Abstract
Agriculture is likely to expand poleward with climate change, encouraging deforestation for agriculture in subarctic regions, which alters soil physical, chemical and biological properties and potentially affects microbial metabolic efficiency. Deciphering how and by which mechanisms land-use change affects microbial carbon use efficiency (CUE) will enable the development of mitigation strategies to alleviate C losses. We assessed CUE using 18O-labelled water in a paired-plot approach on soils collected from 19 farms across the subarctic region of Yukon, Canada, comprising 14 pairs of forest-to-grassland conversion and 15 pairs of forest-to-cropland conversion. Microbial CUE significantly increased following conversion to grassland and cropland. Land-use conversion resulted in a lower estimated abundance of fungi, while the archaeal abundance increased. Interestingly, structural equation modelling revealed that increases in CUE were mediated by a rise in soil pH and a decrease in soil C:N ratio rather than by shifts in microbial community composition, i.e. the ratio of fungi, bacteria and archaea. Our findings indicate a direct control of abiotic factors on microbial CUE via improved nutrient availability and facilitated conditions for microbial growth. Overall, this implies that to a certain extent CUE can be managed to achieve a more efficient build-up of stabilised soil organic C (SOC), as reflected in increased mineral-associated organic C under agricultural land use. These insights may also help constrain SOC models that generally struggle to predict the effects of deforestation, something that is likely to take place more frequently in the subarctic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is likely to lead to agriculture expanding further poleward (Tchebakova et al. 2011; Franke et al. 2021). This will encourage the conversion of natural vegetation to agricultural land, which has been found to negatively affect soil organic C (SOC) stocks in various climate zones (Guo and Gifford 2002; Grünzweig et al. 2004; Don et al. 2011). Subarctic ecosystems might be particularly vulnerable to land-use change as large amounts of terrestrial C, protected by permafrost and low temperatures, will be exposed to decomposition following deforestation (Grünzweig et al. 2004; Peplau et al. 2022). However, agricultural activity might also increase C stocks in the long term in soils previously unaffected by permafrost, probably related to a shift towards stable C forms that reduce decomposability and increased C input from agricultural management (Peplau et al. 2022). Deforestation and conversion of pristine forest to agricultural land largely alter the conditions under which C is cycling, especially in what were originally nutrient-poor and cold subarctic soils.
Deciphering the impact of land-use change on soil microbial processes is crucial for predicting C dynamics and may facilitate the development of mitigation strategies since soil microbes play a key role in biogeochemical cycles. They process organic matter through enzymatic decomposition and transform complex organic into mineral compounds, where C is lost to the atmosphere during the predominant heterotrophic respiration (Schimel and Schaeffer 2012). Microbial C use efficiency (CUE) is a measure of microbial metabolic processes and is defined as the ratio of C allocated to growth to total C metabolised (Manzoni et al. 2012; Sinsabaugh et al. 2013). Under favourable growth conditions, microbial CUE is high and metabolised C is largely directed to microbial growth with relatively low amounts of CO2-C lost to the atmosphere. It is assumed that a high CUE results in the build-up of microbial biomass, which becomes structural material for mineral-associated soil organic matter when it dies (Miltner et al. 2012; Kallenbach et al. 2016; Soong et al. 2020). An increase in CUE is likely to reflect more efficient SOC stabilisation per unit C entering the soil (Liang et al. 2017). Whether CUE increases or decreases after deforestation for agriculture might affect the extent of C losses typically occurring after land-use conversion. Insights into the drivers of alterations in CUE induced by land-use changes would potentially allow the development of mitigation strategies to alleviate these C losses.
Discussions in the literature suggest that soil microbial CUE depends on substrate stoichiometry (Keiblinger et al. 2010; Manzoni et al. 2012; Sinsabaugh et al. 2016), soil pH (Manzoni et al. 2012; Malik et al. 2018; Jones et al. 2019) and microbial community composition (e.g. Soares and Rousk 2019; Saifuddin et al. 2019; Bölscher et al. 2016). For example, CUE is expected to be highest when substrate stoichiometry is close to the microbial stoichiometric needs (Manzoni et al. 2012). Hence, high substrate C:N ratios are correlated with low CUE as microbes respire excess C to mine organic N resources when N is limited (Craine et al. 2007). The positive correlation between CUE and soil pH observed across a wide range of soils is associated with an alleviation of H+-/Al3+-induced stress and a shift in microbial community composition (Jones et al. 2019). It is still a matter of debate whether a small relative abundance of fungi induces higher CUE or vice versa (e.g. Thiet et al. 2006; Soares and Rousk 2019; Domeignoz-Horta et al. 2020).
Land-use change is one of the strongest instantaneous alterations to which an ecosystem and soil can be subjected, altering soil physical, chemical and biological properties (Grünzweig et al. 2004; Smith et al. 2016). Changes occur due to altered vegetation, microclimate and abiotic soil properties induced by management, such as liming, fertilisation and irrigation. Land-use change is thought to drive microbial CUE and possibly the whole ecosystem CUE (Manzoni et al. 2018). Subarctic forest soils usually have high C:N ratios and nutrient limitation (Deluca and Boisvenue 2012), potentially restricting microbial CUE. Changes in the quantity and quality of organic matter soil inputs and increased nutrient availability in fertilised agricultural soils are likely to affect the microbial metabolism by alleviating stoichiometric imbalances. Soil pH might be increased after land-use conversion due to deforestation practices (e.g. pile burning) and agricultural management practices such as liming (Pietikäinen and Fritze 1995; Grover et al. 2021), which could result in higher CUE. Furthermore, CUE could be affected by shifts in soil microbial abundance and diversity induced by changes in soil pH (Wakelin et al. 2008; Rousk et al. 2010a; Zhou et al. 2020). For example, the fungal-to-bacterial (F:B) ratio often declines after deforestation and conversion to agricultural land (Zhou et al. 2018). Therefore, we hypothesised that in subarctic soils, CUE increases upon deforestation for agricultural land use. However, as the potential influencing factors of CUE (i.e. substrate stoichiometry, soil pH, microbial community composition) are closely interrelated, the mechanisms by which land-use change will alter CUE are unclear. This knowledge is crucial in order to upscale land-use change effects on soil C dynamics and also assess whether and how CUE could be deliberately manipulated.
Only a few studies have evaluated CUE under different land-use types. These studies, which differ in their experimental design, climatic zone and methodological approach (e.g. 18O-labelling, calorespirometry, CUE estimation based on PLFA), showed diverging results: some found the highest CUE in forest soils (Bölscher et al. 2016; Canarini et al. 2020; Li et al. 2021a), while others found CUE to be highest in arable soils (Martí-Roura et al. 2019; Soares and Rousk 2019; Zheng et al. 2019). How deforestation and conversion to agricultural land affect soil microbial CUE during the decomposition of soil organic matter in subarctic soils remains largely unstudied. In the rare cases where land-use changes have been studied in a paired-plot design, allowing site effects on CUE to be excluded, the number of sites was restricted to two temperate (Zheng et al. 2019) and six Mediterranean sites (Martí-Roura et al. 2019). Paired-plot approaches have not yet been applied to subarctic soils.
The aim of this study was to provide evidence of the effect of deforestation for agricultural land use on CUE and identify the drivers of this. These results will contribute to the development of management strategies to influence CUE and thus potentially reduce C losses associated with land-use change, especially in particularly vulnerable subarctic soils. Using a unique dataset based on a paired-plot approach on 19 farms across the subarctic Yukon Territory of Canada, we tested the hypothesis that deforestation and subsequent agricultural land use increase microbial CUE. Furthermore, we evaluated the main mechanisms of land-use changes influencing CUE using structural equation modelling (SEM).
Methods
Study area and sampling
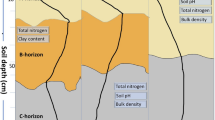
The sampling campaign was conducted from June to August 2019 at 19 sites covering the main centres of agricultural activity in Yukon, Canada (Fig. 1). The study area has a subarctic climate (Table 1) and discontinuous permafrost, and the most prevalent soil types at the studied sites are Cambisols, Fluvisols and Cryosols (IUSS Working Group WRB 2015). The distribution of general soil parameters is depicted in Fig. 2 and shows lower SOC content at a constant N content, and thus a reduced soil C:N ratio as well as a shift towards higher soil pH, following conversion of pristine boreal forest to agricultural land. Detailed information on soil characteristics per site can be retrieved from the repository specified for this study. Land-use history is relatively short as it began at the end of the nineteenth century with the Klondike gold rush; thus, the duration of agricultural land use among the sampled sites ranged from 5 to 125 years.
This study was designed as a paired-plot experiment comprising 48 plots (19 forest, 14 grassland and 15 cropland plots) and thus 29 pairs (14 representing forest to grassland conversion and 15 representing forest to cropland conversion). The forest plots served as a reference for the initial conditions prior to land-use conversion, usually mixed-wood forests of the boreal cordillera ecoregion (Smith et al. 2004). The grassland category comprises pastures and meadows for hay production. Cropland refers to the small-scale agricultural systems between market gardens and cropland that are found in Yukon, with vegetables, greens and herbs. Typically, the market gardens are irrigated and amended with organic fertilisers produced on the farm or near-by (e.g. manure, compost) without further use of mineral fertilisers. Croplands are tilled occasionally to a depth of between 10 and 30 cm. Detailed information on management is given in Peplau et al. (2022).
For each plot, three composite samples were collected within a sampling area of 10 m × 10 m. To compile individual composite samples, the litter layer was removed manuallyand a slide-hammer driven soil corer (6 cm diameter) was used to collect three individual soil cores from the top 10 cm which were then homogenised by hand. Approximately 50 g fresh weight soil of each composite sample was frozen at − 20 °C within 8 h and subsequently stored and shipped frozen.
Determination of 18O-CUE
To assess microbial CUE of the native soil organic matter, the 18O-labelling method was conducted according to the original protocol of Spohn et al. (2016a) with the same modifications as described in Schroeder et al. (2021). In detail, microbial growth is determined based on the incorporation of isotopically labelled water into the newly synthesised DNA. Prior to CUE analysis, frozen samples were thawed and 2 mm-sieved. Samples were pre-incubated at 15 °C for 1 week after adjusting the water content to 45% water-holding capacity (WHC). For CUE analysis, two 300 mg fresh weight aliquots of the pre-incubated soil were weighed into Eppendorf vials, then placed into 20-ml glass vials and crimp-sealed. Labelled water H218O (80 at% 18O) was added to label one aliquot at 20 at% 18O in the final soil water while adjusting the water content to 60%WHC. The same amount of bi-distilled water was added to the natural abundance control. To equalise the starting conditions, vials with labelled samples were evacuated and flushed with standard gas (348 ppm CO2, 1300 kPa) within 1 min of 18O water addition. A gas sample of 20 ml was taken from the labelled samples with a manual gas syringe (SGE Syringe, Trajan Scientific and Medical) after 24-h incubation in the dark at 15 °C. Subsequently, the vials were de-crimped pairwise and soil samples were immediately frozen in liquid N2. Non-labelled and labelled samples were stored at − 80 °C until DNA extraction. Gas samples were analysed using a gas chromatograph equipped with an electron capture detector (Agilent 7890A GC, Agilent Technologies) and respiration flux was calculated from the increase in CO2 concentration within 24-h incubation using the ideal gas equation as described in Schroeder et al. (2021).
DNA was extracted from labelled and non-labelled soil samples using the FastDNA™ SPIN Kit for Soil (MP Biomedicals) following the standard protocol, with an extension of the centrifugation to 15 min in step five (25.155 × g, 15.000 rpm, Sigma 4-16KS). The DNA concentration in the extracts was quantified with the QuantiT PicoGreen dsDNA Kit (Invitrogen). The isotopic signatures of dried DNA extracts (oven-dried at 60 °C in silver capsules) were measured using a high-temperature conversion/elemental analyser (TC/EA) (Thermo Fisher Scientific) coupled with a Delta V Plus isotope ratio mass spectrometer via a ConFloIV interface (Thermo Fisher Scientific).
The increase in 18O in the DNA was calculated based on isotopic signatures of labelled and non-labelled samples and an initial enrichment of 20 at% 18O, and extrapolated to DNA via the relative proportion of oxygen in the DNA (31.21%w/w) to derive the amount of DNA produced within 24 h of incubation (Schroeder et al. 2021). The allocation rate to microbial biomass production (CGrowth) was then derived by multiplying the amount of DNA produced by the conversion factor fDNA, i.e. microbial biomass C over total DNA extracted, where fDNA values were derived for individual field samples. The microbial CUE is defined as microbial biomass C produced, i.e. CGrowth, over the total uptake of C, as an approximation of the sum of microbial biomass C produced and C respired (CGrowth + CRespiration) (Manzoni et al. 2012; Sinsabaugh et al. 2013). Means and standard deviations of microbial growth and respiration rates and fDNA per land-use type for the pairs of land-use conversion are provided in Table S1.
Determination of microbial biomass C and N
After pre-incubation, microbial biomass C and N were determined by the chloroform fumigation extraction method (Brookes et al. 1985; Vance et al. 1987). In brief, fumigation was conducted for 24 h at room temperature in the dark, providing an excess amount (approx. 50 ml for 15 samples) of chloroform (CHCl3) and adjusting a pressure < 200 mbar. To get rid of chloroform artefacts, exsiccators were defumigated thoroughfully in 7 cycles after removing the remaining CHCl3. The non-fumigated and fumigated 5 g aliquots were extracted with 0.5 M K2SO4 in a 1:4 soil-to-extractant ratio (30 min horizontal shaking at 200 rpm) and filtered. Non-purgeable organic C (NPOC) was analysed in a 1:4 v/v extract dilution after the removal of total inorganic C by adding 15% HCl in order to adjust to pH 2–3 and outgassing emerging CO2 for 5 min with artificial air (Dimatoc 2000, DIMATEC Analysetechnik). Microbial biomass C was calculated with a conversion factor of 0.45 (Joergensen 1996). The total N in the extracts was measured in 1:10 v/v dilutions using a Total Nitrogen Analyser TN-100 (Nittoseiko Analytech). Microbial biomass N was calculated using a conversion factor of 0.54 (Joergensen and Mueller 1996). Mean and standard deviation of microbial biomass C and N per land-use type for the pairs of land-use conversion are provided in Table S1.
Microbial abundance by qPCR
The abundances of bacteria, archaea and fungi were estimated from the non-labelled DNA extracts by qPCR using the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories) (Hemkemeyer et al. 2015). The abundance of archaea and bacteria was estimated according to the TaqMan probe approach. Amplification of the 16S rRNA gene of archaea and bacteria was conducted using the primers ARC787F, ARC1059R and BAC338F, BAC805R. The probes ARC915F and BAC516F were used for quantification of the same gene (Yu et al. 2005). Fungal ITS1 sequences were amplified using the primers NS1 and 58A2R and quantified by SYBR Green (Martin and Rygiewicz 2005). Reactions were carried out in duplicate from 50 to 100–fold dilutions of the DNA extracts. Standard curves for the respective domains were generated using DNA from pure cultures of Bacillus subtilis, Methanobacterium oryzae and Fusarium culmorum. The PCR efficiencies were 96.1 ± 1.74% (R2 = 0.998) for archaea, 95.5 ± 1.38% (R2 = 0.999) for bacteria, and 94.2 ± 3.74% (R2 = 0.997) for fungi.
General soil properties
For each plot, soil properties were determined on oven-dried (40 °C) and 2 mm-sieved pooled soil core samples, as described in Peplau et al. (2022). The soil total organic C and N were determined on milled aliquots by dry combustion (LECO TruMac). Additionally, samples with soil pH > 6.5 were analysed for carbonates via stepwise combustion at 450 °C for 12 h (LECO RC612). Water holding capacity was quantified by soaking 10 g soil placed on a cotton wool-padded funnel with water. The water content quantified when water run-off stopped was assumed to resemble 100%WHC. Soil pH was measured in a 1:5 w/v ratio soil to H2O (1 h shaking horizontally, 200 rpm). Following Olsen (1954), extractable P was determined in a 1:20 w/v ratio soil to 0.5 M NaHCO3 solution adjusted to pH 8.5 by horizontally shaking (30 min, 180 rpm) and filtering (hw3, Sartorius Stedim Biotech). Extracts were measured by inductively coupled plasma optical emission spectroscopy (ICP-OES, iCAP 7400 Thermo Fisher Scientific) at a wavelength of 178.284 nm (mode of measurement: axial). Soil texture was measured in accordance with ISO 11277:2009 (clay < 2 µm, silt 2–63 µm, sand > 63 and < 2000 µm), which is based on a combination of sieving and sedimentation of fine particles in a suspension (Köhn 1929).
Specific-surface area and clay mineralogy
The specific surface area (SSA) of bulk soil and clay mineralogy were determined on oven-dried and 2 mm-sieved pooled soil core samples of the reference (forest) plot. The SSA of bulk soil was determined by the multipoint Brunauer, Emmet and Teller (BET) method (Brunauer et al. 1938; ISO 9277, 2010) using a Tristar 3000 (Micromeritics Instrument Corporation) with N2 as the adsorbate at a fixed temperature of 77.3 K (boiling point liquid N2). Prior to SSA analysis, the samples were degassed for 72 h with H2 gas at a relatively low temperature of 50 °C to remove adsorbed contaminants while avoiding irreversible surface changes. Clay mineralogy was determined by X-ray powder diffraction (XRD) analysis on air-dried 40 mg-smears of the samples, following H2O2 removal of organic matter to reduce background noise (Girard et al. 2004). X-ray patterns were recorded on a Bruker D8 Advance Powder Diffractometer equipped with a LYNXEYE detector (Co Kα radiation set at 35 kV and 40 mA). The results of the semi-quantitative analysis of smears were based on the Reference Intensity Ratio method (EVA software, Bruker Nano, Inc.).
Statistics
Statistical analyses and data visualisation were conducted in R v3.6.3 (R Core Team 2020) using RStudio v1.1.463 (RStudio Team 2016). The following packages were used: tidyverse (Wickham et al. 2019), RcolorBrewer (Neuwirth 2014), lme4 (Bates et al. 2015), lmerTest (Kuznetsova et al. 2017), emmeans (Lenth 2021), multcomp (Hothorn et al. 2008), multcompView (Graves et al. 2019), corrplot (Wei and Simko 2021), MVN (Korkmaz et al. 2014), Hmisc (Harrell 2021), ggfortify (Tang et al. 2016), lavaan (Rosseel 2012), lavaan.survey (Oberski 2014), lavaanPlot (Lishinski 2021), ggpmisc (Aphalo 2021), cowplot (Wilke 2020), rgdal (Bivand et al. 2021), sf (Pebesma 2018), ggmap (Kahle and Wickham 2013), rnaturalearth (South 2017) and ggspatial (Dunnington 2021). Unless otherwise stated, the values below are given as mean ± standard deviation.
To test whether CUE, estimated abundances, soil pH and C:N ratio differed significantly between land-use types, a linear mixed-effects model approach was used on forest-grassland (F-G) and forest-cropland (F–C) paired data. The mixed-effects models accounted for the paired character of the experimental design by setting land use as the fixed effect and site as the random effect, allowing for random intercepts. For CUE and estimated abundances, an additional random intercept was introduced for the sets in which samples had been analysed (six different sets containing samples from different sites) to account for potential variation in laboratory conditions. A visual inspection of residual plots was used to check for deviations from homoscedasticity or normality, and data were log-transformed where necessary. Significance of the fixed effect was assessed at a significance level of α = 0.05. Estimated marginal means were calculated and differences between land-use types are given as a compact letter display in the respective figures at a significance level of α = 0.05. The p-values were adjusted according to Tukey. A table of estimated marginal means, standard errors and confidence intervals is provided in the supplementary material (Table S2).
To reduce the complexity of the clay mineralogy data and extract the most important gradients, a principal component analysis (PCA) was conducted on the relative proportions of minerals. The first principal component explained 61.8% of the variation by a gradient of illite and plagioclase content. The second principal component mainly explaining the quartz content accounted for 19.3% of the observed variation. The scores of the first and second principal components were used as a proxy for clay mineralogy for further analyses.
Spearman correlation analysis was conducted on individual land-use types, i.e. forest, grassland and cropland soils, as well as on all available data combined in order to assess the most important drivers of CUE in subarctic soils.
To investigate the mechanisms of land-use mediated changes in CUE, we conducted a SEM analysis (Grace 2006). The SEM was performed on the combined dataset (grassland and cropland pairs) — including a random effect for site — to increase the statistical power. A detailed description of how the SEM analysis was conducted can be found in the supplementary material. In essence, the SEM was built based on an a priori developed conceptual meta model (Figure S1, Table S3) and fitted using the robust maximum-likelihood estimator MLM. Significance and goodness of fit of the SEM were assessed using a combination of model fit indices, i.e. χ2 p-value > 0.05, CFI < 0.95, RMSEA < 0.05, and SRMR < 0.08. Each path in the final model was evaluated for a significant contribution (***p < 0.001, **p < 0.01, *p < 0.05) and the standardised coefficients, explained variance (R2) per response variable and total effects of exogenous variables on CUE were calculated.
Results
Higher CUE in agricultural soils
The conversion of pristine boreal forest to agricultural land significantly increased microbial CUE from 0.25 ± 0.09 (forest) to 0.32 ± 0.12 in grassland soils (30%, p < 0.001), and from 0.24 ± 0.09 (forest) to 0.28 ± 0.11 in cropland soils (26%, p < 0.01) (Fig. 3). Both the SOC-normalised microbial respiration rate (p < 0.001 F-G and F–C) and growth rate (p = 0.051 F-G and p = 0.035 F–C) decreased after conversion to agricultural land. Overall, increases in CUE were associated with greater reductions in CRespiration than in CGrowth. In addition, SOC-normalised microbial biomass C increased by 72 ± 125% (median: 35%) in grassland and by 64 ± 124% (median: 34%) in cropland soils, with the increase being significant in grassland only.
Conversion from pristine boreal forest to agricultural land significantly increased microbial C use efficiency (CUE). Tukey-style box-and-whisker plots of CUE per land use for the paired-plot comparisons. Different letters indicate significant differences between land-use types at a significance level of α = 0.05
Shift in estimated abundance of fungi and archaea under land-use change
The linear mixed-effects models showed a significant reduction in the estimated abundance of fungi (p < 0.001 F-G, p = 0.045 F–C) (Fig. 4). The estimated abundance of bacteria remained unaltered with land-use change, but agricultural practices significantly increased the estimated abundance of archaea (p < 0.001 F-G and F–C). Thus, the main effect of land-use change on the relative abundance of domains was a reduction in the fungi-to-archaea ratio (F:A ratio) (Fig. 5).
Soil microbial community as affected by land-use type. Tukey-style box-and-whisker plots of the estimated abundances of fungi, bacteria and archaea per land-use type for paired-plot comparisons. Estimated abundances are given as the log10-transformed number of gene copies g.−1 soil. Different letters indicate significant differences between land-use types at a significance level of α = 0.05
CUE is correlated with soil pH, soil stoichiometry and microbial community composition
Overall, CUE was correlated with the abundance of archaea, and thus with the fungi-to-archaea and bacteria-to-archaea ratio, as well as with soil pH, soil C:N ratio, clay mineralogy, specific surface area and climate (Fig. 6). Soil pH was only weakly correlated with CUE in forest soils and did not increase over a wide range of soil pH from 4.4 to 7.9. In grassland and cropland, however, CUE was positively correlated with soil pH (Fig. 7A). Microbial CUE was negatively correlated with soil C:N ratio (Fig. 7B). Increases in the abundance of archaea under agricultural land use were linked to increased CUE (Fig. 7C). Furthermore, the abundance of archaea was also positively correlated with soil pH, which in turn was positively linked to CUE (Fig. 7D).
Correlogram of microbial C use efficiency (CUE) and soil and site characteristics given for forest, grassland and cropland soils individually, as well as for all 48 plots combined. The correlation of CUE with soil pH was weaker in forest soils than in agricultural soils. The colour indicates the Spearman’s correlation coefficient ρ and asterisks indicate the significance level (* < 0.05, ** < 0.01, *** < 0.001)
Microbial C use efficiency (CUE) was A positively correlated with soil pH, B negatively correlated with the ratio of soil organic C to total N (C:N ratio), and C positively correlated with the estimated abundance of archaea (log10-transformed number of gene copies g.−1 soil). D Covariance of estimated abundance of archaea and soil pH with different land-use types impeded interpretation of the driver of CUE increases under agricultural land use. Error bars indicate standard deviation from the mean values of field replicates (n = 3)
Changes in CUE are induced by altered soil pH and stoichiometry
The SEM was established to analyse indirect (e.g. via changes in soil microbial community structure, soil pH and C:N ratio) and direct effects of land-use type on CUE. The covariance structure of the model was in close agreement with the observed covariances within our multivariate dataset (model goodness of fit indices: χ2 p-value > 0.05, CFI < 0.95, RMSEA < 0.05, SRMR < 0.08), capturing 31% of the overall variance in CUE. No significant direct effect of land-use type on CUE was found (Fig. 8), indicating that the conceptual model considered all relevant land use-dependent factors. Conversion to agricultural land led to significant increases in soil pH and a reduction in the soil C:N ratio. This was further confirmed by the linear mixed-effects model testing for differences between land-use types in terms of soil pH (p < 0.001 F-G and F–C) and C:N ratio (p < 0.001 F-G and F–C) (Table S2). Furthermore, soil pH was linked to site-specific clay mineralogy, here represented as a composite variable of the first two principal components of the clay mineralogy data, with PC1 representing a gradient of illite and plagioclase and PC2 indicating the quartz content. The SEM and mediation analysis showed that deforestation for agricultural land use affected CUE directly through an increase in soil pH, with an estimated standardised effect size of 0.19 standard deviations (p = 0.023). Microbial community composition was affected by land use–driven changes in soil pH (p < 0.001). A direct relationship between stoichiometry (C:N soil) and microbial community structure could not be detected. However, other land use–specific changes that are not explained by the causal network influenced the F:A ratio, as indicated by a significant path between land use and F:A ratio in the SEM. Smaller C:N ratios upon agricultural land use also mediated increases in CUE, with a standardised effect size of 0.15 standard deviations (p = 0.003). The total standardised effect of land use on CUE was 0.20 standard deviations, due to the contrasting effects of F:A ratio (negative) and C:N ratio and soil pH (both positive) on CUE (Figure S2). The total standardised effect of the mineral phase was − 0.20 standard deviations and was mainly mediated through effects on soil pH. Figure 8 shows standardised coefficients, while unstandardised coefficients are provided in the supplementary material (Table S4). Summarising the results of the SEM, land use–driven changes in CUE were mediated through shifts in soil pH and C:N ratio.
Best fit for the structural equation model (SEM) to the entire dataset showing that the effects of deforestation and conversion to agricultural land on microbial C use efficiency (CUE) are mediated through shifts in soil pH and C:N ratio rather than through the altered relative abundance of fungi, bacteria and archaea. To account for the paired-plot approach, the site was introduced as a random effect. Furthermore, a composite variable (hexagonal box) of the first two principal components of clay mineralogy was included to account for site-related effects on soil pH and C:N ratio. The numbers next to arrows represent the standardised coefficients for each path and R.2 indicates the variance explained by the model for each endogenous variable. To enable reproducibility, the thickness of the edges was set at 10 points times the estimate of the corresponding coefficient. Black indicates a positive effect and red a negative effect. The transparency of the arrow colours indicates the level of significance, with 20% not significant, 50% p < 0.05, 75% p < 0.01 and 100% p < 0.001
Discussion
Deforestation for agriculture increases CUE
Deforestation for agricultural use resulted in a significant increase in CUE of 30% and 26% in subarctic grassland and cropland soils, respectively, which supports our hypothesis. For comparison, in a paired-plot approach using the 18O-labelling method, a higher CUE was found in grassland soils than in forest soils, while cropland soils showed no significant increases in CUE at the two temperate sites studied (Zheng et al. 2019). In another paired-plot approach based on six Mediterranean sites, CUE of forest soils was lower than that of cultivated and abandoned agricultural soils, which was associated with limited nutrient availability and higher C:N ratios in the forest soils (Martí-Roura et al. 2019). Our results are in line with the limited number of available paired-plot studies that show higher CUE after land-use conversion. However, the presented findings contrast with the only other study to have examined the effects of land-use type on CUE specifically in subarctic soils. Bölscher et al. (2016) reported higher substrate use efficiencies in Swedish subarctic forest soils compared with ley farming, grassland and cropland soils. The authors linked the higher substrate use efficiency to greater abundance of fungi and the different microbial community composition overall. However, the significance of their results for C processes in subarctic soils may be limited because of the following: (i) substrate use efficiencies by calorespirometry do not represent the CUE of soil organic matter decomposition and thus do not allow conclusions to be drawn on in situ C cycling (Geyer et al. 2019) and (ii) the forest soil (Haplic Podzol) was not paired with agricultural soils (Eutric Cambisols) and the authors concluded that site effects may have interfered with their findings. Thus, their study design does not allow any conclusions to be made about the effects of land-use change on CUE. Here, we provide evidence that the deforestation of pristine boreal forest and conversion to agricultural land increases microbial CUE during decomposition of soil organic matter.
Abiotic drivers of CUE
Apart from land-use change per se, soil C:N ratio and soil pH explained most of the variability in CUE, with soil pH positively and soil C:N ratio negatively correlated with CUE (Fig. 7A + B). Land-use conversion of natural systems to agricultural land induces large shifts in abiotic soil conditions. The observed increase in soil pH after land-use conversion was expected for the studied soils since deforestation via clear cutting and pile burning (a common practice in subarctic Yukon) has been found to increase soil pH by 1.7 units in a forest soil in northern Finland (Pietikäinen and Fritze 1995). According to a survey, Yukon farmers mostly use organic fertilisers and occasionally wood-ash amendments (Peplau et al. 2022). These additional management practices further explain higher soil pH in agricultural soils because of the following: (i) soil pH can be actively increased by liming or wood-ash amendment (Cruz-Paredes et al. 2017; Grover et al. 2021) and (ii) long-term mineral N fertilisation usually reduces soil pH in agricultural soils (Chien et al. 2008), while application of organic fertilisers does not (Xiao et al. 2021). The latter effect therefore does not counteract the former. The significant decline in C:N ratio with deforestation and conversion to agricultural land observed here has been widely reported (e.g. Murty et al. 2002; Grünzweig et al. 2004; Xu et al. 2013). Changes in soil stoichiometry are related to alterations in the amount and quality of organic matter inputs, changes in decomposition processes and altered nutrient inputs, e.g. via fertilisation (Murty et al. 2002). Boreal forest organic matter inputs are mostly wood-derived and of low quality with high C:N ratios (Deluca and Boisvenue 2012). On a global scale, forest litter exhibits relatively high C:N values of 66 and even higher ratios of 88 have been reported for coniferous forest litter (McGroddy et al. 2004), whereas the C:N ratio of manure and plant amendments is reported to vary between 10 and 29 (Bertrand et al. 2019). Thus, differences in input stoichiometry in forest and agricultural systems may explain lower C:N ratios after land-use conversion.
Overall, CUE was significantly positively correlated with soil pH in this study, which is in line with the results of several other studies reporting a positive correlation between CUE and soil pH in agricultural soils (Malik et al. 2018; Jones et al. 2019; Xiao et al. 2021). It has been suggested that crossing a proposed threshold pH-value of pHH2O = 6.2 (Malik et al. 2018) or pHCaCl2 = 5.5 (Jones et al. 2019) might promote increases in CUE by reducing the trade-off caused by stress alleviation (e.g. H3O+, Al3+). Our results do not suggest a threshold, but the positive correlation of CUE with soil pH holds true for agricultural soils. Interestingly, the subarctic forest soils investigated here did not show a significant relationship between CUE and soil pH, although they covered a soil pH range of 4.4 to 7.9 (Fig. 6, Fig. 7A). This finding is in line with a study reporting that 18O-CUE did not increase over a pHH2O gradient from 3.7 to 6.5 across a forest transect (Wang et al. 2021). Taken together, microbial metabolic efficiency of forest soils might not depend on soil pH across sites. However, a higher CUE was reported for a forest soil after laboratory liming, which was linked to an increased bacterial-to-fungal growth ratio at higher soil pH (Silva-Sánchez et al. 2019). This result suggests different overall CUE depending on the dominance of fungal or bacterial growth. While bacterial growth exerts a narrow pH optimum, fungal growth is less restricted by soil pH (Rousk et al. 2010b). Thus, the absence of CUE dependence on soil pH across different forest soils might be linked to a generally higher abundance of fungi in forest soils. It is also possible that other factors such as soil stoichiometry or the higher chemical complexity of organic matter input from woody vegetation outcompete pH effects on CUE in forest soils.
Soil microbes are largely homeostatic in their biomass C:N:P stoichiometry (Cleveland and Liptzin 2007; Xu et al. 2013) and cope with stoichiometric imbalances between their needs and the available resources by partly adapting biomass C:N:P, e.g. shifts in community composition, excretion of exo-enzymes or by adjusting the CUE by overflow respiration (Craine et al. 2007; Manzoni et al. 2012; Mooshammer et al. 2014; Sinsabaugh et al. 2016). Therefore, it is assumed that the highest CUE can be obtained when substrate stoichiometry is close to microbial biomass stoichiometry (Manzoni et al. 2012). Here, soil C:N and C:P ratios declined with agricultural land use, suggesting an alleviation of stoichiometric imbalances that could explain the facilitated metabolism and higher CUE in the studied subarctic soils. The soil C:N ratio was the parameter that best represented the effects of soil stoichiometry on CUE in the present study, while soil C:P and N:P were not correlated with CUE (Fig. 6). In line with this observation, 20% higher substrate use efficiency has been reported at a substrate C:N ratio of 10 compared with 50, while substrate C:P and C:S ratios were not correlated with CUE (Khan and Joergensen 2019). Corroborating the stoichiometric imbalance concept, N fertilisation has been reported to directly control CUE (Spohn et al. 2016b), while 18O-CUE negatively correlated with the dissolved organic C: dissolved N ratio across six grassland soils but was unaffected by additional N and P supply (Widdig et al. 2020). These diverging results with regard to N-addition effects on CUE suggest that factors other than direct nutrient availability control the relationship between CUE and soil stoichiometry. For example, a positive correlation between fungal CUE and C:N ratios has been found in pure culture media of different C:N:P ratios, while bacterial CUE was highest at low C:N ratios and reduced when stoichiometric imbalances increased (Keiblinger et al. 2010). The authors linked divergent relationships to differences in fungal and bacterial biomass stoichiometry, with reported C:N ratios of 10 for fungi and 4 for bacteria in soils (Sylvia et al. 2005). This suggests that in soils, the relative abundance of fungi and bacteria could affect how CUE is linked to soil C:N ratios.
Biotic drivers of CUE
Here, we found a positive correlation between CUE and archaeal abundance, estimated based on qPCR-based 16S rRNA gene copy numbers, which increased after land-use conversion (Fig. 4, Fig. 7C). Archaeal abundance was positively correlated with soil pH (Fig. 7D, Figure S3). Increases in estimated archaeal abundance with higher soil pH, comparable in magnitude to our observations, have previously been reported (Bengtson et al. 2012; Grover et al. 2021), and soil archaeal community composition has been found to be permanently altered after deforestation of boreal forest (Jurgens and Saano 1999). The crucial role of methanogenic archaea in biogeochemical C cycling under anoxic conditions, i.e. in paddy rice field and peat bogs, is well known. However, under aerobic conditions, there is currently no evidence that archaeal organisms play a major role in soil organic C mineralisation (Offre et al. 2013). Thaumarchaeota (formerly described as mesophilic Crenarchaeota) are the most dominant archaeal phylum in soils (Bates et al. 2015; Zheng et al. 2019). Among these, the subgroup of ammonia-oxidising archaea (AOA) contributes to the nitrification process by oxidising NH3 to NO2− (Offre et al. 2013). Thaumarchaeota are described as depending on the assimilation of inorganic C sources, i.e. CO2 or HCO3−, which were largely abundant in the carbonate-rich soils of the present study. We therefore hypothesise that Thaumarchaeota are the most prevalent archaea phylum in these soils, but this was not investigated. Generally, it can be expected that the relative contribution of archaea to heterotrophic respiration in soils is minor compared with that of fungi and bacteria. Accordingly, the results of the present study’s SEM demonstrate that archaeal abundance had no direct effect on CUE.
In previous studies, microbial CUE has been found to be linked to the fungal-to-bacterial growth ratio, with a higher CUE at lower F:B ratios (Soares and Rousk 2019; Silva-Sanchez et al. 2019). This finding suggests that abiotic factors might mediate shifts in CUE via control of the relative abundances of fungi and bacteria. However, the F:B ratio and fungal abundance were not found to have a strong control on CUE (Fig. 6), which is in line with several other studies (Thiet et al. 2006; Spohn et al. 2016b; Zheng et al. 2019; Domeignoz-Horta et al. 2020). The decline in estimated fungal abundance observed here corroborated the observation that conversion to agricultural land induces a decline in soil microbial F:B ratios (Zhou et al. 2018). While bacterial community is largely shaped by soil pH, fungal abundance and community composition have been associated with changes in soil nutrient status (Lauber et al. 2008). Indeed, the estimated abundance of fungi was linked to soil P content across the studied subarctic soils (Figure S3). Furthermore, relative and estimated fungal abundance was negatively correlated with soil pH. This is consistent with decreasing qPCR-based F:B ratios along an agricultural soil pH gradient ranging from 4.5 to 8.3 due to bacterial growth dominating and suppressing fungal growth at a higher pH (Rousk et al. 2010a,b).
Land use–driven increase in CUE is mediated through abiotic factors, not through a shift in microbial relative abundance
The presented dataset allowed an investigation of the mechanisms by which land-use conversion affects CUE in subarctic soils. Most interestingly, agricultural land use was found to increase CUE via abiotic drivers, i.e. soil pH and soil C:N ratio. While microbial community was shaped by soil pH, it did not affect CUE within our model (Fig. 8). Therefore, it was concluded that the observed correlation between CUE and F:A ratio or archaeal abundance must be interpreted as a pseudo-correlation explained by the strong link between archaeal abundance and soil pH (Fig. 7D). Our results strongly suggest that agricultural management increases CUE by altering microbial physiology, alleviating nutrient limitations through fertilisation or bringing soil pH closer to optimal growing conditions, rather than by shifts in the relative abundance of fungi, bacteria and archaea.
Soil pH was found to have an indirect effect on CUE by its effect on enzymatic activity (Sinsabaugh et al. 2016) or microbial α-diversity (Malik et al. 2018), using SEM approaches. However, a direct effect of soil pH on CUE was not included in these models and thus was not tested. A SEM approach used to test the mechanisms by which pH alters CUE in a grassland with increasing artificial soil acidification from a pHH2O of 6.9 to 5.9 indicates that pH might affect CUE at near neutral pH by alleviating C:N imbalances rather than by induction of Al3+ toxicity or shifts in F:B ratio (Li et al. 2021b). Their finding supports our conclusion that the effect of altered soil pH was not mediated through shifts in the relative abundances of fungi, bacteria and archaea, but through altered nutrient availability, which was further supported by the indirect effect of land-use type on CUE via soil C:N ratio in this study’s SEM.
Implications for C dynamics in subarctic soils under global change
Here, we revealed that land-use conversion of particularly vulnerable subarctic soils affects microbial CUE via altered soil pH and soil stoichiometry. The land-use effect on CUE does not necessarily have to be generally valid, as shown by Zheng et al. (2019) who found that CUE in arable soil was higher compared to a paired forest soil on lime bedrock while it was reduced on silicate bedrock. It is much more the changes in abiotic conditions due to land-use change that alter CUE. For instance, the extent to which pH and C:N are affected by land-use conversion might explain the differences observed between different studies and sites. In the study of Zheng et al. (2019), pH was increased from 4.0 to 5.9 on silicate bedrock while it may have passed a critical threshold at the site on lime bedrock by shifting the pH from 6.1 in forest soil to 8.1 in arable soil. Our results, based on a high number of paired-plots, suggest that it may be possible to influence and control microbial metabolic efficiency directly through targeted management options. Microbial CUE was positively correlated with soil pH in grasslands and croplands; therefore, we hypothesise that CUE can be actively managed by agricultural practices that increase soil pH, e.g. liming. These practices could also include the use of organic fertiliser, which, unlike mineral fertiliser, do not result in soil acidification and have been positively correlated with CUE in a long-term fertilisation experiment (Xiao et al. 2021). Current agricultural practices in Yukon mainly rely on organic fertilisation due to small-scale agriculture including animal stocks and to the rather limited availability of expensive mineral fertilisers. Wood ash is often applied to the soil after deforestation, given the clear-cutting and pile-burning practices, leading to initial increases in soil pH after conversion. The increase in CUE after land-use conversion might thus be related to the specific agricultural practice in the Yukon.
Higher microbial CUE after deforestation for agriculture in subarctic soils might alleviate C losses by a more efficient microbial biosynthesis, since microbial necromass contributes significantly to the stabilised soil C pool (Miltner et al. 2012; Kallenbach et al. 2016; Liang et al. 2017; Sokol et al. 2019). Indeed, we found microbial biomass per unit C to increase in agricultural soils compared with forest soils and to be positively correlated with CUE. Furthermore, for the same sites, Peplau et al. (2022) found that SOC quality was altered after land-use conversion: while the rather labile particulate organic matter pool was depleted, the absolute amount of mineral-associated organic matter increased under cropland and grassland. This would also fit with the results of Angst et al. (2021), who found a higher contribution of microbial-derived compounds in aggregates and mineral-associated organic matter of cropland and grassland soils than in forest soils. At the same time, the Yukon sites show a tendency of SOC replenishment under agricultural land use after initial losses upon deforestation (Peplau et al. 2022), which has similarly previously been observed in Alaska (Grünzweig et al. 2004). All together, these findings point to the importance of microbial anabolism to SOC cycling as a whole, which implies that managing microbial CUE should potentially become an integral part of climate-smart agriculture. At the same time, the in vivo pathway was recently found insufficient to explain mineral-associated SOC formation in temperate forest soils, as the stimulation of microbial growth by high-quality litter enhanced SOC decomposition and thus offset the positive effects via enhanced efficiency on SOC stabilisation (Craig et al. 2022). In the modelling community, the debate about whether and to what extent microbial pools and processes should be represented in soil C models is ongoing (Woolf and Lehmann 2019). It is known that widely used models such as RothC have difficulties in predicting land-use change, particularly deforestation effects on SOC stocks (Gottschalk et al. 2010). The present study, which used a large number of paired plots and a wide range of abiotic soil properties, provides evidence that deforestation of pristine boreal forests and subsequent agricultural land use greatly affects the microbial metabolism, and thus the in vivo C stabilisation pathway (Sokol et al. 2019), through changes in organic matter quality and abiotic soil properties. Reflecting such metabolic shifts in a mechanistic way might or might not lead to more accurate prediction and upscaling of SOC stock change upon land-use change. More accurate predictions are urgently needed, as deforestation is a major contributor to climate change, which in turn will exacerbate land-use changes on the vast subarctic landmass around the world.
Conclusions
Our findings suggest that shifts in physico-chemical soil properties following deforestation and conversion to agricultural land in subarctic soils, i.e. increase in soil pH and lower C:N ratio, may enhance microbial metabolic efficiency directly. The positive relationship between CUE and soil pH in agricultural soils suggests that managing CUE through practices that increase soil pH could alleviate land use change-induced C losses and potentially promote the formation of stable mineral-associated SOC through the in vivo pathway. However, it is unclear how the increases in CUE observed in harmonised laboratory experiments, which are small in absolute numbers, will translate to in situ conditions where differences in water availability and temperature between land-use types may offset effects of soil pH and C:N ratio on metabolic efficiency. Nevertheless, our results imply that introducing abiotic drivers of the microbial metabolic efficiency may improve model predictions of microbial physiology and potentially C dynamics upon land-use change.
Data availability
All data and R code used for this study are freely available at [10.5281/zenodo.6720275].
References
Angst G, Mueller KE, Nierop KG, Simpson MJ (2021) Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol Biochem 156:108189. https://doi.org/10.1016/j.soilbio.2021.108189
Aphalo PJ (2021) ggpmisc: miscellaneous extensions to ‘ggplot2’. R Package Version 0.3.9. https://CRAN.R-project.org/package=ggpmisc. Accessed 01 May 2022
Climate Atlas of Canada. (2019) version 2 Using BCCAQv2 climate model data. www.climateatlas.ca. Accessed 19 August 2021
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bengtson P, Sterngren AE, Rousk J (2012) Archaeal abundance across a pH gradient in an arable soil and its relationship to bacterial and fungal growth rates. Appl Environ Microbiol 78:5906–5911. https://doi.org/10.1128/AEM.01476-12
Bertrand I, Viaud V, Daufresne T, Pellerin S, Recous S (2019) Stoichiometry constraints challenge the potential of agroecological practices for the soil C storage. A Review Agron Sustainable Dev 39:336. https://doi.org/10.1007/s13593-019-0599-6
Bivand R, Keitt T, Rowlingson B (2021) rgdal: bindings for the ‘geospatial’ data abstraction library. R package version 1.5–23. https://CRAN.R-project.org/package=rgdal. Accessed 01 May 2022
Bölscher T, Wadsö L, Börjesson G, Herrmann AM (2016) Differences in substrate use efficiency: impacts of microbial community composition, land use management, and substrate complexity. Biol Fertil Soils 52:547–559. https://doi.org/10.1007/s00374-016-1097-5
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Sci Total Environ 17:837–842. https://doi.org/10.1016/0038-0717(85)90144-0
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. JACS 60:309–319. https://doi.org/10.1021/ja01269a023
Canarini A, Wanek W, Watzka M, Sandén T, Spiegel H, Šantrůček J, Schnecker J (2020) Quantifying microbial growth and carbon use efficiency in dry soil environments via 18O water vapor equilibration. Global Change Biol 26:5333–5341. https://doi.org/10.1111/gcb.15168
Chien SH, Gearhart MM, Collamer DJ (2008) The effect of different ammonical nitrogen sources on soil acidification. Soil Sci 173:544–551. https://doi.org/10.1097/SS.0b013e31817d9d17
Cleveland CC, Liptzin D (2007) C:N: P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252. https://doi.org/10.1007/s10533-007-9132-0
Craig ME, Geyer KM, Beidler KV, Brzostek ER, Frey SD, Stuart Grandy A, Liang C, Phillips RP (2022) Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat Commun 13:1229. https://doi.org/10.1038/s41467-022-28715-9
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113. https://doi.org/10.1890/06-1847.1
Cruz-Paredes C, Wallander H, Kjøller R, Rousk J (2017) Using community trait-distributions to assign microbial responses to pH changes and Cd in forest soils treated with wood ash. Soil Biol Biochem 112:153–164. https://doi.org/10.1016/j.soilbio.2017.05.004
Deluca TH, Boisvenue C (2012) Boreal forest soil carbon: distribution, function and modelling. Forestry 85:161–184. https://doi.org/10.1093/forestry/cps003
Domeignoz-Horta LA, Pold G, Liu X-JA, Frey SD, Melillo JM, DeAngelis KM (2020) Microbial diversity drives carbon use efficiency in a model soil. Nat Commun 11:3684. https://doi.org/10.1038/s41467-020-17502-z
Don A, Schumacher J, Freibauer A (2011) Impact of tropical land-use change on soil organic carbon stocks - a meta-analysis. Global Change Biol 17:1658–1670. https://doi.org/10.1111/j.1365-2486.2010.02336.x
Dunnington D (2021) ggspatial: spatial data framework for ggplot2. https://CRAN.R-project.org/package=ggspatial. Accessed 01 May 2022
Franke JA, Müller C, Minoli S, Elliott J, Folberth C, Gardner C, Hank T, Izaurralde RC, Jägermeyr J, Jones CD, Liu W, Olin S, Pugh TAM, Ruane AC, Stephens H, Zabel F, Moyer EJ (2021) Agricultural breadbaskets shift poleward given adaptive farmer behavior under climate change. Global Change Biol 28:167–181. https://doi.org/10.1111/gcb.15868
Geyer KM, Dijkstra P, Sinsabaugh R, Frey SD (2019) Clarifying the interpretation of carbon use efficiency in soil through methods comparison. Soil Biol Biochem 128:79–88. https://doi.org/10.1016/j.soilbio.2018.09.036
Girard I, Klassen RA, Laframboise RR (2004) Sedimentology Laboratory Manual, Terrain Sciences Division, Geolgocial Survey of Canada, Open File 4823
Gottschalk P, Bellarby J, Chenu C, Foereid B, Smith P, Wattenbach M, Zingore S, Smith J (2010) Simulation of soil organic carbon response at forest cultivation sequences using 13C measurements. Org Geochem 41:41–54. https://doi.org/10.1016/j.orggeochem.2009.04.017
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Graves S, Piepho H-P, Selzer L, with help from Dorai-Raj, Sundar (2019) multcompView: visualizations of paired comparisons. R package version 0.1–8. https://CRAN.R-project.org/package=multcompView. Accessed 01 May 2022
Grover SP, Butterly CR, Wang X, Gleeson DB, Macdonald LM, Tang C (2021) Liming and priming: the long-term impact of pH amelioration on mineralisation may negate carbon sequestration gains. Soil Security 3:100007. https://doi.org/10.1016/j.soisec.2021.100007
Grünzweig JM, Sparrow SD, Yakir D, Chapin FS (2004) Impact of agricultural land-use change on carbon storage in boreal Alaska. Global Change Biol 10:452–472. https://doi.org/10.1111/j.1529-8817.2003.00738.x
Guo LB, Gifford RM (2002) Soil carbon stocks and land use change: a meta analysis. Global Change Biol 8:345–360. https://doi.org/10.1046/j.1354-1013.2002.00486.x
Harrell Jr. FE (2021) Hmisc: Harrell Miscellaneous: with contributions from Charles Dupont and many others. R package version 4.5–0. https://CRAN.R-project.org/package=Hmisc. Accessed 01 May 2022
Hemkemeyer M, Christensen BT, Martens R, Tebbe CC (2015) Soil particle size fractions harbour distinct microbial communities and differ in potential for microbial mineralisation of organic pollutants. Soil Biol Biochem 90:255–265. https://doi.org/10.1016/j.soilbio.2015.08.018
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J Biometrische Zeitschrift 50:346–363. https://doi.org/10.1002/bimj.200810425
International Organization for Standardization (2009) ISO 11277:2009 Soil quality - determination of particle size distribution in mineral soil material - method by sieving and sedimentation
International Organization for Standardization (2010) ISO 9277:2010 Determination of the specific surface area of solids by gas adsorption — BET method
IUSS Working Group WRB (2015) WRB. 2015. World reference base for soil resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Sci Total Environ 28:25–31. https://doi.org/10.1016/0038-0717(95)00102-6
Joergensen RG, Mueller T (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEN value. Soil Biol Biochem 28:33–37. https://doi.org/10.1016/0038-0717(95)00101-8
Jones DL, Cooledge EC, Hoyle FC, Griffiths RI, Murphy DV (2019) pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities. Soil Biol Biochem 138:107584. https://doi.org/10.1016/j.soilbio.2019.107584
Jurgens G, Saano A (1999) Diversity of soil Archaea in boreal forest before, and after clear-cutting and prescribed burning. FEMS Microbiol Ecol 29:205–213. https://doi.org/10.1111/j.1574-6941.1999.tb00612.x
Kahle D, Wickham H (2013) ggmap: Spatial Visualization with ggplot2. R J 5:144–161
Kallenbach CM, Frey SD, Grandy AS (2016) Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat Commun 7:13630. https://doi.org/10.1038/ncomms13630
Keiblinger KM, Hall EK, Wanek W, Szukics U, Hämmerle I, Ellersdorfer G, Böck S, Strauss J, Sterflinger K, Richter A, Zechmeister-Boltenstern S (2010) The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol Ecol 73:430–440. https://doi.org/10.1111/j.1574-6941.2010.00912.x
Khan KS, Joergensen RG (2019) Stoichiometry of the soil microbial biomass in response to amendments with varying C/N/P/S ratios. Biol Fertil Soils 55:265–274. https://doi.org/10.1007/s00374-019-01346-x
Köhn M (1929) Korngrößenanalyse Vermittels Pipettenanalyse. Tonindustrie-Zeitung 53:729–731
Korkmaz S, Goksuluk D, Zararsiz G (2014) MVN: an R package for assessing multivariate normality. R J 6:151–161
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82. https://doi.org/10.18637/jss.v082.i13
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415. https://doi.org/10.1016/j.soilbio.2008.05.021
Lenth RV (2021) emmeans: estimated marginal means, aka least-squares means. R package version 1.5.4.https://CRAN.R-project.org/package=emmeans . Accessed 01 May 2022
Li T, Wang R, Cai J, Meng Y, Wang Z, Feng X, Liu H, Turco RF, Jiang Y (2021) Enhanced carbon acquisition and use efficiency alleviate microbial carbon relative to nitrogen limitation under soil acidification. Ecol Process 10:79. https://doi.org/10.1186/s13717-021-00309-1
Li J, Pei J, Dijkstra FA, Nie M, Pendall E (2021b) Microbial carbon use efficiency, biomass residence time and temperature sensitivity across ecosystems and soil depths. Soil Biol Biochem 154:108117. https://doi.org/10.1016/j.soilbio.2020.108117
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2:17105. https://doi.org/10.1038/nmicrobiol.2017.105
Lishinski A (2021) lavaanPlot: path diagrams for ‘Lavaan’ models via ‘DiagrammeR’. R package version 0.6.2. https://CRAN.R-project.org/package=lavaanPlot. Accessed 01 May 2022
Malik AA, Puissant J, Buckeridge KM, Goodall T, Jehmlich N, Chowdhury S, Gweon HS, Peyton JM, Mason KE, van Agtmaal M, Blaud A, Clark IM, Whitaker J, Pywell RF, Ostle N, Gleixner G, Griffiths RI (2018) Land use driven change in soil pH affects microbial carbon cycling processes. Nat Commun 9:3591. https://doi.org/10.1038/s41467-018-05980-1
Manzoni S, Taylor P, Richter A, Porporato A, Agren GI (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91. https://doi.org/10.1111/j.1469-8137.2012.04225.x
Manzoni S, Čapek P, Porada P, Thurner M, Winterdahl M, Beer C, Brüchert V, Frouz J, Herrmann AM, Lindahl BD, Lyon SW, Šantrůčková H, Vico G, Way D (2018) Reviews and syntheses: carbon use efficiency from organisms to ecosystems – definitions, theories, and empirical evidence. Biogeosciences 15:5929–5949. https://doi.org/10.5194/bg-15-5929-2018
Martin KJ, Rygiewicz PT (2005) Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol 5:28. https://doi.org/10.1186/1471-2180-5-28
Martí-Roura M, Hagedorn F, Rovira P, Romanyà J (2019) Effect of land use and carbonates on organic matter stabilization and microbial communities in Mediterranean soils. Geoderma 351:103–115. https://doi.org/10.1016/j.geoderma.2019.05.021
McGroddy ME, Daufresne T, Hedin LO (2004) Scaling of C:N: P stoichiometry in forests worldwide: implications of terrestrial redfield-type ratios. Ecology 85:2390–2401. https://doi.org/10.1890/03-0351
Miltner A, Bombach P, Schmidt-Brücken B, Kästner M (2012) SOM genesis: microbial biomass as a significant source. Biogeochemistry 111:41–55. https://doi.org/10.1007/s10533-011-9658-z
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A (2014) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22. https://doi.org/10.3389/fmicb.2014.00022
Murty D, Kirschbaum MUF, McMurtrie RE, McGilvray H (2002) Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Global Change Biol 8:105–123. https://doi.org/10.1046/j.1354-1013.2001.00459.x
Neuwirth E (2014) RColorBrewer: colorbrewer palettes. R package version 1.1–2. https://CRAN.R-project.org/package=RColorBrewer. Accessed 01 May 2022
Oberski D (2014) lavaan.survey: an R package for complex survey analysis of structural equation models. J Stat Softw 57. https://doi.org/10.18637/jss.v057.i01
Offre P, Spang A, Schleper C (2013) Archaea in biogeochemical cycles. Annu Rev Microbiol 67:437–457. https://doi.org/10.1146/annurev-micro-092412-155614
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate, vol 939. US Department of Agriculture
Pebesma E (2018) Simple features for R: standardized support for spatial vector data. R J 10:439–446. https://doi.org/10.32614/RJ-2018-009
Peplau T, Schroeder J, Gregorich E, Poeplau C (2022) Subarctic soil carbon losses after deforestation for agriculture depend on permafrost abundance. Global Change Biol. https://doi.org/10.1111/gcb.16307
Pietikäinen J, Fritze H (1995) Clear-cutting and prescribed burning in coniferous forest: comparison of effects on soil fungal and total microbial biomass, respiration activity and nitrification. Sci Total Environ 27:101–109. https://doi.org/10.1016/0038-0717(94)00125-K
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 01 May 2022
Rosseel Y (2012) lavaan: an R package for structural equation modeling. J Stat Softw 48. https://doi.org/10.18637/jss.v048.i02
Rousk J, Brookes PC, Bååth E (2010) Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol Biochem 42:926–934. https://doi.org/10.1016/j.soilbio.2010.02.009
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351. https://doi.org/10.1038/ismej.2010.58
RStudio Team (2016) RStudio: integrated development environment for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/. Accessed 01 May 2022
Saifuddin M, Bhatnagar JM, Segrè D, Finzi AC (2019) Microbial carbon use efficiency predicted from genome-scale metabolic models. Nat Commun 10:3568. https://doi.org/10.1038/s41467-019-11488-z
Schimel JP, Schaeffer SM (2012) Microbial control over carbon cycling in soil. Front Microbiol 3:348. https://doi.org/10.3389/fmicb.2012.00348
Schroeder J, Kammann L, Helfrich M, Tebbe CC, Poeplau C (2021) Impact of common sample pre-treatments on key soil microbial properties. Soil Biol Biochem 160:108321. https://doi.org/10.1016/j.soilbio.2021.108321
Silva-Sánchez A, Soares M, Rousk J (2019) Testing the dependence of microbial growth and carbon use efficiency on nitrogen availability, pH, and organic matter quality. Soil Biol Biochem 134:25–35. https://doi.org/10.1016/j.soilbio.2019.03.008
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett 16:930–939. https://doi.org/10.1111/ele.12113
Sinsabaugh RL, Turner BL, Talbot JM, Waring BG, Powers JS, Kuske CR, Moorhead DL, Follstad Shah JJ (2016) Stoichiometry of microbial carbon use efficiency in soils. Ecol Monogr 86:172–189. https://doi.org/10.1890/15-2110.1
Smith P, House JI, Bustamante M, Sobocká J, Harper R, Pan G, West PC, Clark JM, Adhya T, Rumpel C, Paustian K, Kuikman P, Cotrufo MF, Elliott JA, McDowell R, Griffiths RI, Asakawa S, Bondeau A, Jain AK, Meersmans J, Pugh TAM (2016) Global change pressures on soils from land use and management. Global Change Biol 22:1008–1028. https://doi.org/10.1111/gcb.13068
Smith CA, Meikle JC, Roots CF (eds) (2004) Ecoregions of the Yukon territory: biophysical properties of Yukon landscapes, PARC Technical Bulletin No. 04–01. Agriculture and Agri-Food Canada, PARC Technical Bulletin No. 04–01, Summerland, British Columbia
Soares M, Rousk J (2019) Microbial growth and carbon use efficiency in soil: links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol Biochem 131:195–205. https://doi.org/10.1016/j.soilbio.2019.01.010
Sokol NW, Sanderman J, Bradford MA (2019) Pathways of mineral-associated soil organic matter formation: integrating the role of plant carbon source, chemistry, and point of entry. Global Change Biol 25:12–24. https://doi.org/10.1111/gcb.14482
Soong JL, Fuchslueger L, Marañon-Jimenez S, Torn MS, Janssens IA, Penuelas J, Richter A (2020) Microbial carbon limitation: the need for integrating microorganisms into our understanding of ecosystem carbon cycling. Global Change Biol 26:1953–1961. https://doi.org/10.1111/gcb.14962
South A (2017) rnaturalearth: world map data from natural earth. R package version 0.1.0. https://CRAN.R-project.org/package=rnaturalearth. Accessed 01 May 2022
Spohn M, Klaus K, Wanek W, Richter A (2016) Microbial carbon use efficiency and biomass turnover times depending on soil depth - implications for carbon cycling. Soil Biol Biochem 96:74–81. https://doi.org/10.1016/j.soilbio.2016.01.016
Spohn M, Pötsch EM, Eichorst SA, Woebken D, Wanek W, Richter A (2016) Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol Biochem 97:168–175. https://doi.org/10.1016/j.soilbio.2016.03.008
Sylvia DM, Hartel PG, Fuhrmann JJ, Zuberer DA (eds) (2005) Principles and applications of soil microbiology. Pearson Educational Inc., Upper Saddle River, NJ
Tang Y, Horikoshi M, Li W (2016) ggfortify: unified interface to visualize statistical results of popular R packages. R J 8(2):478–489
Tchebakova NM, Parfenova EI, Lysanova GI, Soja AJ (2011) Agroclimatic potential across central Siberia in an altered twenty-first century. Environ Res Lett 6:45207. https://doi.org/10.1088/1748-9326/6/4/045207
Thiet RK, Frey SD, Six J (2006) Do growth yield efficiencies differ between soil microbial communities differing in fungal:bacterial ratios? Reality check and methodological issues. Soil Biol Biochem 38:837–844. https://doi.org/10.1016/j.soilbio.2005.07.010
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Wakelin SA, Macdonald LM, Rogers SL, Gregg AL, Bolger TP, Baldock JA (2008) Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biol Biochem 40:803–813. https://doi.org/10.1016/j.soilbio.2007.10.015
Wang C, Qu L, Yang L, Liu D, Morrissey E, Miao R, Liu Z, Wang Q, Fang Y, Bai E (2021) Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon. Global Change Biol 27:2039–2048. https://doi.org/10.1111/gcb.15550
Wei T, Simko V (2021) corrplot: visualization of a correlation matrix. R Package Version 0.90. https://github.com/taiyun/corrplot. Accessed 01 May 2022
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen T, Miller E, Bache S, Müller K, Ooms J, Robinson D, Seidel D, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H (2019) Welcome to the Tidyverse. JOSS 4:1686. https://doi.org/10.21105/joss.01686
Widdig M, Schleuss P-M, Biederman LA, Borer ET, Crawley MJ, Kirkman KP, Seabloom EW, Wragg PD, Spohn M (2020) Microbial carbon use efficiency in grassland soils subjected to nitrogen and phosphorus additions. Soil Biol Biochem 146:107815. https://doi.org/10.1016/j.soilbio.2020.107815
Wilke CO (2020) cowplot: streamlined plot theme and plot annotations for ‘ggplot2’. R package version 1.1.1. https://CRAN.R-project.org/package=cowplot. Accessed 01 May 2022
Woolf D, Lehmann J (2019) Microbial models with minimal mineral protection can explain long-term soil organic carbon persistence. Sci Rep 9:6522. https://doi.org/10.1038/s41598-019-43026-8
ArcGIS-basemap ‘World Hillshade’: Credits: Esri; USGS; NGA; NASA; CGIAR; N Robinson; NCEAS; NLS; OS; NMA; Geodatastyrelsen; Rijkswaterstaat; GSA; Geoland; FEMA; Intermap; and the GIS user community. https://doc.arcgis.com/en/data-appliance/7.1/maps/world-hillshade.htm. Accessed 1 March 2022
Xiao Q, Huang Y, Wu L, Tian Y, Wang Q, Wang B, Xu M, Zhang W (2021) Long-term manuring increases microbial carbon use efficiency and mitigates priming effect via alleviated soil acidification and resource limitation. Biol Fertil Soils 57:925–934. https://doi.org/10.1007/s00374-021-01583-z
Xu X, Thornton PE, Post WM (2013) A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Global Ecol Biogeogr 22:737–749. https://doi.org/10.1111/geb.12029
Yu Y, Lee C, Kim J, Hwang S (2005) Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 89:670–679. https://doi.org/10.1002/bit.20347
Zheng Q, Hu Y, Zhang S, Noll L, Böckle T, Richter A, Wanek W (2019) Growth explains microbial carbon use efficiency across soils differing in land use and geology. Soil Biol Biochem 128:45–55. https://doi.org/10.1016/j.soilbio.2018.10.006
Zhou Z, Wang C, Luo Y (2018) Effects of forest degradation on microbial communities and soil carbon cycling: a global meta-analysis. Global Ecol Biogeogr 27:110–124. https://doi.org/10.1111/geb.12663
Zhou Z, Wang C, Luo Y (2020) Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat Commun 11:3072. https://doi.org/10.1038/s41467-020-16881-7
Acknowledgements
We are very grateful to the Yukon farmers for giving us the opportunity to sample their land, and who provided useful insights into subarctic agriculture. Thanks also to the Geological Survey Yukon and Whitehorse local Jeannie McLorie for offering facilities for soil sample drying and sieving. This study was conducted under the Yukon Scientists and Explorers Act Licence No. 6800-20-1151, and we thank all Yukon First Nations for granting us permission to conduct our scientific work on their traditional land. We would also like to thank the technical staff at the Thünen Institute involved in this study: Frank Hegewald (sampling), Kerstin Gilke (GC), Claudia Wiese (CN), Arne Heidkamp and the team of the Soil Monitoring Laboratory (soil texture, Olsen-P), Sabine Wathsack (Cmic), Andrea Niemeyer (Nmic), Britta Müller, Karin Trescher and Jana Usarek (DNA extraction, qPCR). Special thanks to Dr. Jens Dyckmans and his colleagues at the Centre for Stable Isotope Research and Analysis of the University of Göttingen. We thank Dr. Jeanne Percival of the Geological Survey of Canada, Natural Resources Canada for conducting the mineralogical analyses.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was part of the ‘Breaking the Ice’ project funded by the German Research Foundation [grant number 401106790]. Funding for EG was provided by the Science and Technology Branch of Agriculture and Agri-Food Canada [Project J-001756 ‘Biological Soil Carbon Stabilisation’].
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schroeder, J., Peplau, T., Pennekamp, F. et al. Deforestation for agriculture increases microbial carbon use efficiency in subarctic soils. Biol Fertil Soils 60, 17–34 (2024). https://doi.org/10.1007/s00374-022-01669-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-022-01669-2