Abstract
Due to the heterogeneous nature of soil pore structure, processes such as nitrification and denitrification can occur simultaneously at microscopic levels, making prediction of small-scale nitrous oxide (N2O) emissions in the field notoriously difficult. We assessed N2O+N2 emissions from soils under maize (Zea mays L.), switchgrass (Panicum virgatum L.), and energy sorghum (Sorghum bicolor L.), three potential bioenergy crops in order to identify the importance of different N2O sources to microsite production, and relate N2O source differences to crop-associated differences in pore structure formation. The combination of isotopic surveys of N2O in the field during one growing season and X-ray computed tomography (CT) enabled us to link results from isotopic mappings to soil structural properties. Further, our methodology allowed us to evaluate the potential for in situ N2O suppression by biological nitrification inhibition (BNI) in energy sorghum. Our results demonstrated that the fraction of N2O originating from bacterial denitrification and reduction of N2O to N2 is largely determined by the volume of particulate organic matter occluded within the soil matrix and the anaerobic soil volume. Bacterial denitrification was greater in switchgrass than in the annual crops, related to changes in pore structure caused by the coarse root system. This led to high N-loses through N2 emissions in the switchgrass system throughout the season a novel finding given the lack of data in the literature for total denitrification. Isotopic mapping indicated no differences in N2O-fluxes or their source processes between maize and energy sorghum that could be associated with the release of BNI by the investigated sorghum variety. The results of this research show how differences in soil pore structures among cropping systems can determine both N2O production via denitrification and total denitrification N losses in situ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
N2O is a highly potent greenhouse gas with a substantial global warming impact that can also harm the stratospheric ozone layer (Ravishankara et al. 2009; Tian et al. 2020). Agriculture is responsible for the majority (60%) of anthropogenic N2O emissions (Syakila and Kroeze 2011), through management practices like tillage and fertilizer application (Butterbach-Bahl and Dannenmann 2011; McGill et al. 2018; McSwiney and Robertson 2005). Biofuels from cellulosic bioenergy feedstocks make it possible to reduce the climate impact of fossil fuel energy consumption; their positive contribution to mitigating climate change, however, might be diminished by greenhouse gas (GHG) emissions, including N2O (Oates et al. 2016; Qin et al. 2015; Walter et al. 2015; Wightman et al. 2015). While N2O production is known to be affected by plant species composition (Butterbach-Bahl and Dannenmann 2011; Stehfest and Bouwman 2006) we do not fully understand the mechanisms influencing N2O emission from soils under different vegetation systems well enough to develop effective solutions for curbing overall GHG emissions.
In soils, N2O is a product of an array of N transformations (Robertson and Groffman 2015), with heterotrophic bacterial denitrification (bD), autotrophic nitrification (Ni), nitrifier denitrification (nD), and fungal denitrification (fD) regarded as primary sources (Butterbach-Bahl et al. 2013; Müller et al. 2014). Even though these processes may drastically differ from each other in terms of conditions necessary for their occurrence and the microorganisms involved, the extremely high micro-scale heterogeneity of the soil matrix enables them to produce N2O in a close spatial proximity (Braker and Conrad 2011; Rohe et al. 2021). Disentangling the specific drivers responsible for enhanced N2O emissions in the field is therefore notoriously difficult.
Availability of O2 is one of the major physical factors controlling N2O fluxes (Bollmann and Conrad 1998; Groffman et al. 1988; Rohe et al. 2021). For example, O2 discriminates between N2O production via denitrification, i.e. the anoxic reduction of nitrate (NO3-) to N2 with N2O as an intermediate, which takes place in the absence of O2, and nitrification, where N2O is a by-product during the oxidation of hydroxylamine (NH2OH) to nitrite (NO2-), which requires aerobic conditions. In contrast to bD, the major end product of fD is N2O since fungal denitrifiers lack N2O reductase (Baggs 2011; Philippot et al. 2011). In contrast to coupled nitrification-denitrification where the formation of nitrate and subsequent nitrate reduction are conducted by different microorganisms, in nD these tasks are performed by the same organism, the process benefitting from low organic C and O2 but high N availability (Braker and Conrad 2011; Wrage et al. 2001).
Denitrification is thus favored by wet conditions, available C and nitrate, and is often a major N2O source during high N2O flux events (Baggs 2011; Wrage et al. 2004). Yet, denitrification also can make surprisingly sizeable contributions to N2O fluxes from relatively dry soils. The latter is an outcome of local anaerobicity observed within decomposing plant residues or within soil matrix, e.g., centers of soil aggregates (Schlüter et al. 2018; Wrage et al. 2001). Occurrence and proliferation of anaerobic microsites within the soil matrix, which we will refer to as anaerobic soil volume fraction, is hard to quantify directly, but can be assessed indirectly through measures of diffusivity as well as model simulations based on air-filled pore volumes (Andersen and Petersen 2009; Balaine et al. 2013). Anaerobic soil volume fraction can also be manipulated in bulk (whole) soil samples by controlling the water filled pore space (WFPS) (Chen et al. 2016; Kim et al. 2022; Kravchenko et al. 2017) The WFPS 70-80% has been shown to be optimal for denitrification (Butterbach-Bahl et al. 2013). However, such bulk measurements do not consider pore structure, a key factor controlling the microscale distribution patterns in anaerobic soil volume fraction. Recent advancements in X-ray computed tomography (X-ray CT) imaging allowed visualization of anaerobic soil volume fraction at micro-scales and demonstrated that anaerobic soil volume fraction, quantified via distances to air-filled pores, can predict N2O emissions as well as magnitudes of complete denitrification (Kravchenko et al. 2018; Rabot et al. 2015; Rohe et al. 2021).
Not only O2 influx, but also O2 demand is highly controlled by the pore architecture. Intense microbial activity boosts oxygen consumption, creating local anaerobic micro-sites which stimulates N2O production (Kim et al. 2020, 2021; Kravchenko et al. 2017). Microbial activity’s impact on N2O emissions thus depends on pore architecture in a complex not yet fully understood manner. Some studies suggest that narrow macropores (30-150 μm diameter (Ø)) can provide a perfect environment for microbial decomposers, hence, for close spatial coupling of N2O production and emission during decomposition of fresh plant residues (Kim et al. 2020, 2021, 2022; Kravchenko et al. 2017). Yet, lower availability of O2 stimulated by greater distances from pores to decomposing soil particulate organic matter (POM) can enhance N2O emissions (Ortega-Ramírez et al. 2023).
Plant roots play the most important role in shaping soil pore architecture through direct formation of biopores and indirect repacking and rearranging of soil solids (Lucas et al. 2019, 2022). Biopores formed by roots range from ~30 μm to 5000 μm, i.e. span three orders of magnitude (Yunusa and Newton 2003). Narrow biopores (e.g., 30-150 μm Ø) can be particularly important due to their oversize contribution to the overall connectivity of the pore system (Lucas et al. 2020). Differences in root architectures in plants of different species or plant communities can have a sizeable influence on narrow macropore formation (Bacq-Labreuil et al. 2019; Bodner et al. 2014; Lucas et al. 2022), hence potentially influencing the size of anaerobic soil volume fraction. For example, a comparison of several bioenergy cropping systems demonstrated that polyculture vegetation communities decreased soil anaerobic soil volume fraction compared to monoculture maize and switchgrass, and that such decreases were associated with lower N2O emissions (Kravchenko et al. 2018).
The other two important routes through which roots can affect N2O emissions are through direct alterations of soil N balance through N uptake vs. N inputs via exudation and rhizodeposition (Jones et al. 2009; Moreau et al. 2019), and by shaping the composition and functions of the soil microbiome and rhizosphere processes (Berendsen et al. 2012; Hinsinger et al. 2009). However, the actual significance of plant-microbe interactions for the soil N-cycle, e.g. through the release of labile C as drivers of rhizosphere denitrification, is currently not well understood (Baggs 2011; Moreau et al. 2019; Philippot et al. 2009). Certain plant species, e.g., sorghum a promising bioenergy crop, can produce and release biological nitrification inhibitors (BNIs) into the soil, potentially suppressing nitrification, through the reduction of ammonia-oxidizing bacteria and archea (Li et al. 2021; Sarr et al. 2020; Subbarao et al. 2007, 2015; Tesfamariam et al. 2014). However, sizes and ecological impacts of biological nitrification inhibition of sorghum in the field are yet to be determined by measurements of in-situ gross nitrification and denitrification, such as through isotopic techniques (Nardi et al. 2022).
With few exceptions, for example, Rohe et al. (2021), previous studies measured only the emitted N2O, lacking the ability to identify the processes that led to its production or to assess the full extent of denitrification. Therefore, while N losses in the soil in the form of N2 can be substantial, their measurements are complicated by a high atmospheric N2 background (Lewicka-Szczebak et al. 2017; Yu et al. 2020). By analyzing the isotopic signatures of N2O, including the δ18O value of oxygen, the bulk δ15N value, and the intramolecular distribution of 15N in N2O (site preference, SP), it is possible to gain insights into the origins of N2O emissions (Yu et al. 2020). A way to derive quantitative information on N2O sources from such isotopic analyses is isotopic mapping using δ15NSP / δ18O (Yu et al. 2020). Based on the isotopic enrichment of residual N2O during the reduction to N2, it further allows to derive denitrification product ratio [N2O / (N2O + N2)] (pr) and thus to quantify complete denitrification through N2O+N2 fluxes (Lewicka-Szczebak et al. 2017).
The objectives of the study were 1) to conduct field monitoring of N2O and comparisons of N2O + N2 emissions and their N2O component from the soils under energy sorghum, maize and switchgrass crops, grown for bioenergy stock production; 2) to distinguish among the prevalent pathways of N2O production in the soils of these crops; and 3) to elucidate the potential role of soil pore structure for influencing N2O + N2 emissions and their sources. We collected N2O throughout the growing season using static flux chambers and implemented δ15NSP / δ18O isotope mapping to estimate the relative contribution of different microbial pathways to N2O production as well as to quantify the reduction of N2O to N2. X-ray CT imaging of undisturbed soil cores allowed us to quantify pore structure and elucidate its contribution to micro-environmental conditions prevalent within the soil matrix. We also hypothesized that the reported ability of sorghum roots, in contrast to maize, to reduce nitrification potential by BNI (Subbarao et al. 2007; Tesfamariam et al. 2014) will be manifested in the field through greater N2O production via denitrification than nitrification pathway. We also hypothesized that a capacity of switchgrass to reduce narrow macropores and increase the anaerobic soil volume (Kravchenko et al. 2019, 2022) will result in an increased importance of complete denitrification, as compared to that in the other two crops.
Methods
Field design and management
The DOE-Great Lakes Bioenergy Research Center (GLBRC) Biofuel Cropping System Experiment (BCSE) site was established in 2008 at the Kellogg Biological Station (KBS) Long-term Ecological Research site (Robertson and Hamilton 2015) in Hickory Corners, Michigan [42°23′47′′ N, -85°22′26′′ W, 288 m a.s.l.]. Site soils are loamy, well-drained Alfisols developed on glacial outwash with loess inputs. The experiment is a randomized complete block design with five replicate blocks. We evaluated N2O emissions from three systems during the 2021 field season: Monocultures of switchgrass (P. virgatum L. variety Cave-in-rock), maize (Zea mays L., Pioneer P0306Q) and energy sorghum (Sorghum bicolor L., TAM 17651). Before 2018, the energy sorghum plots contained continuous maize + cover crops.
Seeding and fertilizer application differed between the treatments (Fig. 2a, top row). Details on the agricultural management in 2021 and before can be found on https://aglog.kbs.msu.edu/. In summary, maize was seeded on the 15th of May with a starter fertilizer supplying 34 kg N ha-1 and energy sorghum was seeded on the 19th of May with 56 kg N ha-1. At the end of June an additional 137 kg N ha-1 (28% Urea Ammonium Nitrate, UAN) was injected in the middle of the rows of the two crops. The switchgrass plots were sprayed with 28% UAN on the 13th of May supplying 56 kg N ha-1 with no further N additions. All systems were managed without tillage.
N2O sampling
Two static (closed-cover) flux chambers were installed within 2 m of one another in each of the five blocks in May 2021 and removed only for agronomic operations for a total of 10 chambers per cropping system. Each chamber consisted of a cylindrical metal base and an airtight plastic lid (surface area = 641 cm2, headspace volume = 16.6 L) and was hammered 5 cm deep in the soil. Atmospheric pressure within the sealed chamber was maintained by a piece of coiled stainless-steel tubing (0.5 m X 0.32 cm OD and 0.18 cm ID) extending from the interior to exterior of the chamber. Gas samples were taken from an approx. 50 cm long and 0.6 mm outer diameter polyurethane tubing connected to the headspace (Fig. 1a). During sampling a pre-evacuated 250 ml glass bottle was connected to a steel needle at the end of the tubing for 1 min to assure diffusive equilibration. Additionally, a 30 ml pre-evacuated glass bottle was filled. Chamber closing times ranged between 70 min and 470 min to ensure sufficient N2O concentrations for later isotopic characterization. Initial closures were adjusted to the expected fluxes, i.e. with the shortest closing times for maize directly after fertilization and the longest for switchgrass before fertilization and timed to complete sampling by noon. Additionally, 250 ml atmospheric gas samples were taken on the respective sampling days in plot 1 of each of the respective plant treatments at the height of the top of the chamber.
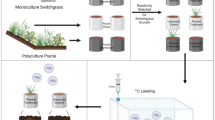
Photographs and CT visualizations. a) Collecting N2O from a static flux chamber at the beginning of the season. b) A soil core taken within the base of the chamber. c) An image slice from a CT-scanned soil core showing pores, roots, particulate organic matter (POM), and soil solid matrix identified on the image. d) Visualization of the root system of maize, energy sorghum and switchgrass within the soil core obtained using X-ray CT and e) visualization of the soil matrix (turquoise) and macropores (black) as well as the visualization of POM (brown) within the 3D distance map to air-filled macropores.
Sampling was conducted throughout the 2021 season with 7-8 sampling events per crop. Some of the sampling events took place on a regular basis, i.e., once a month, throughout the growing season, with the first sampling on 12th of May and the last on 25th of August. Other sampling events targeted anticipated enhanced GHG emissions, including samplings at 3, 7, 14, and 30 days after N fertilization and sampling on the day of the first large rain event (>30 mm, Fig. 2a) that followed a long May-June drought.
a) Daily precipitation (blue bars) and mean daily temperature (red line). Shadow represents max. and min temperature range. b) Water filled pore space (WFPS). c) N2O+N2 fluxes during the cropping season for the three bioenergy crops. The darkest parts of the bars represent the fraction of N2O. The top row shows the sampling days for the three studied crops and marks the dates of seeding and fertilization events. Whiskers show the standard errors of the means. Different letters mark significant differences among the crops within the same sampling date (p<0.05). On some of the sampling dates N fluxes were too low to provide reliable isotopic values, thus no values are reported on c). Note that N2 was calculated based on the pr derived from the isotopic mapping approach.
N2O flux calculations
Measurements of N2O concentration in the 30 ml samples were carried out using a gas chromatograph (GC-ECD,Shimadzu GC-2014) with an analytical precision of approx. 2%. Using these concentrations, the N2O fluxes were calculated based on an increase in the N2O concentration from that of air using the ambient mean of the measured N2O concentration of 335 ppbv during the closing time. For all samples with N2O concentrations > 300 ppbv, the paired taken 250 ml samples were used for isotopic characterization (see below). In that case, the N2O concentration from respective analyses was used to calculate N2O fluxes.
Isotopic characterisation
An Elementar IsoPrime 100 stable isotope ratio mass spectrometer (IRMS) interfaced to a Trace Gas inlet system (Elementar; Mt. Laurel, NJ) was used to measure δ15Nbulk, δ15Nα, δ15Nβ and δ18O of N2O as previously described (Sutka et al. 2003). By analyzing the mass-to-charge (m/z) 44, 45, and 46 in intact N2O+ molecular ions, we determined the bulk δ15N and δ18O isotope signatures, while the δ15Nα values were detected by the m/z 30 and 31 of NO+ fragment ions generated in the mass spectrometer. N2O is a linear molecule consisting of two N atoms (NNO), with one of the N atoms in the central position (α site) and the other at the terminal position (β site). The distribution of 15N within the N2O molecule is called site preference (SP) and is defined as the difference in δ15N values between the α (δ15Nα) and β (δ15Nβ) sites. The isotopic values are presented as deviation from the 15N/14N and 18O/16O ratios of atmospheric N2 and the Vienna Standard Mean Ocean Water (VSMOW), respectively. The analytical precision determined as standard deviation (1σ) of primary standards measurements was 0.5‰ for δ15Nbulk and δ18O, 0.4‰ for δ15Nα, and δ15Nβ and 0.6‰ SP. The δ15Nbulk, δ15Nα, δ15Nβ , δ18O and SP values of the two laboratory N2O primary standards are -0.69‰, 11.51‰, -12.88‰, 40.16‰ and 24.39‰ and -0.77‰, -1.12‰, -0.42‰, 39.17‰ and -0.70‰, respectively as determined by calibration against international reference material USGS51 and USGS52 (Ostrom et al. 2018). The dilution of the ambient air in the flux chamber was corrected based on the increase in the N2O concentration from that of the measured mean ambient concentration and the respective isotopic values throughout the season (δ15N 6.1±0.2, δ15NSP 13.4±0.5, δ18O 41.7 ±0.2). To assure high accuracy, we further analyzed only isotopic readings of samples with N2O concentrations greater than 130% of the ambient N2O concentration.
We followed the "δ15NSP / δ18O isotope mapping technique" to estimate the relative contribution of the different microbial N2O production pathways to the total N2O+N2 production (Lewicka-Szczebak et al. 2020; Yu et al. 2020). We used recently summarized data from Yu et al. (2020) to map the endmembers, i.e. the microbial source isotope values for bD/ nD and Ni processes and between bD/nD and fD as well as the N2O reduction (Tab. S1). The δ18O values were corrected for by the mean δ18O of annual precipitation water (-7.3 ‰) derived from the Waterisotopes Database (http://waterisotopesDB.org. Accessed 01.11.2022). Adding the endmembers into the δ15NSP / δ18O isotope plot allows us to derive the different slopes of the mixing line between bD+nD and fD or Ni, as well as the reduction line for isotpic enrichment of residual N2O. The latter allows us to calculate the denitrification product ratio [N2O / (N2O + N2)] (pr) and thus to derive total N2O+N2 emissions. Note that bD and nD cannot be distinguished by this method.
We followed a protocol (Lewicka-Szczebak et al. 2017, 2018) taking into account the sample position in the and δ15NSP / δ18O map using a mixing equation for the bacterial fraction and the Rayleigh equation for N2O reduction. In short, to derive the relative contributions of the endmembers, two scenarios are assumed, of which we present mean values: In the first, N2O is produced by bD and partially reduced; then a mixing of residual N2O with unreduced N2O from Ni or fD appears. In the second, these processes happen vice versa. The δ18O endmembers for Ni and fD taken from Yu et al. (2020) are sufficiently differentiated to allow both mixing-lines between bD-NI and bD-fD to be distinguished (Lewicka-Szczebak et al. 2020). Since multiple mixing curves cannot be evaluated simultaneously with the equations of Lewicka-Szczebak (2018), we distinguished between bD-NI and bD-fD mixing as two possible instances of end-member mixing as suggested in Lewicka-Szczebak et al. (2020). In the event that the samples were located below the mean reduction line, the calculation results provide the fraction of bD values slightly higher than 1, which were set to 1 for further summaries.
WFPS and N forms
Soil moisture was measured (0 – 10 cm depth) at three locations close to each chamber at every sampling event using a volumetric soil moisture sensor (HydroSense II, Campbell Scientific, Logan UT, USA).
Additionally, we took disturbed soil samples (~100g) from around the chambers at various time points throughout the season (Tab. S2), including sample dates before and after fertilization as well as after the rain event. The soil samples were stored at -20°C before extraction. For this, a homogenized sample of approx. 10 g of fresh soil was extracted with 0.1 mol KCl. Available NH4+ and NO3- were analyzed in the MSU soil test laboratory according to Sinsabaugh et al. (2000) and Doane and Horwáth (2003), respectively.
After scanning the undisturbed cores with X-ray CT (see below), we derived the bulk density of the cores gravimetrically. The water filled pore space was then calculated based on the measured water contents and the bulk density within the different plots. In addition, soil of these cores was used to measure the pH-value (in water).
Weather data (daily precipitation and temperature) are from https://lter.kbs.msu.edu/datatables/12.
X-ray CT
After the last sampling campaign in late August, one intact soil core (5 cm Ø, 5 cm height) was taken from 1 to 6 cm depth under the base of each static chamber (Fig. 1b). These cores were subjected to X-ray CT shortly after collection. The soil cores were scanned using an X-ray microtomograph (X3000, North Star Imaging, Rogers, USA) at 75 kV and 470 μA. Since the samples were scanned using a continuous subpiX mode, a resolution of 18.2 μm could be achieved, although the respective energy settings resulted in a larger focal spot on the VarianL07 detector panel (size 1920 * 1536 pixels). During a scan with four subimages (2 rows and 2 columns), 2880 projections were acquired at 3 frames per second with an average of 2 frames. The 3D image reconstruction was performed with the efX reconstruction software.
Image processing and analyses
The reconstructed images were cut into cubes of 1850x1850x2300 voxels in Fiji (V. 153n, Schindelin et al. 2012). This was done, to avoid analyzing disturbed regions at the core walls. Then the images were segmented into four classes, namely pores, soil matrix, POM and roots (Fig. 1c). For this, we used a random forest classifier trained in ILASTIK (Berg et al. 2019) to pore segments, soil matrix, and a class that includes roots and POM. To train the classifier, we used subvolumes of five randomly chosen images for annotation. The out-of-bag error was <0.01. We were not able to further compare image-based POM to POM conventionally analyzed, but a similar protocol was used by Schlüter et al. (2022) to show good agreement of image-based POM and conventionally analyzed POM. Moreover, visual expectation of the images showed no over-segmentation of POM particles, although under-segmentation could potentially happen for small POM particles due to our resolution of 18.2 μm. Such small POM particles, however, seem to be distributed more evenly in the soil matrix (Schlüter et al. 2022) and therefore would not affect the analyzed distribution of POM. After segmenting all plant residues, we further differentiated between POM and roots in Fiji, in which objects of the mixed class were assigned to roots (Fig. 1d) only if they were connected to the outer boundary of the image and were larger than 10.000 voxels, i.e. approx. 0.06 cm3. For the latter, we used the “connected components labeling” and the “size opening” functions of the plugin MorphoLibJ (Version 1.4.3, Legland et al. 2016).
The anaerobic soil volume fraction, i.e., the volume fraction of air distance larger than a threshold (Fig. 1e), was calculated as written in Rohe et al. (2021) by computing the Euclidean Distance Transform for the pore image. In addition to computing the anaerobic soil volume fraction and the visible porosity (pores > 0.036 mm), we computed the Γ-indicator as a third metric to estimate oxygen supply. For this, the pore image was labelled using the connected component labelling from the plugin BoneJ2 plugin (V. 7.10, Domander et al. 2021). This image was used to calculate the Γ-indicator, which is a metric of pore connectivity (Lucas et al. 2020).
To measure the volume of pores between 0.036 mm and 0.15 mm Ø, we used the local thickness method (Hildebrand and Rüegsegger 1997) in Fiji. We refer to the volume of pores between 0.036 mm and 0.15 mm Ø by pores < 0.15 mm Ø and report their volume relative to the volume of the soil core. Additionally, the image of the Euclidean Distance Transform (Fig. 1e) was also used to calculate the mean distance of POM to macropores similar to Ortega-Ramírez et al. (2023). Note that one image of the switchgrass cores contained a massive volume of roots, as only the root sod was sampled (Fig. 1d). This sample was handled as an outlier, as the large root volume (>10 %) led to unreliable information on the soil matrix. It was also excluded from the bulk density estimation.
Statistical analysis
Effects of the plant treatment (maize vs. switchgrass vs. energy sorghum) on the studied flux data and isotopic characteristics as well soil structural properties derived from X-ray CT scans were investigated using linear mixed model approach implemented in the lme4-package (Bates et al. 2015) of R (V. 4.1.1). These models extend simple linear models to include the non-independent nature of our sample hierarchical structure, i.e. the different chambers within one plot. The random effects assigned consisted of the treatment plots, used as an error term for testing the plant treatment effect, and the flux chambers nested within the plots, used as an error term. Additionally, for the studied flux data and isotopic characteristics, the time point of sampling was added as a fixed factor to the model. Because on some dates only maize and energy sorghum were sampled, we constructed two models, the first including only dates with these two plant treatments, while the second contained only sampling campaigns of all three plant treatments. The assumptions of normality and homogeneity of variances were assessed using normal probability plots of the residuals and Levene’s tests for equal variances, respectively. When the normality assumption was found to be violated, the data were logarithmically transformed; when the equal variance assumption was violated, the unequal variance models were fitted using the package ‘nlme’ in R, respectively.
To address our second research question concerning the evaluating soil structural properties as predictors of denitrification, we computed the correlation matrix of Pearson’s correlation showing coefficients in R using the ‘corrplot’ package. In addition to the correlations including data from all sampling days, we computed these correlation matrixes for specific days for which all data (pore structure, N2O, and soil chemistry) were available.
Due to the different management strategies of bioenergy crops we did not analyze the pore structural correlations with the total fluxes across the plant systems. For this, we computed plant treatment specific linear regressions of parameters derived from X-ray CT (anaerobic soil volume fraction, distance of POM to macropores, Pores <150μm) with the mean N2+N2O emissions of the flux chambers. In addition, linear regression show the response of the fraction of bD and the pr to the anaerobic soil volume fraction and the distance of POM to macropores across the plant systems, where the fraction of bD as well as the pr were log scaled. Available NH4+ and NO3- were analyzed only in one sample per plot only, that is, no mixed effect model was necessary and we used an analysis of variance (ANOVA) in conjunction with Tukey’s HSD test implemented in the ‘agricolae’ package (Mendiburu and Yaseen 2020).
Results
N2O+N2 fluxes
At the beginning of the season (early May), the perennial system switchgrass was wetter compared to maize and energy sorghum (Fig. 2b) systems. Fertilization of switchgrass during this time led to a peak in N2O+N2 emissions with fluxes > 100 g N day-1 ha-1, with N2O less than 25 g N day-1 ha-1 (Fig. 2c). Due to low precipitation (Fig. 2a), by late May WFPS had dropped substantially (Fig. 2b) and N2O emissions were barely detectable (Fig. 2c). The large rain event of mid-June led to high fluxes of N2O+N2 in all three systems, with both N2O (approx. 75 g N day-1 ha-1) and N2O+N2 emissions significantly higher in energy sorghum compared to maize and switchgrass. Shortly after the first large rain event, the two annual crops were fertilized, and multiple heavy rains followed within two days, greatly increasing soil water contents. The N2O+N2 emissions after fertilization did not differ between energy sorghum and maize and were substantially lower than the emission peaks observed after the first rain event of 18 June. Seven days after this fertilization their N2O+N2 fluxes again increased substantially (approx. 100 g N day-1 ha-1), with a particularly large share of N2O (approx. 50 g N day-1 ha-1). Interestingly, after the low June fluxes and despite the lack of fertilization, N2O+N2 emissions from switchgrass increased in early July and then again in late August, with a continuously low share of N2O.
The mean N2O+N2 fluxes for the entire season did not differ significantly among the three crops (Table 1). Mean N2O fluxes were significantly higher in energy sorghum (18.6 g N day-1 ha-1) compared to maize (14.6 g N day-1 ha-1), while numerically the lowest in switchgrass stands (9.2 g N day-1 ha-1).
Soil NH4+ and NO3- concentrations did not significantly differ among the systems at any point during the season (Table S2) except in July when the soil of maize plots had significantly higher NO3- concentrations compared to energy sorghum and switchgrass.
Isotopic Characterization
On most dates when isotopic characterization was performed for all three cropping systems switchgrass had higher δ18O, δ15Nbulk, and lower δ15NSP values compared to maize and energy sorghum (Fig. S1). Only in late August, the N2O from switchgrass had significantly lower δ18O values compared to the two other plants. Consequently, in the isotopic mapping of δ18O / δ15NSP the maize and energy sorghum data are distributed mainly in-between the fD-bD mixing line and the reduction line, while the switchgrass data are mostly clustered around the reduction line (Fig. 3). High fluxes were directly associated with a higher fraction of bD (Fig. S2), thus yellow to red points in Fig. 3 are found only close to the reduction line.
Reasoned summarized isotopic endmember values from Yu et al. (2020) enable us to differentiate between two potential mixing lines (bD-Ni and bD-fD) and thus we must select the appropriate mixing scenario (Lewicka-Szczebak et al. 2020). In this study, the pr and the fraction of bD are nearly identical for both mixing scenarios (Fig. 4, Fig. S3). This is because of the generally high fraction of bD especially in switchgrass. As most points lay far below the bD-fD mixing line and are distant from Ni, indicating the low importance of Ni, we focus our analysis on the results in Fig. 4, assuming a primary mixing of bD and fD. That said, the influence of Ni (Fig. S3) cannot be excluded for all points and later is discussed separately.
The calculated values of the fraction of bD and pr from Fig. 3 revealed a high importance of bD in switchgrass systems and a large share of N2O’s being reduced to N2 as compared to maize and energy sorghum systems (Table, 1, Fig. 4). An exception was the late August sampling date, where the fraction bD in switchgrass became numerically lower and the pr numerically higher than those in maize and energy sorghum. While throughout the season energy sorghum and maize did not differ from each other in terms of either the fraction of bD and pr, shortly after fertilization energy sorghum had a significantly lower bD relative contribution compared to maize (Fig. 4). If we assume the mixing of bD and Ni only (Fig. S3), this would suggest that the importance of Ni was higher in sorghum systems compared to maize that particular sampling event, while on all other days there was no difference between the two crops in the relative contributions of Ni and bD to N2O production. Indeed, the corresponding points can be found above the bD-fD mixing line in the subplot of energy sorghum (Fig. 3).
Correlation with pore structural properties
We conducted a sensitivity test and correlated the volume fraction of varying minimum distances to pores with the N2O+N2 fluxes to derive the anaerobic soil volume fraction (Rohe et al. 2021). While there was no significant correlation for switchgrass throughout all distances, the two other plants had significant correlations for a range of minimum distances (Fig. S4). For computing the final anaerobic soil volume fraction, we used the distance >0.41 mm away from pores, for which the lowest mean p-value for the two annual plants was found.
pH-values, bulk density, macroporosity (percent of pores >40 μm Ø), Г-indicator (connectivity of the pore system), and percent POM were not significantly different among the three systems (Table 1). Yet, switchgrass had significantly higher root volumes and anaerobic soil volume fraction compared to energy sorghum and maize. Furthermore, switchgrass decreased the <150 μm Ø pores and increased the mean distance from POM to macropores.
Several of these properties were correlated with each other (Fig. S2a). The anaerobic soil volume fraction and the distance of POM to macropores were highly positively correlated, and both were negatively correlated to pores <150 μm Ø and positively to the root volume.
The distance of POM to macropores was positively correlated to N2O+N2 emissions in soils of maize and energy sorghum, but not in switchgrass (Fig. 5a). The same pattern was observed for the association between anaerobic soil volume fraction and N2O+N2 emissions (Fig. S5a). The abundance of <150 μm Ø pores was related to N2O+N2 (negatively) only in sorghum soils (Fig. S5b). In addition, only in switchgrass was the root volume positively associated with the N2O+N2 emissions throughout the season (Fig. 5b).
Relationship of the distance of POM to macropores (a) and roots (b) with N2O+N2 fluxes. Each point represents mean values of fluxes throughout the season for one static flux chamber. Stars indicate the significant association between the two parameters for the specific plant; p<0.05 =*, p<0.01 =**, ns = non-significant. Shadows show 95% confidence level interval for the predictions of the linear model.
Associations between N2O+N2 emissions and pore structure characteristics in the studied crops varied throughout the season (Fig. S2). For example, N2O+N2 emissions 7 days after fertilization (20th of May) were positively correlated to < 150 μm Ø pores in switchgrass (Fig. S5c). The unexpectedly high N2O+N2 emissions in switchgrass soil in late August was positively associated with the volume of roots observed within intact soil cores (Fig. S5d, p < 0.1), while not with other pore structural properties (not shown).
When examined across all three systems, there was a nonlinear positive trend of the fraction of bd’s increasing with increases in the anaerobic soil volume fraction (Fig. S6a) and the distance of POM to macropores (Fig. 6a). The fraction of bD was particularly low in soils of energy sorghum and maize when the soil anaerobic soil volume fraction was at its lowest and the distance from POM was at its highest, with bd fraction’s as low as <55% in some energy sorghum plots. Note that this distance represents the mean of all POM within a given sample. The fraction of bD increased to >90% in switchgrass and plateaued after the distance of POM to macropores exceeded 0.1 mm. The trend was opposite for pr, which in energy sorghum and maize soils exceeded 60% at small anaerobic soil volume fractions and short distances to POM, and then decreased to <50% with increasing the distance of POM to macropores. Yet, pr was <40% in switchgrass soils across the entire range of observed distances of POM to macropores. Note that the mean WFPS during the season did correlate with the total N2O + N2 emissions, but not with the fraction of bD and pr (Fig. S2a).
Influence of the distance of POM to macropores on (a) fraction of bacterial denitrification (bD) and the (b) denitrification product ratio (pr). Each point represents mean values of fluxes throughout the season for one static flux chamber. Stars indicate the significant association between the two parameters; p<0.05 =*, p<0.01 =**, ns = non-significant. Shadows show 95% confidence level interval for the predictions of the linear model.
Discussion
The effects of the pore structure on denitrification and N2 production across bioenergy systems.
Our observations support the notion of high importance of bD in N2O production, especially during events of high emissions (Fig. 3, Fig. 4a, Di Liang and Robertson 2021; Gao et al. 2023; Kravchenko et al. 2018; Ostrom et al. 2021). Furthermore, high anaerobic soil volume fractions and the distance of POM to macropores lead to a high amount of N2O being reduced to N2 (Fig. 6b,d). The reason for this could be that N2O formed in the anaerobic soil volume fraction, e.g. at hotspots of occluded POM, can potentially be reduced to N2 before it reaches the air-filled pore space (Braker and Conrad 2011; Rohe et al. 2021). As expected, high emissions of N2O+N2 corresponded to fertilizer application dates and changing soil moisture (Fig. 2a). The change in pore structure, however, led to high N2O emissions only in energy sorghum and maize, e.g. after fertilization (28th of June and 6th of July), where a relatively high denitrification pr [N2O / (N2O + N2)] (Fig. 4b) resulted in N2O fluxes > 50 g N day-1 ha-1 (Fig. 2c). Thus, mean N2O emissions increased in the order switchgrass < maize < energy sorghum (Table 1).
In rainfed areas of the US, bioenergy systems using sorghum were found to have comparable N2O emissions to those using maize (Kent et al. 2020). Indeed, in our study the two systems behaved very similarly with 1) a peak after fertilization and 2) the reduced importance of bD one month after fertilzation (Fig. 3a) as measured on the same fields two years before for maize (Fig. 2c, Ostrom et al. 2021), while the plots of switchgrass deviated from the others not only due to management strategies, e.g. different timing and amount of fertilzation. Switchgrass was found to have lower N2O emissions compared to maize (Tab. 1 and as shown before by Abraha et al. 2018 and Ostrom et al. 2021). The large anaerobic soil volume fractions in switchgrass, which were associated with a high fraction of bD and a low pr (Tab. 1, Fig. S6), lead to relatively low N2O emissions in switchgrass throughout the season (Fig. 2c). Even after the fertilization event in switchgrass (20th of May), N2O emissions were low (<25 g N day-1 ha-1), while N2O+N2 emissions peaked at approx. 100 g N day-1 ha-1 (Fig. 2c). In such a system with high relative contribution of bD, gross N2O consumption can exceed N2O production, which can make denitrification a net N2O sink (Philippot et al. 2011). Note that due to error propagation the calculation of N2 emissions from isotopic mapping tend to be imprecise especially at low fluxes, where methodological uncertainties are highest. A comparison to the 15N tracing technique applied to the field, however, showed that the mapping technique offers valid qualitative information about the N2 emissions, which are due to the high atmospheric N2 background rarely measured (Lewicka-Szczebak et al. 2020).
However, the distance threshold for anaerobic conditions was set to 0.41 mm, based on a sensitivity analyses before further evaluation (Fig. S4). This is more than double than that found by Kravchenko et al. (2018), but also about 1/10 of the 5 mm found by Rohe et al. (2021). The latter’s large distances, however, were related to repacked soil conditions, which can create completely different conditions with trapped gas pockets and reduced hotspots due to missing POM as compared to structured soil (Rohe et al. 2021). When, locally, the O2 demand exceeds the O2 supply, denitrification is favored (Rohe et al. 2021). Our results confirm former studies that show the capacity for X-ray CT to estimate the anaerobic soil volume fraction to describe O2 availability (Kravchenko et al. 2018; Rabot et al. 2015; Rohe et al. 2021) and additionally to quantify local hotspots created by POM (Kim et al. 2020; Kravchenko et al. 2018; Ortega-Ramírez et al. 2023). These hotspots are microsites of high O2 demand and unless connected to an air-filled macropore, anaerobic conditions develop that allow denitrification to occur. The anaerobic soil volume fraction and the distance of POM to macropores were highly correlated in our study (Fig. S2a), making both parameters equally good predictors of N2+N2O fluxes (Fig. 5a, Fig S5a) and of the activity of denitrifying bacteria (Fig. S6, Fig. S6a).
The highly negative correlation of pores <150 μm Ø with the anaerobic soil volume fraction (Fig. 2a) is in agreement with previous findings showing the importance of these pores to reduce the volume of anaerobic microsites (Kravchenko et al. 2018). Therefore, the <150 μm Ø pores were negatively associated with N2O+N2 fluxes in energy sorghum, while no significant association was found in the two other plant systems (Fig. S5b). This is in contrast to the laboratory incubation experiments with fresh residue additions or roots of young recently terminated plants (Kim et al. 2020, 2022; Kravchenko et al. 2017). In these studies, N2O emissions were enhanced by greater presence of <150 μm pores Ø and an associated enhanced creation of hotspots due to the sponge effect of fresh decomposing plant and reveals that in the field the effect of the anaerobic soil volume fraction can counteract the sponge effect. The intact cores of our study contained residues of old as well as young roots as well as POM in a wide range of decomposition stages and thus provided a more realistic assessment of field processes.
The anaerobic soil volume fraction varies under changing water contents at the same time that local hotspots created through POM will change O2 demand (Kravchenko et al. 2018; Rabot et al. 2015; Rohe et al. 2021; Schlüter et al. 2019). The relationship of bD with the anaerobic soil volume fraction and related structural properties derived from the cores taken in late August is therefore not on all days significant (Fig. S2). This was true, for instance, on 18 June, i.e. the day of the large rain event, when the anaerobic soil volume fraction was potentially much higher as estimated by our image analysis. On this day, however, Г-indicator, i.e. the connectivity of the pore space, was negatively correlated with the N2O + N2 fluxes. The potential lower infiltration in plots with low connectivity increased the anaerobic soil volume fraction during the rain event and led, in combination with the higher amounts of available NO3- (Fig. S2d), to the burst of N2O + N2 especially in energy sorghum (Fig. 2c).
Despite these variabilities, our findings highlight the value of our method to connect N2O production pathways during a crop season to microscale properties. In the future, pore scale modelling could be used to simulate the anaerobic soil volume fraction under contrasting conditions throughout the year and therefore improve the predictability of N2O+N2 emissions. To summarize, we have discovered a strong correlation between the anaerobic soil volume fraction and the distance of POM to macropores with the average fraction of bD in the bioenergy systems.
How the large roots system of the perennial switchgrass changes the N-cycle.
Switchgrass had lower N2O emissions compared to maize (Tab. 1), making it a promising biofuel crop option for mitigating climate change due to its low greenhouse gas emissions (Monti et al. 2012). Switchgrass as a perennial crop builds its large root system over several years and then maintains it, while most of the root system of sorghum and maize degrades shortly after harvest. The large volume of switchgrass roots create a pore structure dominated by large root-holding macropores at the expense of narrow macropores (Tab. 1), leading to larger anaerobic soil volume fraction and consequently high potential for bD (Fig. S6a, Fig. 4a). In addition, large volumes of POM generated from switchgrass massive root system serve as sources for denitrification (Fig. 6a). Thus, despite being supplied with only 1/3 of the N fertilizer, switchgrass showed numerically the highest N2O+N2 emissions compared to the annual systems (Tab. 1, Fig. 6a). Even though continuous measurements of the gas fluxes would be necessary to obtain unequivocal estimations for the entire season, our intermittently sampled data strongly suggest that switchgrass loses large amounts of the applied fertilizer N as N2. Higher N2O+N2 emissions from switchgrass is a novel finding given the lack of data in the literature for total denitrification.
Despite the high fraction of bD in switchgrass we found no significant association of the anaerobic soil volume fraction and the total N2+N2O emitted. One reason could be that we only used 5 cm cores from the topsoil and therefore were not able to include potential subsoil properties guiding denitrification (Shcherbak and Robertson 2019). Another could be that the lower application of urea and the large root system in switchgrass systems enhance the role of the root system’s affecting N-availability and consequently N2O+N2 production (Fig. 5b). Plant roots can modify the N balance in multiple ways; for example, the direct release of exudates can lead to priming effects, and the allocation of C to ectomycorrhizal fungi can increase the mineralization of org-N (Moreau et al. 2019). Such processes would release N in close proximity to switchgrass roots, i.e. the rhizosphere, which is potentially often compacted (Tab.1) and would result in local anaerobic hotpots’ driving complete denitrification (N2O reduction to N2). Note that the availability of such labile C form also drives denitrification (Baggs 2011; Groffman et al. 1988; Wrage et al. 2004). In addition, switchgrass is known to harness free living N-fixing bacteria (Roley et al. 2021), which could additionally fuel N2 + N2O emissions.
After full aboveground development and pollination switchgrass undergoes senescence. During this period remobilization of plant N into roots prior to harvest occurs (Yang and Udvardi 2018). During the sampling of N2O in late August, when the plant flowered, we found a decreasing importance of bD (Fig. 3a) and an increase in pr (Fig. 3b), while N2O+N2 fluxes were high (Fig. 2c). Interestingly, during this sampling date the root volume of switchgrass was positively correlated with total fluxes (Fig. S5d, p<0.1). Considering that switchgrass roots, when grown in monoculture, are predominantly located in large macropores (Lucas et al. 2023), we can surmise that N2O formed during the decomposition of senescent roots with elevated N content (Yang et al. 2016) readily escapes to the atmosphere, leading to the observed increases in pr. From this, it follows that the N source for N2O formation has shifted from the anaerobic soil volume fraction/matrix to the roots and their rhizodeposition, which however needs further investigation.
In summary, switchgrass with its massive root system stimulates denitrification both directly, by releasing labile N into the compacted rhizosphere, and indirectly, by forming large amounts of POM at large distances from macropores. This resulted in large N loses through N2O+N2 emissions.
The impact of BNI for the investigated sorghum variety
Our expectation was that 4 years of continuous cropping of energy sorghum with its potential for BNI (Subbarao et al. 2007) will lead to greater N2O production through the denitrification pathway relative to the nitrification pathway by reducing gross nitrification as compared to maize (Nardi et al. 2022). Indeed, energy sorghum seem to be capable of reducing the amount of ammonia oxidizing bacteria under field conditions (Bozal-Leorri et al. 2023). However, during the study season, the denitrification-based N2O gross production from the soil under energy sorghum was not greater than that from maize (Table 1). Moreover, on none of the sampling dates energy sorghum had significantly higher bD fraction than maize, while three days after fertilization (25th of June) the fraction of bD from sorghum soil was significantly lower compared to maize, suggesting a greater importance of nitrification (Fig. S3a) or fungal denitrification (Fig. 4a) there. The fertilization events with 28% urea-ammonium nitrate fertilizer and the rain event resulted in occasionally relatively high NH4+-concentrations in the soils of both energy sorghum and maize systems (Tab. S2). The presence of NH4+ in the rhizosphere could have stimulated the exudation of BNIs (Subbarao et al. 2015). Note that the largest N2O+N2 emissions from energy sorghum occurred at the beginning of the season (Fig. 2c), while large effects on gross nitrification by BNIs requires root proliferation as found only later in the growing season. This is because sorgoleone, a major BNI component of sorghum, is hydrophobic and thus likely to be restricted to the rhizosphere (Dayan et al. 2010; Subbarao et al. 2013). But even hydrophilic-BNIs that can diffuse into the soil (Gao et al. 2022) would impact nitrification in a limited soil volume if the root system is still under development.
However, the release of BNIs by sorghum can be highly variable depending on genotype (Gao et al. 2022; Sarr et al. 2020; Subbarao et al. 2015; Tesfamariam et al. 2014). As there are, to the best of our knowledge, no detailed analyses of BNIs releases by the variety used in our study (TAM 17651) we cannot draw any conclusions about other sorghum varieties. Thus, additional research with other varieties by using the isotopoic mapping approach is needed to investigate the effect of BNI by sorghum on gross-denitrification on the field. In summary, the importance of bD was already high for all plants throughout the season (Fig. 4a), and we could not identify any effect of potential nitrification inhibition by sorghum, which would have led to even higher importance of bD.
Conclusions
For the first time, we were able to link microscale properties with N2O+N2 emissions and production pathways in an agricultural cropping season. We show that the anaerobic soil volume fraction and the distance of POM to macropores derived from X-ray CT are important factors for bacterial denitrification and can be used to cover local variability in N2O+N2 emissions in the field. The large changes in these microscale properties measured across and within the investigated plant systems highlight the effect of certain plant species on pore structure and their potential use to mitigate GHG emissions. Although our study reflects fluxes from only a single season, and a multi-year observation period would be needed to assess long-term effects of cropping histories and soil structural differences on N2O+N2 emissions in field settings, results nonetheless demonstrate the feasibility and usefulness of N2O+N2 monitoring in combination with pore structural analysis in the field. Results also show the value of a future research focus on root traits’ leading to changes in pore structure and the distribution of POM, with the potential to reduce N loses by N2O+N2. Finally, our data questions the importance of BNI for the investigated sorghum variety, as we found no evidence for reduced gross nitrification.
References
Abraha M, Gelfand I, Hamilton SK, Chen J, Robertson GP (2018) Legacy effects of land use on soil nitrous oxide emissions in annual crop and perennial grassland ecosystems. Ecol Appl 28:1362–1369. https://doi.org/10.1002/eap.1745
Andersen AJ, Petersen SO (2009) Effects of C and N availability and soil-water potential interactions on N2O evolution and PLFA composition. Soil Biol Biochem 41:1726–1733. https://doi.org/10.1016/j.soilbio.2009.06.001
Bacq-Labreuil A, Crawford J, Mooney SJ, Neal AL, Ritz K (2019) Cover crop species have contrasting influence upon soil structural genesis and microbial community phenotype. Sci Rep 9:7473. https://doi.org/10.1038/s41598-019-43937-6
Baggs EM (2011) Soil microbial sources of nitrous oxide: recent advances in knowledge, emerging challenges and future direction. Curr Opin Environ Sustain 3:321–327. https://doi.org/10.1016/j.cosust.2011.08.011
Balaine N, Clough TJ, Beare MH, Thomas SM, Meenken ED, Ross JG (2013) Changes in Relative Gas Diffusivity Explain Soil Nitrous Oxide Flux Dynamics. Soil Sci Soc Am J 77:1496–1505. https://doi.org/10.2136/sssaj2013.04.0141
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends in Plant Sci 17:478–486. https://doi.org/10.1016/j.tplants.2012.04.001
Berg S, Kutra D, Kroeger T, Straehle CN, Kausler BX, Haubold C, Schiegg M, Ales J, Beier T, Rudy M, Eren K, Cervantes JI, Xu B, Beuttenmueller F, Wolny A, Zhang C, Koethe U, Hamprecht FA, Kreshuk A (2019) ilastik: interactive machine learning for (bio)image analysis. Nat Methods 16:1226–1232. https://doi.org/10.1038/s41592-019-0582-9
Bodner G, Leitner D, Kaul H-P (2014) Coarse and fine root plants affect pore size distributions differently. Plant Soil 380:133–151. https://doi.org/10.1007/s11104-014-2079-8
Bollmann A, Conrad R (1998) Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Glob Chang Biol 4:387–396. https://doi.org/10.1046/j.1365-2486.1998.00161.x
Bozal-Leorri A, Corrochano-Monsalve M, Arregui LM, Aparicio-Tejo PM, González-Murua C (2023) Evaluation of a crop rotation with biological inhibition potential to avoid N2O emissions in comparison with synthetic nitrification inhibition. J Environ Sci (China) 127:222–233. https://doi.org/10.1016/j.jes.2022.04.035
Braker G, Conrad R (2011) Diversity, structure, and size of N2O-producing microbial communities in soils--what matters for their functioning? Adv Appl Microbiol 75:33–70. https://doi.org/10.1016/B978-0-12-387046-9.00002-5
Butterbach-Bahl K, Dannenmann M (2011) Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr Opin Environ Sustain 3:389–395. https://doi.org/10.1016/j.cosust.2011.08.004
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci 368:20130122. https://doi.org/10.1098/rstb.2013.0122
Chen H, Williams D, Walker JT, Shi W (2016) Probing the biological sources of soil N2O emissions by quantum cascade laser-based 15N isotopocule analysis. Soil Biol Biochem 100:175–181. https://doi.org/10.1016/j.soilbio.2016.06.015
Dayan FE, Rimando AM, Pan Z, Baerson SR, Gimsing AL, Duke SO (2010) Sorgoleone. Phytochemistry 71:1032–1039. https://doi.org/10.1016/j.phytochem.2010.03.011
de Mendiburu F, Yaseen M (2020) agricolae: Statistical Procedures for Agricultural Research. R package version 1.4.0, https://myaseen208.github.io/agricolae/https://cran.r-project.org/package=agricolae
Di L, Robertson GP (2021) Nitrification is a minor source of nitrous oxide (N2 O) in an agricultural landscape and declines with increasing management intensity. Glob Chang Biol 27:5599–5613. https://doi.org/10.1111/gcb.15833
Doane TA, Horwáth WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36:2713–2722. https://doi.org/10.1081/AL-120024647
Domander R, Felder AA, Doube M (2021) BoneJ2 - refactoring established research software. Wellcome Open Res 6:37. 10.12688/wellcomeopenres.16619.2
Gao X, Uno K, Sarr PS, Yoshihashi T, Zhu Y, Subbarao GV (2022) High-sorgoleone producing sorghum genetic stocks suppress soil nitrification and N2O emissions better than low-sorgoleone producing genetic stocks. Plant Soil 477:793–805. https://doi.org/10.1007/s11104-022-05474-6
Gao N, Zhang F, Bo Q, An T, Gao J, Wei X, Yue S, Shen Y, Li S (2023) Microbial and isotopomer analysis of N2O production pathways in a calcareous film-mulched farmland. Biol Fertil Soils 59:407–422. https://doi.org/10.1007/s00374-023-01711-x
Groffman PM, Tiedje JM, Robertson GP, Christensen S (1988) Denitrification at different temporal and geographical scales: proximal and distal controls. In: Wilson JR (ed) Advances in nitrogen cycling in agricultural ecosystems: Proceedings of a Symposium on Advances in Nitrogen Cycling in Agricultural Ecosystems, held in Brisbane, Australia, 11-15 May 1987. C.A.B. International, Oxon, pp 174–192
Hildebrand T, Rüegsegger P (1997) A new method for the model-independent assessment of thickness in three-dimensional images. J Micr 185:67–75. https://doi.org/10.1046/j.1365-2818.1997.1340694.x
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152. https://doi.org/10.1007/s11104-008-9885-9
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33. https://doi.org/10.1007/s11104-009-9925-0
Kent J, Hartman MD, Lee DK, Hudiburg T (2020) Simulated biomass sorghum GHG reduction potential is dimilar to maize. Environ Sci Technol 54:12456–12466. https://doi.org/10.1021/acs.est.0c01676
Kim K, Guber A, Rivers M, Kravchenko A (2020) Contribution of decomposing plant roots to N2O emissions by water absorption. Geoderma 375:114506. https://doi.org/10.1016/j.geoderma.2020.114506
Kim K, Kutlu T, Kravchenko A, Guber A (2021) Dynamics of N2O in vicinity of plant residues: a microsensor approach. Plant Soil 462:331–347. https://doi.org/10.1007/s11104-021-04871-7
Kim K, Gil J, Ostrom NE, Gandhi H, Oerther MS, Kuzyakov Y, Guber AK, Kravchenko AN (2022) Soil pore architecture and rhizosphere legacy define N2O production in root detritusphere. Soil Biol Biochem 166:108565. https://doi.org/10.1016/j.soilbio.2022.108565
Kravchenko AN, Toosi ER, Guber AK, Ostrom NE, Yu J, Azeem K, Rivers ML, Robertson GP (2017) Hotspots of soil N2O emission enhanced through water absorption by plant residue. Nature Geosci 10:496–500. https://doi.org/10.1038/ngeo2963
Kravchenko AN, Guber AK, Quigley MY, Koestel J, Gandhi H, Ostrom NE (2018) X-ray computed tomography to predict soil N2O production via bacterial denitrification and N2O emission in contrasting bioenergy cropping systems. GCB Bioenergy 10:894–909. https://doi.org/10.1111/gcbb.12552
Kravchenko AN, Guber AK, Razavi BS, Koestel J, Quigley MY, Robertson GP, Kuzyakov Y (2019) Microbial spatial footprint as a driver of soil carbon stabilization. Nat Commun 10:3121. https://doi.org/10.1038/s41467-019-11057-4
Kravchenko AN, Richardson JA, Lee JH, Guber AK (2022) Distribution of Mn Oxidation States in Grassland Soils and Their Relationships with Soil Pores. 56:16462–16472 Environ Sci Technol. https://doi.org/10.1021/acs.est.2c05403
Legland D, Arganda-Carreras I, Andrey P (2016) MorphoLibJ: integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 32:3532–3534. https://doi.org/10.1093/bioinformatics/btw413
Lewicka-Szczebak D (2018) Mapping approach model after Lewicka-Szczebak et al. (2017) - detailed description of calculation procedures. 10.13140/RG.2.2.17478.52804
Lewicka-Szczebak D, Augustin J, Giesemann A, Well R (2017) Quantifying N2O reduction to N2 based on N2O isotopocules – validation with independent methods (helium incubation and 15N gas flux method). Biogeosciences 14:711–732. https://doi.org/10.5194/bg-14-711-2017
Lewicka-Szczebak D, Lewicki MP, Well R (2020) N2O isotope approaches for source partitioning of N2O production and estimation of N2O reduction – validation with the 15N gas-flux method in laboratory and field studies. Biogeosciences 17:5513–5537. https://doi.org/10.5194/bg-17-5513-2020
Li Y, Zhang Y, Chapman SJ, Yao H (2021) Biological nitrification inhibition by sorghum root exudates impacts ammonia-oxidizing bacteria but not ammonia-oxidizing archaea. Biol Fertil Soils 57:399–407. https://doi.org/10.1007/s00374-020-01538-w
Lucas M, Santiago JP, Chen J, Guber A, Kravchenko A (2023) The soil pore structure encountered by roots affects plant-derived carbon inputs and fate. New Phytol. https://doi.org/10.1111/nph.19159
Lucas M, Schlüter S, Vogel H-J, Vetterlein D (2019) Roots compact the surrounding soil depending on the structures they encounter. Sci Rep 9:16236. https://doi.org/10.1038/s41598-019-52665-w
Lucas M, Vetterlein D, Vogel H-J, Schlüter S (2020) Revealing pore connectivity across scales and resolutions with X-ray CT. Eur J Soil Sci. https://doi.org/10.1111/ejss.12961
Lucas M, Nguyen LTT, Guber A, Kravchenko AN (2022) Cover crop influence on pore size distribution and biopore dynamics: Enumerating root and soil faunal effects. Front Plant Sci 13:928569. https://doi.org/10.3389/fpls.2022.928569
McGill BM, Hamilton SK, Millar N, Robertson GP (2018) The greenhouse gas cost of agricultural intensification with groundwater irrigation in a Midwest U.S. row cropping system. Glob Chang Biol 24:5948–5960. https://doi.org/10.1111/gcb.14472
McSwiney CP, Robertson GP (2005) Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Glob Chang Biol 11:1712–1719. https://doi.org/10.1111/j.1365-2486.2005.01040.x
Monti A, Barbanti L, Zatta A, Zegada-Lizarazu W (2012) The contribution of switchgrass in reducing GHG emissions. Glob. Change Biol. Bioenergy 4:420–434. https://doi.org/10.1111/j.1757-1707.2011.01142.x
Moreau D, Bardgett RD, Finlay RD, Jones DL, Philippot L (2019) A plant perspective on nitrogen cycling in the rhizosphere. Funct Ecol 33:540–552. https://doi.org/10.1111/1365-2435.13303
Müller C, Laughlin RJ, Spott O, Rütting T (2014) Quantification of N2O emission pathways via a 15N tracing model. Soil Biol Biochem 72:44–54. https://doi.org/10.1016/j.soilbio.2014.01.013
Nardi P, Müller C, Pietramellara G, Subbarao GV, Nannipieri P (2022) Recommendations about soil Biological Nitrification Inhibition (BNI) studies. Biol Fertil Soils 58:613–615. https://doi.org/10.1007/s00374-022-01645-w
Oates LG, Duncan DS, Gelfand I, Millar N, Robertson GP, Jackson RD (2016) Nitrous oxide emissions during establishment of eight alternative cellulosic bioenergy cropping systems in the North Central United States. GCB Bioenergy 8:539–549. https://doi.org/10.1111/gcbb.12268
Ortega-Ramírez P, Pot V, Laville P, Schlüter S, Amor-Quiroz DA, Hadjar D, Mazurier A, Lacoste M, Caurel C, Pouteau V, Chenu C, Basile-Doelsch I, Henault C, Garnier P (2023) Pore distances of particulate organic matter predict N2O emissions from intact soil at moist conditions. Geoderma 429:116224. https://doi.org/10.1016/j.geoderma.2022.116224
Ostrom NE, Gandhi H, Coplen TB, Toyoda S, Böhlke JK, Brand WA, Casciotti KL, Dyckmans J, Giesemann A, Mohn J, Well R, Yu L, Yoshida N (2018) Preliminary assessment of stable nitrogen and oxygen isotopic composition of USGS51 and USGS52 nitrous oxide reference gases and perspectives on calibration needs. Rapid Commun Mass Spectrom 32:1207–1214. https://doi.org/10.1002/rcm.8157
Ostrom PH, DeCamp S, Gandhi H, Haslun J, Ostrom NE (2021) The influence of tillage and fertilizer on the flux and source of nitrous oxide with reference to atmospheric variation using laser spectroscopy. Biogeochemistry. https://doi.org/10.1007/s10533-020-00742-y
Philippot L, Hallin S, Börjesson G, Baggs EM (2009) Biochemical cycling in the rhizosphere having an impact on global change. Plant Soil 321:61–81. https://doi.org/10.1007/s11104-008-9796-9
Philippot L, Andert J, Jones CM, Bru D, Hallin S (2011) Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Glob Chang Biol 17:1497–1504
Qin Z, Zhuang Q, Zhu X (2015) Carbon and nitrogen dynamics in bioenergy ecosystems: 2. Potential greenhouse gas emissions and global warming intensity in the conterminous United States. GCB Bioenergy 7:25–39. https://doi.org/10.1111/gcbb.12106
Rabot E, Lacoste M, Hénault C, Cousin I (2015) Using X-ray Computed Tomography to Describe the Dynamics of Nitrous Oxide Emissions during Soil Drying. Vadose Zone Journal 14:vzj2014.12.0177. https://doi.org/10.2136/vzj2014.12.0177
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. https://doi.org/10.1126/science.1176985
Robertson GP, Groffman PM (2015) Nitrogen Transformations. In: Paul EA (ed) Soil Microbiology. Elsevier, Ecology and Biochemistry (Fourth Edition), pp 421–446
Robertson GP, Hamilton SK (2015) Long-term ecological research in agricultural landscapes at the Kellogg Biological Station LTER site: conceptual and experimental framework. In: Hamilton SK, Doll JE, Robertson GP (Eds) The Ecology of Agricultural Landscapes: Long-Term Research on the Path to Sustainability. Oxford, UK, pp 1–32
Rohe L, Apelt B, Vogel H-J, Well R, Wu G-M, Schlüter S (2021) Denitrification in soil as a function of oxygen availability at the microscale. Biogeosciences 18:1185–1201. https://doi.org/10.5194/bg-18-1185-2021
Roley SS, Ulbrich TC, Robertson GP (2021) Nitrogen Fixation and Resorption Efficiency Differences Among Twelve Upland and Lowland Switchgrass Cultivars. Phytobiomes J 5:97–107. https://doi.org/10.1094/PBIOMES-11-19-0064-FI
Sarr PS, Ando Y, Nakamura S, Deshpande S, Subbarao GV (2020) Sorgoleone release from sorghum roots shapes the composition of nitrifying populations, total bacteria, and archaea and determines the level of nitrification. Biol Fertil Soils 56:145–166. https://doi.org/10.1007/s00374-019-01405-3
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Schlüter S, Henjes S, Zawallich J, Bergaust L, Horn M, Ippisch O, Vogel H-J, Dörsch P (2018) Denitrification in Soil Aggregate Analogues-Effect of Aggregate Size and Oxygen Diffusion. Front. Environ. Sci. 6. https://doi.org/10.3389/fenvs.2018.00017
Schlüter S, Zawallich J, Vogel H-J, Dörsch P (2019) Physical constraints for respiration in microbial hotspots in soil and their importance for denitrification. Biogeosciences 16:3665–3678. https://doi.org/10.5194/bg-16-3665-2019
Schlüter S, Leuther F, Albrecht L, Hoeschen C, Kilian R, Surey R, Mikutta R, Kaiser K, Mueller CW, Vogel H-J (2022) Microscale carbon distribution around pores and particulate organic matter varies with soil moisture regime. Nat Commun 13:2098. https://doi.org/10.1038/s41467-022-29605-w
Shcherbak I, Robertson GP (2019) Nitrous Oxide (N2O) Emissions from Subsurface Soils of Agricultural Ecosystems. Ecosystems 22:1650–1663. https://doi.org/10.1007/s10021-019-00363-z
Sinsabaugh R, Reynolds H, Long T (2000) Rapid assay for amidohydrolase (urease) activity in environmental samples. Soil Biol Biochem 32:2095–2097. https://doi.org/10.1016/S0038-0717(00)00102-4
Stehfest E, Bouwman L (2006) N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agroecosyst 74:207–228. https://doi.org/10.1007/s10705-006-9000-7
Subbarao GV, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, Lascano C, Berry WL (2007) Biological nitrification inhibition (BNI)—is it a widespread phenomenon? Plant Soil 294:5–18. https://doi.org/10.1007/s11104-006-9159-3
Subbarao GV, Nakahara K, Ishikawa T, Ono H, Yoshida M, Yoshihashi T, Zhu Y, Zakir HAKM, Deshpande SP, Hash CT, Sahrawat KL (2013) Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 366:243–259. https://doi.org/10.1007/s11104-012-1419-9
Subbarao GV, Yoshihashi T, Worthington M, Nakahara K, Ando Y, Sahrawat KL, Rao IM, Lata J-C, Kishii M, Braun H-J (2015) Suppression of soil nitrification by plants. Plant Sci 233:155–164. https://doi.org/10.1016/j.plantsci.2015.01.012
Sutka RL, Ostrom NE, Ostrom PH, Gandhi H, Breznak JA (2003) Nitrogen isotopomer site preference of N2O produced by Nitrosomonas europaea and Methylococcus capsulatus Bath. Rapid Commun Mass Spectrom 17:738–745. https://doi.org/10.1002/rcm.968
Syakila A, Kroeze C (2011) The global nitrous oxide budget revisited. GHG Measure Manage 1(1):17–26. https://doi.org/10.3763/ghgmm.2010.0007
Tesfamariam T, Yoshinaga H, Deshpande SP, Srinivasa Rao P, Sahrawat KL, Ando Y, Nakahara K, Hash CT, Subbarao GV (2014) Biological nitrification inhibition in sorghum: the role of sorgoleone production. Plant Soil 379:325–335. https://doi.org/10.1007/s11104-014-2075-z
Tian H, Xu R, Canadell JG, Thompson RL, Winiwarter W, Suntharalingam P, Davidson EA, Ciais P, Jackson RB, Janssens-Maenhout G, Prather MJ, Regnier P, Pan N, Pan S, Peters GP, Shi H, Tubiello FN, Zaehle S, Zhou F et al (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586:248–256. https://doi.org/10.1038/s41586-020-2780-0
Walter K, Don A, Flessa H (2015) Net N2O and CH4 soil fluxes of annual and perennial bioenergy crops in two central German regions. Biomass Bioenergy 81:556–567. https://doi.org/10.1016/j.biombioe.2015.08.011
Wightman JL, Duxbury JM, Woodbury PB (2015) Land Quality and Management Practices Strongly Affect Greenhouse Gas Emissions of Bioenergy Feedstocks. Bioenerg. Res. 8:1681–1690. https://doi.org/10.1007/s12155-015-9620-3
Wrage N, Velthof G, van Beusichem M, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732. https://doi.org/10.1016/S0038-0717(01)00096-7
Wrage N, Lauf J, Del Prado A, Pinto M, Pietrzak S, Yamulki S, Oenema O, Gebauer G (2004) Distinguishing sources of N2O in European grasslands by stable isotope analysis. Rapid Commun Mass Spectrom 18:1201–1207. https://doi.org/10.1002/rcm.1461
Yang J, Udvardi M (2018) Senescence and nitrogen use efficiency in perennial grasses for forage and biofuel production. J Exp Bot 69:855–865. https://doi.org/10.1093/jxb/erx241
Yang J, Worley E, Ma Q, Li J, Torres-Jerez I, Li G, Zhao PX, Xu Y, Tang Y, Udvardi M (2016) Nitrogen remobilization and conservation, and underlying senescence-associated gene expression in the perennial switchgrass Panicum virgatum. New Phytol 211:75–89. https://doi.org/10.1111/nph.13898
Yu L, Harris E, Lewicka-Szczebak D, Barthel M, Blomberg MRA, Harris SJ, Johnson MS, Lehmann MF, Liisberg J, Müller C, Ostrom NE, Six J, Toyoda S, Yoshida N, Mohn J (2020) What can we learn from N2O isotope data? - Analytics, processes and modelling. Rapid Commun Mass Spectrom 34:e8858. https://doi.org/10.1002/rcm.8858
Yunusa IAM, Newton PJ (2003) Plants for amelioration of subsoil constraints and hydrological control: the primer-plant concept. Plant Soil 257:261–281. https://doi.org/10.1023/A:1027381329549
Acknowledgments
We thank Stacey Vanderwulp and Kevin Kahrmark for their assistance with field flux chamber installation and sample collection. We thank Maxwell Oerther for his help during soil sampling and processing, Michelle Quigley for her support during the X-ray CT scans. The authors gratefully acknowledge the help of Hasand Gandhi and Samuel DeCamp with the isotopic analysis. We thank the anonymous reviewers for their suggestions, which greatly improved the manuscript.
This research was funded in part by the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number DE-SC0018409, by the NSF LTER Program (DEB 1027253) at the Kellogg Biological Station, and by Michigan State University AgBioResearch. The Great Lakes Bioenergy Research Center provided the open access funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1397 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lucas, M., Gil, J., Robertson, G.P. et al. Changes in soil pore structure generated by the root systems of maize, sorghum and switchgrass affect in situ N2O emissions and bacterial denitrification. Biol Fertil Soils (2023). https://doi.org/10.1007/s00374-023-01761-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00374-023-01761-1