil biogeochemical processes are mediated by microorganisms (Nannipieri et al. 2003) and the sustainability of soils relies on microbial communities that mediate the nutrient supply to the vegetation (Bier et al. 2015; Geisseler et al. 2010). Consequently, knowledge on factors, such as cobalamin, that control microbial diversity, activity, and physiology may help to understand their biogeochemical functions (Lu et al. 2020), especially in the rhizosphere (Wallner et al. 2022; Yasuda et al. 2022). The importance of cobalamin-producing soil bacteria and archaea suggests that soils play an important role in governing cobalamin supply to the many microorganisms that do not produce but need this coenzyme. However, the knowledge on microbial producers and consumers of cobalamin in soils is still limited, despite recent advances (Hallberg et al. 2022; Lu et al. 2020).
Cobalamin is predominantly produced in soils by microorganisms belonging to the bacterial phyla Proteobacteria and Actinobacteria, as stated above, but also to the phyla Firmicutes (Gram-positive) and Nitrospirae (Gram-negative) as well as to the archaeal phylum Thaumarchaeota (Lu et al. 2020). Production and remodelling of cobalamin and other corrinoids are key functions of soil prokaryotes that shape soil microbial communities and control soil biogeochemistry (Hallberg et al. 2022; Lu et al. 2020). However, less than 10% of bacterial and archaeal species possess the genetic potential for cobalamin synthesis, as demonstrated by metagenomic analysis (Lu et al. 2020). Consequently, cobalamin must be shared in microbial communities because most organisms that use cobalamin lack the ability of its de novo synthesis. One possibility is the transfer of cobalamin via an ATP-binding cassette transport system (Fang et al. 2017), but other largely unknown exchange mechanisms might exist.
Cobalamin in fungi
Fungi and plants were deemed devoid of cobalamin. Orłowska et al. (2021) demonstrated that all non-Dikarya fungal lineages utilize cobalamin, which is supported by the genomic presence of enzymes, which modify and depend on cobalamin similar to those found in animal homologs. Cobalamin usage was probably lost in Mucoromycotina at the base of Dikarya evolution. Only Glomeromycotina (part of Glomeromycota) and Blastocladiomycota (formerly part of Chytridiomycota) have a complete genomic presence of cobalamin-dependent pathways. However, the source of cobalamin in these non-Dikarya fungi is still unknown; it is most likely of bacterial origin, e.g., endo-hyphal bacteria. All components required for the cobalamin de novo synthesis were found in the symbiosis between the arbuscular mycorrhizal fungi (AMF) Gigaspora margarita (Glomeromycotina) and the β-Proteobacterium Candidatus Glomeribacter gigasporarum (Ghignone et al. 2012; Venice et al. 2020).
More information exists on the cobalamin concentration of fungal sporocarps in comparison to plants, see Table 2 and references (Mattila et al. 2001; Rózsa et al. 2019; Turło et al. 2008). Thus, Rózsa et al. (2019) measured up to 9060 µg cobalamin g−1 DW under optimal cultivation conditions. However, in most cases, the cobalamin concentration of commercially available mushrooms varied around 10 µg g−1 dry weight (Mattila et al. 2001; Watanabe et al. 2012), depending on the cultivation conditions and sporocarp part (Koyyalamudi et al. 2009). Especially secondary decomposer fungi, such as Agaricus bisporus or A. blazei, which are cultivated on horse or chicken manure compost (Mattila et al. 2001; Watanabe et al. 2012), contain high cobalamin concentrations. Also, primary decomposer cultivated on bed logs, such as the shiitake mushroom Lentulina edodes, contains considerable cobalamin concentrations (Bito et al. 2014). In contrast, wood decomposing and ecto-mycorrhizal fungi sampled in the forest exhibit often relatively low cobalamin concentrations (Watanabe et al. 2012).
In contrast to sporocarps of edible mushrooms, nothing is known on the cobalt and cobalamin content of fungal hyphae, although fungi generally dominate the soil microbial biomass (Joergensen and Wichern 2008; Khan et al. 2016). In addition, AMF and ectomycorrhizal fungi control Co uptake and transfer to their host vegetation, and the same might be true for cobalamin.
Methods for cobalamin determination
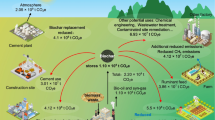
A limited knowledge about the cobalamin content in soils is associated with challenging methods of determination. The methodological approaches can be divided into four steps (Fig. 2): (1) sample homogenization, (2) extraction, (3) concentration and purification, and (4) cobalamin analysis (Nakos 2016).
General flow chart (not drawn to scale) for cobalamin determination according to Nakos (2016): I. Homogenization techniques for a) plant materials and b) soil. II. Extraction followed by III. Purification and concentration. IV. Analysis of cobalamin, where different combinations of each might be applied: a) bacterial growth rate; Ib) TLC = thin layer chromatography, IIb) HPLC = high performance liquid chromatography; c) BSA = bovine serum albumin, IF = intrinsic factor
Sample homogenization
Organic tissue such as plant residues (Bito et al. 2013; Mozafar 1994), insect material (Schmidt et al. 2019), and fungal sporocarps (Bito et al. 2014; Koyyalamudi et al. 2009) were often freeze-dried or shock frozen with liquid N2, followed by a vigorous homogenization, e.g., using Ultra Turrax dispersers. However, in some studies, fresh samples were employed for plant material (Mozafar and Oertli 1992; Katsura et al. 2021) and fungal sporocarps (Watanabe et al. 2012), which were crushed, mixed, and extracted. Mozafar (1994) obtained cobalamin from air-dried and sieved soil (< 1 mm). Lu et al. (2020) measured cobalamin in soils collected from the Canadian MetaMicro-Biome Library (Neufeldt et al. 2011), the Charitable Research Reserve (Lu et al. 2017), and the Craibstone pH plots (Kemp et al. 1992). However, Lu et al. (2017) did not give information on the storage conditions and the sample pre-treatment.
Extraction of organic tissues
Sample dry weight for cobalamin extraction varied between 2 g for plant tissue (Bito et al. 2013) and insect samples (Schmidt et al. 2019), 5 g for fungal sporocarps (Bito et al. 2014; Koyyalamudi et al. 2009; Watanabe et al. 2012), and 10 g for fermented poultry manure (Katsura et al. 2021). Extraction was usually conducted in a sodium acetate buffer with a concentration of 5 mM (Katsura et al. 2021), 50 mM (Schmidt et al. 2019) or 57 mM (Bito et al. 2014; Watanabe et al. 2012) at pH 4.0 (Schmidt et al. 2019), pH 4.5 (Katsura et al. 2021, or pH 4.8 (Bito et al. 2013). The extractants often contained low concentrations of KCN (Bito et al. 2013, 2014; Mozafar and Oertli 1992; Watanabe et al. 2014) or NaCN (Koyyalamudi et al. 2009; Nakos 2016) to substitute the various upper ligands of cobalamin by cyano-cobalamin. In one case, cobalamin was extracted from fungal mycelium with 80% aqueous isopropanol containing 1% Na2S2O5 (w/v) at pH 5.5 (Turło et al. 2008). Under these conditions aquo-cobalamin is converted into a reasonably more stable sulfito-cobalamin, while adenosyl, methyl and cyano-forms would remain unchanged, at least without a decisive illumination of the sample (Pratt 1972).
The extraction was often carried out at 100 °C (Bito et al. 2013, 2014; Schmidt et al. 2019; Watanabe et al. 2012), sometimes followed by an autoclaving step to release protein-bound cobalamin (Bito et al. 2013, 2014; Mozafar and Oertli 1992; Watanabe et al. 2012). For this reason, 1 g of pepsin and 0.25 g of Taka-diastase were added in one study (Schmidt et al. 2019). The extraction was sometimes carried out under N2 stream and in the dark or strong light protection (Schmidt et al. 2019).
Extraction of soil
In an early attempt (Mozafar and Oertli 1992; Mozafar 1994), 1 g soil was extracted with 50 mL of a buffer, containing 400 mM acetate and 3 mM KCN, for 10 min at room temperature, followed by autoclaving for 30 min.
Recently, Lu et al. (2020) analyzed several soil samples following the cobalamin extraction procedure of Heal et al. (2017). They described their method in the supplementary material but did not explicitly mention soil samples. Most likely, Lu et al. (2020) used the organic solvent extraction protocol for environmental samples (Heal et al. 2017). This was based on approaches of Rabinowitz and Kimball (2007) and Kido Soule et al. (2015), paired with physical bead beating under strict light protection. In brief, Nylon membrane filters (0.2 µm) were placed into tubes containing 100 and 400 μm beads of equal volume, before 1 mL of a cold (–20 °C) acidic acetonitrile / methanol / water mixture (40 / 40 / 20 with 0.1% formic acid) was added according to Rabinowitz and Kimball (2007). Then, the samples were bead beaten for 40 s three times during a 20 min period and kept at –20 °C when possible. After centrifugation at 5000 g and supernatant removal, the filter was rinsed once with the 40 / 40 / 20 solvent and twice with methanol, followed by centrifuging after each step. The combined supernatants and rinses were dried under N2 or vacuum with less than 40 °C heat.
Purification and concentration
Cobalamin containing extracts were often purified and concentrated with Sep-Pak Plus C18 cartridges (Waters) (Bito et al. 2013, 2014; Katsura et al. 2021), which consist of a silica-bonded hydrocarbon chains with a high affinity for most hydrophobic analytes present in aqueous solutions. In one case, Turło et al. (2008) passed the samples through an activated aluminium oxide column, washed with sulfuric acid solution of approximately pH 4. For high-performance liquid chromatography (HPLC) and ultra-HPLC analysis (Bito et al. 2013; 2014; Katsura et al. 2021; Schmidt et al. 2019), the cobalamin containing filtrates were passed through an EASI-EXTRACT-Immunoaffinity Vitamin B12 Column (P80, R-Biopharm) and subsequently purified with different solvents. This column contains a gel suspension with monoclonal antibodies specific for cyano-cobalamin.
Analysis of cobalamin
Bunbury et al. (2022) quantified cobalamin by measuring the growth response of Salmonella typhimurium (AR3612) at an optical density at 600 nm (Raux et al. 1996). This optical density was compared with a standard curve of cultures grown on standardized cobalamin concentrations. A similar method was applied by Katsura et al. (2021), just using the growth response of cobalamin-dependent Lactobacillus leichmannii ATCC 7830. However, this method is not suitable, as L. leichmanii can use pseudo-vitamin B12 as well (Watanabe et al. 1998, 1999).
A protein-binding assay can be used for the accurate determination of cobalamin (Lau et al. 1965; Mozafar and Oertli 1992; Mozafar 1994; Watanabe et al. 1998), particularly for the correct differentiation from its inactive analogues (Fedosov et al. 2023). Several automated platforms exist for measuring B12 by a competitive binding assay involving the cobalamin-specific protein intrinsic factor and provide high-throughput technology for massive measurements (Ispir et al. 2015), which are widely applicable in medical laboratories.
Bito et al. (2013) and Katsura et al. (2021) performed bioautography of cobalamin compounds according to Tanioka et al. (2008). Bioautography is a technique to isolate active organic molecules on a thin-layer chromatogram (TLC), followed by a biological detection system (Dewanjee et al. 2015). After concentration and purification, 2 µL of the extracts was spotted on a silica gel TLC plate, which was developed with a mixture of 2-propanol / NH4OH (28%) / water (7 / 1 / 2 v/v) in the dark at 25 °C (Bito et al. 2013). After the TLC sheet was dried, the agar-containing basal medium and precultured cobalamin-dependent Escherichia coli 215 were overlaid and then incubated at 37 °C for 12 h, followed by spraying with a methanol solution.
Reversed phase HPLC or ultra HPLC are commonly combined with UV detection, using a large variety of methods. These HPLC systems make it possible to quantify the different forms of cobalamin and pseudo-cobalamin, especially in combination with a triple quadrupole mass spectrometer (MS) (Heal et al. 2017). Sometimes an HPLC–MS/MS system was applied, coupled to a positive electrospray ionization (ESI) detection system (Bito et al. 2013, 2014; Koyyalamudi et al. 2009).
Conclusions
Cobalt, as well as soil bacteria and archaea, are of vital importance for the cobalamin turnover in terrestrial ecosystems, especially in soils of those agricultural land use systems that receive faecal organic fertilizers. The importance of soil bacteria and archaea for cobalamin production suggests that soils play an important role in governing cobalamin supply to many organisms that need this vitamin. The recent advances should encourage more scientists to accept the analytical challenge of measuring cobalt and cobalamin in soil and their interaction with arable but especially horticultural crops.
References
Alpers DH, Russell-Jones G (2013) Gastric intrinsic factor: the gastric and small intestinal stages of cobalamin absorption. A personal journey. Biochimie 95:989–994
Arif N, Yadav V, Singh S, Singh S, Ahmad P, Mishra RK, Sharma S, Tripathi DK, Dubey NK, Chauhan DK (2016) Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front Environ Sci 4:69. https://doi.org/10.3389/fenvs.2016.00069
Bakkaus E, Gouget B, Gallien JP, Khodja H, Carrot F, Morel JL, Collins R (2005) Concentration and distribution of cobalt in higher plants: the use of micro-PIXE spectroscopy. Nucl Instrum Methods Phys Res Sect B 231:350–356. https://doi.org/10.1016/j.nimb.2005.01.082
Balabanova L, Averianova L, Marchenok M, Son O, Tekutyeva L (2021) Microbial and genetic resources for cobalamin (vitamin B12) biosynthesis: from ecosystems to industrial biotechnology. Int J Mol Sci 22:4522. https://doi.org/10.3390/ijms22094522
Banerjee P, Bhattacharya P (2021) Investigating cobalt in soil-plant-animal-human system: dynamics, impact and management. J Soil Sci Plant Nutr 21:2339–2354. https://doi.org/10.1007/s42729-021-00525-w
Bastida F, Eldridge DJ, García C, Png GK, Bardgett RD, Delgado-Baquerizo M (2021) Soil microbial diversity–biomass relationships are driven by soil carbon content across global biome. ISME J 15:2081–2091. https://doi.org/10.1038/s41396-021-00906-0
Basu M (2011) Effect of cobalt, Rhizobium and phosphobacterium inoculation on growth attributes, yield, quality and nutrient uptake of summer groundnut (Arachis hypogeae L.). Am J Exp Agric 1:21–26. https://doi.org/10.9734/AJEA/2011/003
Bertrand EM, McCrow JP, Moustafa A, Zheng H, McQuaid JB, Delmont TO, Post AF, Sipler RE, Spackeen JL, Xu K, Bronk DA, Hutchins DA, Allen AE (2015) Phytoplankton–bacterial interactions mediate micronutrient colimitation at the coastal Antarctic sea ice edge. PNAS 112:9938–9943. https://doi.org/10.1073/pnas.1501615112
Bier RL, Bernhardt ES, Boot CM, Graham EB, Hall EK, Lennon JT, Nemergut DR, Osborne BB, Ruiz-González C, Schimel JP, Waldrop MP, Wallenstein MD (2015) Linking microbial community structure and microbial processes: an empirical and conceptual overview. FEMS Microbiol Ecol 91:fiv113. https://doi.org/10.1093/femsec/fiv113
Biswas S, Dey R, Mukherjee S, Banerjee PC (2013) Bioleaching of nickel and cobalt from lateritic chromite overburden using the culture filtrate of Aspergillus niger. Appl Biochem Biotechnol 170:1547–1559. https://doi.org/10.1007/s12010-013-0289-9
Bito T, Ohishi N, Hatanaka Y, Takenaka S, Nishihara E, Yabuta Y, Watanabe F (2013) Production and characterization of cyanocobalamin-enriched lettuce (Lactuca sativa L.) grown using hydroponics. Int J Agric Food Chem 61:3852–3858. https://doi.org/10.1021/jf305033s
Bito T, Teng F, Ohishi N, Takenaka S, Miyamoto E, Sakuno E, Terashima K, Yabuta Y, Watanabe F (2014) Characterization of vitamin B12 compounds in the fruiting bodies of shiitake mushroom (Lentinula edodes) and bed logs after fruiting of the mushroom. Mycosci 55:462–468. https://doi.org/10.1016/j.myc.2014.01.008
Bouguerra S, Gavina A, da Graça RM, Rocha-Santos T, Ksibi M, Pereira R (2019) Effects of cobalt oxide nanomaterial on plants and soil invertebrates at different levels of biological organization. J Soils Sed 19:3018–3034. https://doi.org/10.1007/s11368-019-02285-8
Bunbury F, Deery E, Sayer AP, Bhardwaj V, Harrison EL, Warren MJ, Smith AG, (2022) Exploring the onset of B12-based mutualisms using a recently evolved Chlamydomonas auxotroph and B12-producing bacteria. Environ Microbiol 24:3134–3147. https://doi.org/10.1111/1462-2920.16035
Bundy RM, Tagliabue A, Hawco NJ, Morton PL, Twining BS, Hatta M, Noble AE, Cape MR, John SG, Cullen JT, Saito MA (2020) Elevated sources of cobalt in the Arctic Ocean. Biogeosci 17:4745–4767. https://doi.org/10.5194/bg-17-4745-2020
Cappuyns V, Mallaerts T (2014) Background values of cobalt in Flemish and European soils. Geol Belg 17:107–114
Cembranel AS, Sampaio SC, Remor MB, Gotardo JT, Dalla Rosa PM (2017) Geochemical background in an Oxisol. Engenharia Agrícola 37:565–573. https://doi.org/10.1590/1809-4430-Eng.Agric.v37n3p565-573/2017
Chander K, Dyckmans J, Joergensen RG, Meyer B, Raubuch M (2001) Different sources of heavy metals and their long-term effects on soil microbial properties. Biol Fertil Soils 34:241–247. https://doi.org/10.1007/s003740100406
Coleman RD, Coleman RL, Rice EL (1971) Zinc and cobalt bioconcentration and toxicity in selected algal species. Bot Gazette 132:102–109. http://www.jstor.org/stable/2474045
Collins RN, Kinsela AS (2011) Pedogenic factors and measurements of the plant uptake of cobalt. Plant Soil 339:499–512. https://doi.org/10.1007/s11104-010-0584-y
Combs Jr GF, McClung JP (2022) The vitamins. Fundamental aspects in nutrition and health, 6th ed. Elsevier, Amsterdam, The Netherlands
Combes S, Gidenne T, Cauquil L, Bouchez O, Fortun-Lamothe L (2014) Coprophagous behavior of rabbit pups affects implantation of cecal microbiota and health status. J Anim Sci 92:652–665. https://doi.org/10.2527/jas.2013-6394
Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438:90–93. https://doi.org/10.1038/nature04056
Danchin A, Braham S (2017) Coenzyme B12 synthesis as a baseline to study metabolite contribution of animal microbiota. Microb Biotechnol 10:688–701. https://doi.org/10.1111/1751-7915.12722
Defarge N, Spiroux de Vendômois J, Séralinia GE (2018) Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol Reports 5:156–163. https://doi.org/10.1016/j.toxrep.2017.12.025
De Temmerman L, Vanongeval L, Boon W, Hoenig M, Geypens M (2003) Heavy metal content of arable soils in Northern Belgium. Water Air Soil Poll 148:61–76. https://doi.org/10.1023/A:1025498629671
Dewanjee S, Gangopadhyay M, Bhattacharya N, Khanra R, Dua TK (2015) Bioautography and its scope in the field of natural product chemistry. J Pharmaceut Anal 5:75–84. https://doi.org/10.1016/j.jpha.2014.06.002
Donaldson MS (2000) Metabolic Vitamin B12 status on a mostly raw vegan diet with follow-up using tablets, nutritional yeast, or probiotic supplements. Ann Nutr Metab 44:229–234. https://doi.org/10.1159/000046689
Fang H, Kang J, Zhang D (2017) Microbial production of vitamin B12: a review and future perspectives. Microb Cell Fact 16:15. https://doi.org/10.1186/s12934-017-0631-y
Fedosov SN, Nexo E, Heegaard CW, Goldin J, Mason JB (2023) Protein binding assays for an accurate differentiation of vitamin B12 from its inactive analogue. A study on edible cricket powder. Food Chem X 19:100824. https://doi.org/10.1016/j.fochx.2023.100824
Gad N, Mohammed AM, Bekbayeva LK (2013) Response of cowpea (Vigna Anguiculata) to cobalt nutrition. ME J Sci Res 14:177–184. https://doi.org/10.5829/idosi.mejsr.2013.14.2.2008
Gál J, Hursthouse A, Tatner P, Stewart F, Welton R (2008) Cobalt and secondary poisoning in the terrestrial food chain: Data review and research gaps to support risk assessment. Environ Int 34:821–838. https://doi.org/10.1016/j.envint.2007.10.006
Geisseler D, Horwath WR, Joergensen RG, Ludwig B (2010) Nitrogen uptake by soil microorganisms – A review. Soil Biol Biochem 42:2058–2067. https://doi.org/10.1016/j.soilbio.2010.08.021
Ghignone S, Salvioli A, Anca I, Lumini E, Ortu G, Petiti L, Cruveiller S, Bianciotto V, Piffanelli P, Lanfranco L, Bonfante P (2012) The genome of the obligate endobacterium of an AM fungus reveals an interphylum network of nutritional interactions. ISME J 6:136–145. https://doi.org/10.1038/ismej.2011.110
Grzyb J, Pawlak K (2021) Staphylococci and fecal bacteria as bioaerosol components in animal housing facilities in the Zoological Garden in Chorzów. Environ Sci Poll Res 28:56615–56627. https://doi.org/10.1007/s11356-021-14594-y
Hallberg ZF, Seth EC, Thevasundaram K, Taga ME (2022) Comparative analysis of corrinoid profiles across host-associated and environmental samples. Biochem 61:2791–2796. https://doi.org/10.1021/acs.biochem.2c00367
Hartmann M, Frey B, Mayer J, Mäder P, Widmer F (2015) Distinct soil microbial diversity under long-term organic and conventional farming. ISME J 9:1177–1194. https://doi.org/10.1038/ismej.2014.210
Hashshama SA, Freedman DL (2003) Adsorption of vitamin B12 to alumina, kaolinite, sand and sandy soil. Water Res 37:3189–3193. https://doi.org/10.1016/S0043-1354(03)00131-3
He E, Baas J, Van Gestel CAM (2015) Interaction between nickel and cobalt toxicity in Enchytraeus crypticus is due to competitive uptake. Environ Toxicol Chem 34:328–337. https://doi.org/10.1002/etc.2802
Heal KR, Qin W, Ribalet F, Bertagnolli AD, Coyote-Maestas W, Hmelo LR, Moffett JW, Devola AH, Armbrust EV, Stahl DA, Ingalls AE (2017) Two distinct pools of B12 analogs reveal community interdependencies in the ocean. PNAS 114:364–369. https://doi.org/10.1073/pnas.1608462114
Helliwell KE, Lawrence AD, Holzer A, Kudahl UJ, Sasso S, Kräutler B, Scanlan DJ, Warren MJ, Smith AG (2016) Cyanobacteria and eukaryotic algae use different chemical variants of vitamin B12. Current Biol 26:999–1008. https://doi.org/10.1016/j.cub.2016.02.041
Helliwell KE, Pandhal J, Cooper MB, Longworth J, Kudahl UJ, Russo DA, Tomsett EV, Bunbury F, Salmon DL, Smirnoff N, Wright PC, Smith AG (2018) Quantitative proteomics of a B12-dependent alga grown in coculture with bacteria reveals metabolic tradeoffs required for mutualism. New Phytol 217:599–612. https://doi.org/10.1111/nph.14832
Hoover SR, Jasewicz LB, Proges N (1951) Vitamin B12 in activated sewage sludge. Science 114:213. https://doi.org/10.1126/science.114.2956.213
Hu X, Wei X, Ling J, Chen J (2021) Cobalt: an essential micronutrient for plant growth? Front Plant Sci 12:768523. https://doi.org/10.3389/fpls.2021.768523
Huwait EA, Kumosani TA, Moselhy SS, Masawi RM, Yaghmour SS (2015) Relationship between soil cobalt and vitamin B12 levels in the liver of livestock in Saudi Arabia: role of competing elements in soils. Afri Health Sci 993–8. https://doi.org/10.4314/ahs.v15i3.38
Ilunga Kabeya F, Pongrac P, Lange B, Faucon MP, van Elteren JT, Šala M, Šelih VS, Vanden Eeckhoudt E, Verbruggen N (2018) Tolerance and accumulation of cobalt in three species of Haumaniastrum and the influence of copper. Environ Exp Bot 149:27–33. https://doi.org/10.1016/j.envexpbot.2018.01.018
Iram A, Awan TH, Tanveer A, Akbar N, Saleem MF, Safdar ME (2017) Optimization of cobalt and nitrogen for improving seed yield, protein content and nitrogen use efficiency in mungbean. J Environ Agric 2:173–179
Islam A, Ikeguchi A, Naide T (2019) Concentrations of aerosol numbers and airborne bacteria, and temperature and relative humidity, and their interrelationships in a tie-stall dairy barn. Animals 9:1023. https://doi.org/10.3390/ani9121023
İspir E, Serdar MA, Ozgurtas T, Gulbahar O, Akın KO, Yesildal F, Kurt İ (2015) Comparison of four automated serum vitamin B12 assays. Clin Chem Lab Med 53:1205–1213. https://doi.org/10.1515/cclm-2014-0843
Jacobson SL, Ross SR, Bloomsmith MA (2016) Characterizing abnormal behavior in a large population of zoo-housed chimpanzees: prevalence and potential influencing factors. PeerJ 4:e2225. 10.7717%2Fpeerj.2225
Jaleel CA, Jayakumar K, Chang-Xing Z, Iqbal M (2009) Low concentration of cobalt increases growth, biochemical constituents, mineral status and yield in Zea mays. J Sci Res 1:128–137. https://doi.org/10.3329/jsr.vlil.1226
Joergensen RG, Wichern F (2008) Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol Biochem 40:2977–2991. https://doi.org/10.1016/j.soilbio.2008.08.017
Kandil H (2007) Effect of cobalt fertilizer on growth, yield and nutrients status of faba bean (Vicia faba L.) plants. J Appl Sci Res 3:867–872
Karaoglu L, Pehlivan E, Egri M, Deprem C, Gunes G, Genc MF (2010) Temel I (2010) The prevalence of nutritional anemia in pregnancy in an east Anatolian province, Turkey. BMC Public Health 10:329. https://doi.org/10.1186/1471-2458-10-329
Katsura H, Koseki K, Bito T, Takenaka S, Watanabe F (2021) Characterization of vitamin B12 compounds in fermented poultry manure fertilizers. Agriculture 11:627. https://doi.org/10.3390/agriculture11070627
Kemp JS, Paterson E, Gammack SM, Cresser MS, Killham K (1992) Leaching of genetically modified Pseudomonas fluorescens through organic soils: influence of temperature, soil pH, and roots. Biol Fertil Soils 13:218–224. https://doi.org/10.1007/BF00340579
Keshavarza H, Moghadam RSG (2017) Seed priming with cobalamin (vitamin B12) provides significant protection against salinity stress in the common bean. Rhizosphere 3:143–149. https://doi.org/10.1016/j.rhisph.2017.04.010
Khan KS, Mack R, Castillo X, Kaiser M, Joergensen RG (2016) Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 271:115–123. https://doi.org/10.1016/j.geoderma.2016.02.019
Kido Soule MC, Longnecker K, Johnson WM, Kujawinski EB (2015) Environmental metabolomics: analytical strategies. Mar Chem 177:374–387. https://doi.org/10.1016/j.marchem.2015.06.029
Kikuchi Y, Toyofuku M, Ichinaka Y, Kiyokawa T, Obana N, Nomura N, Taoka A (2022) Physical properties and shifting of the extracellular membrane vesicles attached to living bacterial cell surfaces. Microbial Spectrum 10:02165–02222. https://doi.org/10.1128/spectrum.02165-22
Kimbrel JA, Samo TJ, Ward C, Nilson D, Thelen MP, Siccardi A, Zimba P, Lane TW, Mayali X (2019) Host selection and stochastic effects influence bacterial community assembly on the microalgal phycosphere. Algal Res 40:101489. https://doi.org/10.1016/j.algal.2019.101489
Klessa DA, Dixon J, Voss RC (1989) Soil and agronomic factors influencing the cobalt content of herbage. Res Dev Agric 6:25–35
Kobayashi M, Shimizu S (1999) Cobalt proteins. Eur J Biochem 261:1–9. https://doi.org/10.1046/j.1432-1327.1999.00186.x
Kosiorek M, Wyszkowski M (2019) Remediation of cobalt-polluted soil after application of selected substances and using oat (Avena sativa L.). Environ Sci Poll Res 26:16762–16780. https://doi.org/10.1007/s11356-019-05052-x
Koyyalamudi SR, Jeong SC, Cho KY, Pang G (2009) Vitamin B12 is the active corrinoid produced in cultivated white button mushrooms (Agaricus bisporus). J Agric Food Chem 57:6327–6333. https://doi.org/10.1021/jf9010966
Lau KM, Gottlieb C, Wasserman LR, Herbert V (1965) Measurement of serum vitamin B12 level using radioisotope dilution and coated charcoal. Blood 26:202–214. https://doi.org/10.1182/blood.V26.2.202.202
Li Z, McLaren RG, Metherell AK (2004) The availability of native and applied soil cobalt to ryegrass in relation to soil cobalt and manganese status and other soil properties. New Zealand J Agric Res 47:33–43. https://doi.org/10.1080/00288233.2004.9513568
Linhares D, Pimentel A, Borges C, Cruz JV, Garcia P, dos Santos RA (2019) Cobalt distribution in the soils of São Miguel Island (Azores): from volcanoes to health effects. Sci Tot Environ 684:715–721. https://doi.org/10.1016/j.scitotenv.2019.05.359
Lison D (2015) Cobalt. Chapter 36. In: Nordberg GF, Fowler BA, Nordberg M (Eds) Handbook on the toxicology of metals, 4th ed. Elsevier, Amsterdam, pp 743–763. https://doi.org/10.1016/B978-0-444-59453-2.00034-2
Lu X, Seuradge BJ, Neufeld JD (2017) Biogeography of soil Thaumarchaeota in relation to soil depth and land usage. FEMS Microbiol Ecol 93:fiw246. https://doi.org/10.0000/0003/4914/6003
Lu X, Heal KR, Ingalls AE, Doxey AC, Neufeld JD (2020) Metagenomic and chemical characterization of soil cobalamin production. ISME J 14:53–66. https://doi.org/10.1038/s41396-019-0502-0
Lwalaba JW, Louisa LT, Zvobgoa G, Richmonda MEA, Fua L, Naza S, Mwambaa M, Mundendeb RPM, Zhang G (2020) Physiological and molecular mechanisms of cobalt and copper interaction in causing phyto-toxicity to two barley genotypes differing in Co tolerance. Ecotoxicol Environ Safety 187:109866. https://doi.org/10.1016/j.ecoenv.2019.109866
Mattila P, Könkö K, Eurola M, Pihlava JM, Astola J, Vahteristo L, Hietaniemi V, Kumpulainen J, Valtonen M, Piironen V (2001) Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J Agric Food Chem 49:2343–2348. https://doi.org/10.1021/jf001525d
McGrath D, Fleming GA (2007) Trace elements and heavy metals in Irish soils. Teagasc, Agriculture and Food Development Authority, Wexford. https://www.teagasc.ie/media/website/publications/2011/Trace_Elements.pdf
Medyńska-Juraszek A, Ćwieląg-Piasecka I, Jerzykiewicz M, Trynda J (2020) Wheat straw biochar as a specific sorbent of cobalt in soil. Materials 13:2462. https://doi.org/10.3390/2Fma13112462
Minz A, Sinha AK, Kumar R, Kumar B, Deep KP, Kumar SB (2018) A review on importance of cobalt in crop growth and production. Int J Curr Microbiol App Sci 7:2978–2984
Morton JD (1986) Vitamin B12 and copper in blood serum and liver of hoggets in relation to cobalt and copper status of west coast South Island soils and pasture, New Zealand. J Exp Agric 14:485–489. https://doi.org/10.1080/03015521.1986.10423072
Mozafar A, Oertli JJ (1992) Uptake of a microbially-produced vitamin (B12) by soybean roots. Plant Soil 139:23–30. https://doi.org/10.1007/BF00012838
Mozafar A (1994) Enrichment of some B-vitamins in plants with application of organic fertilizers. Plant Soil 167:305–311. https://doi.org/10.1007/BF00007957
Nakos M (2016) Quantitative determination of vitamin B12 in plants. PhD thesis, University of Hannover
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670. https://doi.org/10.1046/j.1365-2389.2003.00556.x
Narendrula R, Nkongolo KK, Beckett P (2012) Comparative soil metal analyses in Sudbury (Ontario, Canada) and Lubumbashi (Katanga, DR-Kongo). Bull Environ Contam Toxicol 88:187–192. https://doi.org/10.1007/s00128-011-0485-7
Neufeld J, Engel K, Cheng J, Moreno-Hagelsieb G, Rose D, Charles T (2011) Open resource metagenomics: a model for sharing metagenomic libraries. Stand Genom Sci 5:203–210. https://doi.org/10.4056/sigs.1974654
Noble AE, Ohnemus DC, Hawco NJ, Lam PJ, Saito MA (2017) Coastal sources, sinks and strong organic complexation of dissolved cobalt within the US North Atlantic GEOTRACES transect GA03. Biogeosci 14:2715–2739. https://doi.org/10.5194/bg-14-2715-2017
Nygård T, Steinnes E, Røyset O (2012) Distribution of 32 elements in organic surface soils: contributions from atmospheric transport of pollutants and natural sources. Water Air Soil Pollut 223:699–713. https://doi.org/10.1007/s11270-011-0895-5
Oh S, Cave G, Lu C (2021) Vitamin B12 (cobalamin) and micronutrient fortification in food crops using nanoparticle technology. Front Plant Sci 12:668819. https://doi.org/10.3389/fpls.2021.668819
Okamoto N, Nagao F, Umebayashi Y, Bito T, Prangthip P, Watanabe F (2021) Pseudovitamin B12 and factor S are the predominant corrinoid compounds in edible cricket products. Food Chem 347:129048. https://doi.org/10.1016/j.foodchem.2021.129048
Orłowska M, Steczkiewicz K, Muszewska A (2021) Utilization of cobalamin is ubiquitous in early-branching fungal phyla. Genome Biol Evol 13. https://doi.org/10.1093/gbe/evab043
Paterson JP, Klessa DA, MacPherson A (1991) An investigation into the methods of improving the cobalt status of soil, herbage and grazing ruminants and its field assessment. Livestock Prod Sci 28:139–149. https://doi.org/10.1016/0301-6226(91)90004-A
Pratt JM (1972) Inorganic chemistry of vitamin B12. Academic Press, London, UK
Rabinowitz JD, Kimball E (2007) Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Anal Chem 79:6167–6173. https://doi.org/10.1021/ac070470c
Raux E, Lanois A, Levillayer F, Warren MJ, Brody E, Rambach A, Thermes C (1996) Salmonella typhimurium cobalamin (vitamin B12) biosynthetic genes: functional studies in S. typhimurium and Escherichia coli. J Bacteriol 178:753–767. https://doi.org/10.1128/jb.178.3.753-767.1996
Robertson DE (1970) The distribution of cobalt in Oceanic waters. Geochim Cosmochim Acta 34:553–567. https://doi.org/10.1016/0016-7037(70)90016-5
Rosenberg E, Zilber-Rosenberg I (2016) Microbes drive evolution of animals and plants: the hologenome concept. Mbio 7:e01395. https://doi.org/10.1128/mbio.01395-15
Roth JR, Lawrence JG, Rubenfield M, Kieffer-Higgins S (1993) Church GM (1993) Characterization of the cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium. J Bacteriol 175:3303–16. https://doi.org/10.1128/2Fjb.175.11.3303/3316.1993
Rowley CA, Kendall MM (2019) To B12 or not to B12: Five questions on the role of cobalamin in host-microbial interactions. PLoS Pathog 15:e1007479. https://doi.org/10.1371/journal.ppat.1007479
Rózsa S, Maniutiu DN, Posta G, Gocan TM, Andreica I, Bogdan I, Rózsa M, Lazar V (2019) Influence of the culture substrate on the Agaricus blazei Murrill mushrooms vitamins content. Plants 8:316. https://doi.org/10.3390/plants8090316
Saaltink R, Griffioen J, Mol G, Birke M (2014) Geogenic and agricultural controls on the geochemical composition of European agricultural soils. J Soils Sedim 14:121–137. https://doi.org/10.1007/s11368-013-0779-y
Sakamaki T (2010) Coprophagy in wild bonobos (Pan paniscus) at Wamba in the Democratic Republic of the Congo: a possibly adaptive strategy? Primates 51:87–90. https://doi.org/10.1007/s10329-009-0167-9
Schmidt A, Call LM, Macheiner L, Mayer HK (2019) Determination of vitamin B12 in four edible insect species by immunoaffinity and ultra-high performance liquid chromatography. Food Chem 281:124–129. https://doi.org/10.1016/j.foodchem.2018.12.039
Seymour JR, Amin SA, Raina J-B, Stocker R (2017) Zooming in on the phycosphere: the ecological interface for phytoplankton–bacteria relationships. Nat Microbiol 2:1–12. https://doi.org/10.1038/nmicrobiol.2017.65
Smith KA (1990) Manganese and cobalt. In: Alloway BJ (ed) Heavy metals in soils. Blackie, London, pp 197–221
Srivastava P, Bolan N, Casagrande V, Benjamin J, Adejumo SA, Sabir M, Farooqi ZUR, Ullah S (2022) Chapter 5 – Cobalt in soils: sources, fate, bioavailability, plant uptake, remediation, and management. In: Kumar V, Sharma A, Setia R (Eds) Appraisal of metal(loids) in the ecosystem. Elsevier, Amsterdam, pp 81–104. https://doi.org/10.1016/B978-0-323-85621-8.00007-8
Stahmann KP (2019) Vitamins and vitamin-like compounds: microbial production. Schmidt TM (Ed) Encyclopedia of Microbiology, 4th ed. Elsevier, Amsterdam, pp 569–580. https://doi.org/10.1016/B978-0-12-809633-8.13017-1
Stepanova VA, Pokrovsky OS, Viers J, Mironycheva-Tokareva NP, Kosykh NP, Vishnyakova EK (2015) Elemental composition of peat profiles in western Siberia: Effect of the micro-landscape, latitude position and permafrost coverage. Appl Geochem 53:53–70. https://doi.org/10.1016/j.apgeochem.2014.12.004
Tanioka Y, Yabuta Y, Miyamoto E, Inui H, Watanabe F (2008) Analysis of vitamin B12 in food by silica gel 60 TLC and bioautography with vitamin B12-dependent Escherichia coli 215. J Liq Chromatogr Relat Technol 31:1977–1985
Titcomb TJ, Tanumihardjo SA (2019) Global concerns with B vitamin statuses: biofortification, fortification, hidden hunger, interactions, and toxicity. Comp Rev Food Sci Food Safety 18:1968–1984. https://doi.org/10.1111/1541-4337.12491
Turło J, Gutkowska B, Herold F, Krzyczkowski W, Błażewicz A, Kocjan R (2008) Optimizing vitamin B12 biosynthesis by mycelial cultures of Lentinula edodes (Berk.) Pegl. Enzyme Microb Technol 43:369–374. https://doi.org/10.1016/j.enzmictec.2008.05.005
Tyler G (2004) Vertical distribution of major, minor, and rare elements in a Haplic Podzol. Geoderma 119:277–290. https://doi.org/10.1016/j.geoderma.2003.08.005
Venice F, Desirò A, Silva G, Salvioli A, Bonfante P (2020) The mosaic architecture of NRPS-PKS in the arbuscular mycorrhizal fungus Gigaspora margarita shows a domain with bacterial signature. Front Microbiol 11:581313. https://doi.org/10.3389/fmicb.2020.581313
Wallner A, Busset N, Lachat J, Guigard L, King E, Rimbault I, Mergaert P, Béna G, Moulin L (2022) Differential genetic strategies of Burkholderia vietnamiensis and Paraburkholderia kururiensis for root colonization of Oryza sativa subsp. japonica and O. sativa subsp. indica, as revealed by transposon mutagenesis sequencing. Appl Environ Microbiol 88:1–18. https://doi.org/10.1128/aem.00642-22
Walsh PT, McCreless E, Pedersen AB (2013) Faecal avoidance and selective foraging: do wild mice have the luxury to avoid faeces? Anim Behav 86:559–566. https://doi.org/10.1016/2Fj.anbehav.2013.06.011
Wardle DA (1998) Controls of temporal variability of the soil microbial biomass: a global scale synthesis. Soil Biol Biochem 30:1627–1637. https://doi.org/10.1016/S0038-0717(97)00201-0
Watanabe F, Bito T (2018) Vitamin B12 sources and microbial interaction. Experim Biol Med 243:148–158. https://doi.org/10.1177/1535370217746612
Watanabe F, Takenaka S, Abe K, Tamura Y, Nakano Y (1998) Comparison of a microbiological assay and a fully automated chemiluminescent system for the determination of vitamin B12 in food. J Agric Food Chem 46:1433–1436. https://doi.org/10.1021/jf970807j
Watanabe F, Katsura H, Takenaka S, Fujita T, Abe K, Tamura Y, Nakatsuka T, Nakano Y (1999) Pseudovitamin B12 is the predominant cobamide of an algal health food, Spirulina tablets. J Agric Food Chem 47:4736–4741. https://doi.org/10.1021/jf990541b
Watanabe F, Schwarz J, Takenaka S, Miyamoto E, Ohnishi N, Nelle E, Hochstrasser R, Yabuta Y (2012) Characterization of vitamin B12 compounds in the wild edible mushrooms black trumpet (Craterellus cornucopioides) and golden chanterelle (Cantharellus cibarius). J Nutr Sci Vitaminol 58:438e441. https://doi.org/10.3177/jnsv.58.438
Watanabe F, Yabuta Y, Tanioka Y, Bito T (2013) Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J Agric Food Chem 61:6769–6775. https://doi.org/10.1021/jf401545z
Watanabe F, Yabuta Y, Bito T, Teng F (2014) Vitamin B12-containing plant food sources for vegetarians. Nutrients 6:1861–1873. https://doi.org/10.3390/nu6051861
Waterman RC, Kelly WL, Larson CK, Petersen MK (2017) Comparison of supplemental cobalt form on fibre digestion and cobalamin concentrations in cattle. J Agric Sci 155:832–838. https://doi.org/10.1017/S0021859617000107
Wendling LA, Kirby JK, McLaughlin MJ (2009) Aging effects on cobalt availability in soils. Environ Toxicol Chem 28:1609–1617. https://doi.org/10.1897/08-544.1
Wei Z, Xie X, Xue M, Valencak TG, Liu J, Sun H (2021) The effects of non-fiber carbohydrate content and forage type on rumen microbiome of dairy cows. Animals 11:3519. https://doi.org/10.3390/ani11123519
Yasuda M, Dastogeer KMG, Sarkodee-Addo E, Tokiwa C, Isawa T, Shinozaki S, Okazaki S (2022) Impact of Azospirillum sp. B510 on the rhizosphere microbiome of rice under field conditions. Agronomy 12:1367. https://doi.org/10.3390/agronomy12061367
Yin L, Bauer CE (2013) Controlling the delicate balance of tetrapyrrole biosynthesis. Philos Trans R Soc Lond B Biol Sci 368:20120262. https://doi.org/10.1098/2Frstb.2012.0262
Young RS (1979) Cobalt in biology and biochemistry. Academic Press, London
Zhang XP, Deng W, Yang XM (2002) The background concentrations of 13 soil trace elements and their relationships to parent materials and vegetation in Xizang (Tibet) China. J Asian Earth Sci 21:167–174. https://doi.org/10.1016/S1367-9120(02)00026-3
Zohny EAM (2002) Cobalt in alluvial Egyptian soils affected by industrial activities. J Environ Sci 14:34–38
Zupančič N, Skobe S (2014) Anthropogenic environmental impact in the Mediterranean coastal area of Koper/Capodistria, Slovenia. J Soils Sedim 14:67–77. https://doi.org/10.1007/s11368-013-0770-7
Acknowledgements
We gratefully acknowledge the numerous useful comments and suggestions of Ulrike Strothmann (Lossenheim) and Sophie Forster (Innsbruck). We also thank the careful and extensive suggestions and the comments of the anonymous reviewers as well as Mick Locke for correcting our English. Furthermore, we would like to give credit to Servier and DBCLS for their artwork, which is licensed under CC-BY 3.0 and 4.0, respectively. Modified versions are part of Fig. 2.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jörgensen, A.M., Joergensen, R.G. Soil contribution to the cobalamin (vitamin B12) supply of terrestrial organisms. Biol Fertil Soils 60, 613–625 (2024). https://doi.org/10.1007/s00374-024-01828-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-024-01828-7