Abstract
Soil fungi are vital for regulating ecosystem carbon balance and productivity, by driving processes related to soil carbon and nutrient cycling. The rate and capacity of fungi-mediated processes are linked to fungal biomass dynamics and identifying the drivers of fungal biomass is important for predicting ecosystem responses to environmental changes. Here, ergosterol-based fungal biomass estimates and ITS2-based fungal community composition profiles were used to assess biomass of fungal guilds. Effects of forest management (thinning), environmental factors (soil chemical properties, microclimate, weather and forest stand composition) and season were related to the fungal biomass dynamics to identify the guild-specific drivers of biomass. Biomass of most fungal guilds increased with nutrient availability (nitrogen and potassium in particular) and decreased with forest thinning, and variation in total biomass was mainly driven by variation in mycorrhizal biomass. Most fungal guilds reached a minimum in biomass during summer except for mycorrhizal and root-associated ascomycetes, which instead reached a minimum during winter. Mycorrhizal fungi and root-associated ascomycetes displayed similar spatiotemporal variability in biomass. Yeasts and moulds were the only fungi displaying strong linkages with microclimate, whereas pathogenic and moss-associated fungi largely diverged in their responses to the environmental factors. The results of our study highlight that environmental factors related to the availability of soil nutrients may have an overall stronger effect on variation in biomass of fungal guilds in Mediterranean Pinus pinaster forests than direct influences of microclimate, weather and forest management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forests are major reservoirs for terrestrial carbon (C) and are a major component of the global primary production (Pan et al. 2011). In forests, soil fungi contribute extensively to processes regulating soil C and nutrient cycles, affecting the ecosystem C balance and productivity (Baldrian 2017). Saprotrophic fungi are major decomposers of soil organic matter (Chapin et al. 2011), symbiotic fungi contribute to the deposition of recalcitrant C (Godbold et al. 2006; Clemmensen et al. 2013) and enhance plant growth by improving nutrient uptake (Smith and Read 2008), whereas pathogenic fungi shape the vegetation composition and play an important role in maintaining biodiversity (Maron et al. 2011). Mould and yeasts are often generalist taxa with the ability to grow fast to rapidly colonise and use a wide spectrum of C sources (Algora Gallardo et al. 2021). Both mould and yeasts are contributors to soil aggregate formation, and yeasts may solubilise soil nutrients to make them more readily available for plants (Martin and Anderson 1943; Botha 2011). As microbial process and activity vary with microbial composition and biomass (Treseder et al. 2012; Graham et al. 2016; Awad et al. 2019), information on how the biomass of fungal guilds vary with environmental factors is important to improve our understanding of how ecosystem processes and functions may shift under climate change.
Climate change projections predict that Mediterranean ecosystems will be subjected to a 1.6 to 8.3 °C increase in mean annual temperature and a 5 to 10% decrease in precipitation by 2085 (Nogués-Bravo et al. 2008). Drought and rainfall fluctuations have strong control over fungal community composition (Sayer et al. 2017; Meisner et al. 2018), and the responses to drought vary between fungal species and guilds (Shi et al. 2002; Castaño et al. 2018). Soil physical and chemical factors interact, shaping the soil microbiome and influencing the reciprocal exchange of resources between plants and fungal communities which feedback on ecosystem functions and responses to climate change (Reynolds et al. 2003; Putten et al. 2013). Soil microclimate is coupled with weather conditions, and the soil biogeochemical cycles are strongly interlinked with soil chemical characteristics. However, the links between fungal community responses to environmental changes and ecosystem processes remain unclear (Treseder et al. 2012; Koide et al. 2014), and there is a lack of understanding of how effects of microclimate and soil chemistry relate to fungal biomass dynamics and related processes.
In Mediterranean terrestrial ecosystems, about half of the photosynthetic C is allocated belowground to roots and ectomycorrhizal fungi (Gill and Finzi 2016), which is the dominant functional guild in many Mediterranean forests (Castaño et al. 2018). Belowground C allocation is dependent on several factors, including deficiency of soil mineral nutrients associated with photosynthesis (Ericsson 1995; Gill and Finzi 2016). For example, deficiencies of potassium (K) and magnesium (Mg) typically result in a reduced belowground C allocation, whereas increased C allocation is observed under conditions of low plant-available nitrogen (N) and phosphorus (P) (Ericsson 1995). Growth of mycorrhizal mycelium has been shown to be affected by the interaction between N and P, with P starvation leading to a high mycelial production under ample N supply (Wallander and Nylund 1992; Ekblad et al. 1995). In addition to N and P availability, mycorrhizal biomass dynamics likely relate to variation in belowground C allocation (Wallander 1995; Ekblad et al. 2013; Hagenbo et al. 2017) and thus depends on host plant net C assimilation and overall performance. Water is usually the most limiting factor in Mediterranean forests, and the availability of nutrients affects the water status of plants. For example, P and K plays a central role in osmoregulation and stomatal control, hence are important for maintaining a high water-use efficiency and avoiding water stress during drought (Sardans and Peñuelas 2007, 2015; Fernández et al. 2011). Availability of Ca is important for plant water uptake by regulating water channel activity and facilitating water transport across cell membranes (White and Broadley 2003), and ample Ca supplies during dry conditions have been shown to improve plant water status by increasing the expression of membrane proteins (aquaporins) (Galmés et al. 2007; Wang et al. 2016). Since Ca is also important for regulating cell extension, particularly in roots, a shortage of Ca may impair root growth and reduce their capacity to access deep soil layers which usually hold water reserves during the dry season (Schulze et al. 1996; Brum et al. 2017).
The contribution of environmental factors on fungal biomass and community dynamics vary with forest types and geography. In temperate forests, soil conditions (e.g. pH, soil temperature and soil moisture content) and resource availability (C in fine roots, inorganic N, soil C:N) appear to be the main drivers of the ectomycorrhizal biomass, whereas the biomass of saprotrophic fungi seems to be mainly regulated by the availability of resources and forest stand composition (Awad et al. 2019). Similarly, in alpine Picea abies (L.) Karts. stands, community composition of mycorrhizal and saprotrophic fungi has been shown to relate to forest stand characteristics, soil pH and soil C:N (Vašutová et al. 2017). However, the main factors regulating fungal biomass in Mediterranean forests are uncertain but opposed to temperate forests; i.e., non-mycorrhizal fungi seem to be more sensitive towards variation in microclimate (e.g. soil temperature and moisture content) than mycorrhizal fungi (Castaño et al. 2018).
In the present study, objective (1) was to identify potential drivers of fungal biomass dynamics in Mediterranean forest ecosystems. In short, we investigated the relationships between environmental variables related to soil conditions, microclimate and weather against guild-specific fungal biomass variation, which was estimated from soil ergosterol content and ITS2-based fungal community composition. The study was conducted over thinned and control Pinus pinaster (Aiton) forest stands to compare the relative importance between forest management and environmental factors in regulating biomass dynamics of different fungal guilds. Objective (2) was to disentangle direct linear responses of microclimate from bimodal relationships between growth and season, as we recognised that both microclimate and season may be important for regulating fungal biomass but may do so over different time scales (Alday et al. 2017; Castaño et al. 2018). This was done by studying seasonality in biomass in parallel to changes in microclimate and weather and implementing partial least squares regression analyses.
Compared to free-living fungi (e.g. yeast and moulds), mycorrhizal fungi are likely to have better access to groundwater through hydraulic lift which involves the transport of water from deeper soil layers into shallower surface layers (Querejeta et al. 2003). Based on the abundance of free-living fungi declining more than mycorrhizal fungi after dry periods (Castaño et al. 2018) and the assumption that mycorrhizal fungi have better access to deep water reservoirs than free-living taxa, we hypothesised (1) that biomass of non-mycorrhizal fungi would primarily relate to variation in microclimate and weather. Furthermore, given the fact that mycorrhizal fungi represent a large component of the soil fungal biomass in Mediterranean forests (Castaño et al. 2018) and that the growth usually exceeds several hundred kilogrammes per hectare over 2–4 months (Hagenbo et al. 2021), we hypothesised that (2) the overall biomass dynamics are primarily driven by variation in mycorrhizal biomass. Furthermore, based on previous observation of excess N and P starvation resulting in an increase in mycorrhizal mycelial biomass (Wallander and Nylund 1992; Ekblad et al. 1995), we also hypothesised (3) that the mycorrhizal biomass is positively correlated with combined low extractable P and high extractable N availability. Finally, based on K and Ca being important for plant water status and thus important for maintaining photosynthesis and belowground C allocation (Ericsson 1995), we also hypothesised (4) that fungal biomass would correlate positively with the availability of K and Ca.
Materials and methods
Study sites
The study was conducted at 15 long-term monitoring plots, composed of 60-year-old P. pinaster stands, ranging in altitude from 594 to 1013 m above sea level, distributed over 300 ha and located in the protected area of Poblet, in Northeastern Spain (41°21′ 6.4728″ E, 1°2′ 25.7496″ N). The stands were selected to be distant enough (at least 0.2 km apart) to be treated as independent replicates. In 13 stands, an approximate area of 40 × 40 m was subjected to thinning in July–August of 2009, resulting in paired subplots (26 in total) representing thinned and un-thinned (control) treatments. The thinning operations were conducted to assess the effects of forest management and variation in stand basal area on fungal biomass dynamics. The reduction in stand basal area by means of forest thinning varied over five different thinning intensities, representing a uniform distribution between 0 and 77% removal of the total stand basal area (Bonet et al. 2012). Felled trees were removed and Quercus spp. resprouts appearing punctually the following years were also removed to maintain similar conditions between thinned and un-thinned subplots. For further details see Bonet et al. (2012). The sparse understorey was mainly composed by Quercus ilex (L.) with occasional occurrences of Erica arborea (L.) and Arbutus unedo (L.). Herbaceous species were infrequent. The soil has a sandy loam texture and is classified as a Calcaric Cambisol (IUSS Working Group WRB 2015). The mean annual temperature is 11.8 °C and the mean annual precipitation is 667 mm, with a summer drought usually occurring between July–September, lasting approximately 3 months. Variability in soil chemistry, microclimate and weather of the site are described in Table 1 and correlation between the factors is presented in Fig. S1 in Supporting Information.
Meteorological data
Air temperature, relative humidity of the air (RH), soil temperature and volumetric soil water content were measured in the middle of the subplots using Decagon 5™ probes (Decagon Devices Inc., Pullman, WA, USA). Measurements of air temperature and relative humidity were done at 1.3 m height and measurements of soil temperature and soil moisture content were done in the upper 10 cm of the soil. The meteorological data were based on measurements taken every minute and then averaged over 2 h and recorded in a data logger (EM50, Decagon Devices). The data was downloaded and processed using the DATATRAC III software (Pullman, WA, USA) and calculated as monthly averages. Yearly precipitation, as well as air- and soil temperature data were obtained over the same period for each plot following the DAYMET methodology (Thornton et al. 2000), as implemented in the R package ‘meteoland’ (De Cáceres et al. 2018). In short, daily precipitation and temperature were estimated for each plot by averaging the values of several Catalan and Spanish meteorological stations, applying weighting factors that depended on the geographic proximity to the target plot and correcting for differences in elevation between the station and the plot.
Soil sampling and processing
Soil was sampled from all plots in November of 2009 and monthly between June 2013 and May 2014, with a final sampling in November 2014, to facilitate comparisons between years. At each sampling, eight soil cores (12 cm long and 5 cm in diameter) were systematically sampled by collecting soil cores from each 10-m-side of the plot. Upon sampling, intact and partially decomposed needles were discarded, as fungal community composition in needle materials diverge from that of the soil (Lindahl et al. 2007). Soil samples were then stored at 4 °C for < 24 h and sieved through a 3-mm mesh, followed by freeze-drying and pooling, to obtain a single composite for each site, subplot and sampling time point. Pooled samples were then thoroughly ground using a mortar and pestle and transferred to − 20 °C storage until further analysis.
Soil chemical analyses
Organic matter contents of the pooled soil samples, representing the same subplot, were analysed according to the Walkley–Black method (Walkley and Black 1934), and extractable pools of inorganic N and P were analysed using the Kjeldahl method (Kjeldahl 1883) and the Olsen method (Olsen et al. 1954), respectively. Plant-available pools of K, Ca, Mg and Na were extracted using ammonium acetate and quantified using inductively coupled plasma optical emission spectroscopy (ICP-OES). Cation-exchange capacity of the soils was determined by titration using ammonium acetate, as described by Chapman (1965). Soil pH was measured in a 1:2.5 soil-deionised water slurry. All soil chemistry analyses were performed by Applus Agroambiental (Applus, Madrid, Spain).
Fungal community analysis
From pooled soil samples representing the same site, fungal DNA was extracted, amplified, sequenced and bioinformatically analysed as described by Castaño et al. (2018). DNA was extracted from a subsample of 0.5 g using the NucleoSpin NSP soil kit (Macherey–Nagel, Duren, Germany), and PCR amplified in a 2720 Thermal Cycler (Life Technologies, Carlsbad, CA, USA) using the primers gITS7 (Ihrmark et al. 2012) and ITS4 (White et al. 1990), elongated with unique identification tags. The number of PCR cycles was optimised for individual samples, amplification was done using triplicates and negative controls were carried out to detect potential contaminations of DNA or PCR products. The sample PCR products were then purified using the AMPure kit (Beckman Coulter Inc., Brea, CA, USA) and quantified using a Qubit fluorometer (Life Technologies). Equal amounts of DNA from each sample were pooled and further purified using the EZNA Cycle Pure kit (Omega Bio-Tek, Norcross, GA, USA). PCR products were checked for quality using a BioAnalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) and sequenced using Pacific Biosciences RSII sequencing (Pacific Biosciences, Menlo Park, CA, USA) at SciLifeLab (National Genomics Infrastructure, Uppsala, Sweden) using 28 single molecule, real-time (SMRT) cells. Pacific Biosciences sequencing was selected as the sequencing method as the bias related to PCR fragment lengths is low and thus suitable for analysing ITS2 sequences which differ in length (Castaño et al. 2020). Sequences were quality filtered by the SCATA pipeline (scata.mykopat.slu.se) and clustered into species hypotheses (SHs; Kõljalg et al. 2013) using single linkage clustering. Taxonomic assignations were confirmed by using neighbour-joining phylogenetic trees (Castaño et al. 2018). The 550 most common SHs were assigned putative names and grouped into the following functional guilds: ectomycorrhizal, moulds, yeasts, saprotrophs, pathogens, moss-associated fungi and root-associated ascomycetes, based on the UNITE and DEEMY databases, as well as other published literature.
Fungal biomass quantification
Fungal biomass in samples was determined by quantifying the fungal-specific biomass marker ergosterol. In short, ergosterol was extracted as described by Nylund and Wallander (1992) and chromatographically analysed as described by Hagenbo et al. (2017). Fungal biomass was calculated by assuming an ergosterol content of 3 µg mg−1 (Salmanowicz and Nylund 1988), and a correction factor (1/0.62) was applied to compensate for unextracted ergosterol (Montgomery et al. 2000). Estimates of total fungal biomass in samples were multiplied by the relative abundances of each aforementioned functional guild to obtain estimates of guild-specific fungal biomasses.
Statistical analysis
Partial least squares (PLS) regression was performed using the ‘mdatools’ package (Kucheryavskiy 2019) in R v3.5.3 (R Core Team 2013) to interpret relationships between fungal guild-specific biomasses and environmental variables of soil chemistry (nutrient availability, cation-exchange capacity, organic matter content and soil pH), microclimate (soil temperature, soil moisture content and altitude) and weather (precipitation, air temperature and relative air humidity) (Table 1), as well as forest stand density and management (stand basal area, thinned vs non-thinned plots). PLS is used similarly to multiple regression analysis but is a multivariate modelling approach in which a number of latent orthogonal factors (PLS components) are created, explaining the maximum variation in predictor- and response matrices. The latent variables are estimated consecutively and their statistical significances are evaluated by cross-validation (Wold 1978). PLS is appropriate when the explanatory variables are few and collinear (Wold et al. 2001). For all PLS models, we used tenfold cross-validation of the data, which is commonly used to evaluate model performance (McLachlan et al. 2004). Thus, the observations were randomly partitioned into 10 equal-sized subsamples and the fitting procedure was repeated for each subsample against the rest of the data, which was used as training data for the model. The average performance from 10 repetitions was then calculated to produce a single estimation of the overall model performance. All analyses were conducted using the SIMPLS algorithm, and jackknife resampling was performed during the cross-validation procedure to obtain inference statistics for the regression coefficients. The reliability of the PLS models was assessed by comparing their explained cross-validated variance (Q2), and the importance of the individual explanatory variables was assessed using coefficient’s p values, correlation loadings and importance in projection values (VIP). The effect of random spatiotemporal variation was accounted for by including stand and sampling time (year and month) as categorical variables in all PLS regressions. Predictor and response variables were standardised by subtraction of the sample mean and then divided by the standard deviation, according to standard PLS modelling procedure.
Results
Composition of soil fungal biomass and its relationship with season
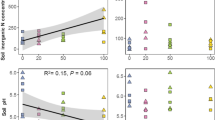
Total fungal biomass was on average 2.6 and 3.5 mg g−1 dry weight soil across thinned and non-thinned control plots and dominated by mycorrhizal fungi which on average contributed by 53.9% of the total fungal biomass (Fig. 1). On average, across thinned and control plots, unknown fungi (taxa with unknown function), moulds, yeasts, saprotrophs, root- and moss-associated fungi contributed by 27.0%, 7.0%, 4.4%, 3.5%, 2.0% and 1.7%, respectively, of the total fungal biomass, whereas the contribution of pathogenic fungi was < 1% (Fig. 1). Over control and thinned plots, fungal biomass varied over the season and biomass of mycorrhizal fungi and root-associated ascomycetes reached a minimum in February, whereas fungi from other guilds reached a seasonal minimum in August (Table 2).
Biomass of all fungal guilds predicted from variables of soil and microclimate
Using PLS regression to test relationships between the biomass of all fungal guilds against all the environmental factors (variables related to soil chemistry, climatic conditions and forest stand characteristics, as well as the effect of season and random site variation) resulted in a PLS model with one significant component, altogether accounting for 7.3% of the variance (R2) in fungal biomass, and were 1.0% of the cross-validated variance (Q2; model’s predictive ability) was explained (Fig. 2a). In a second PLS regression, excluding variables related to climatic conditions and forest stand characteristics, resulted in a model (R2 = 7.0) explaining 0.7% (Q2) of the biomass variation of all fungal guilds (Fig. 2b). Conversely, a PLS regression excluding variables related to soil chemistry yielded a model (R2 = 9.1) with a poor capacity (Q2 = − 0.5) to predict variation in biomass of the fungal guilds (Fig. 2c).
Partial least squares (PLS) correlation loading plots for models explaining relationships between biomass of different fungal guilds in relation to a all environmental variables, b soil properties and c variables related to climatic conditions. Blue and red colours of circles indicate explanatory and response variables, respectively. Values of R2 indicate the percentage of variation explained by the models and values of Q2 represent cross-validated explained variances and indicate how well the models predict new data. The areas of the circles are proportional to the variable importance (VIP) scores in the models and their placements indicate loading scores of the different factors. All projections (a–c) resulted in one significant component (PLS 1)
Predictability was improved when the total biomass and the biomass of separate fungal guilds were tested individually against all the environmental factors (Table 3), due to loosened model constraints. PLS projection of total biomass yielded a model (R2 = 44.5) where 32.5% of Q2 was explained (Fig. 3a), and the first and the second component accounted for 24.2% and 20.3% of the variance in total biomass, respectively (Fig. 4a). Overall, variation in soil chemistry, climatic conditions and forest stand characteristics respectively explained 19.9%, 5.1% and 2.4% of the variance in total biomass, whereas the effect of season and random site variation accounted for 5.4% and 11.7%, respectively (Fig. 4a).
Partial least squares (PLS) correlation loading plots for models explaining relationships between environmental variables (blue circles) and variation in a total fungal biomass and biomass of b mycorrhizal fungi, c root-associated ascomycetes, d yeast fungi, e moulds, f pathogenic fungi and g unknown fungi in Pinus pinaster forests. Factors significantly explaining variation in biomass are sized according to the variable importance (VIP) scores in the models and their placements indicate their loading scores. Values of R2 indicate the percentage of variation explained by the models and values of Q2 represent cross-validated explained variances and indicate how well the models predict new data
Composition of partial least squares regression (PLS) components explaining seasonal variation in biomass of different fungal guilds in Mediterranean Pinus pinaster forest. Variables of soil chemistry (orange bars) are represented by nutrient availability, pH and soil organic matter content and variables of climatic conditions (blue bars) are represented by relative humidity, soil moisture content, air- and soil temperature, as well as precipitation
Predictability was high for PLS regressions describing biomass of mycorrhizal- and pathogenic fungi, with Q2 values of 37.8 and 32.0, and R2 values of 48.8 and 44.8 (Table 3, Figs. 3 and 4), and composed by two and three significant components, respectively. Variables of soil chemistry and climatic conditions respectively explained 22.0% and 6.1% of the variance in mycorrhizal fungal biomass and 18.0% and 8.5% of the biomass variation of pathogenic fungi. The effect of season accounted for 6.7% and 8.8% of the variation in biomass of mycorrhizal and pathogenic fungi, respectively (Fig. 4). The performance was lower for PLS models describing the biomass of yeasts, unknown fungi, moulds, root-associated ascomycetes and moss-associated fungi and resulted in models with two significant components and Q2 and R2 values ranging from 23.3 to 11.9 and 37.0 to 30.4, respectively (Table 3). Predictability was the lowest for a PLS model describing biomass of saprotrophic fungi, with one significant component and Q2 and R2 values of − 37.1 and 5.7, respectively (Table 3).
On average across all PLS regressions, variables related to soil chemistry, climatic conditions and forest stand characteristics respectively explained 14.6%, 5.5% and 1.5% (first and second component) of fungal guild’s biomass variances, whereas the effect of season explained 5.3% of the variances (Fig. 5).
Averaged explained variance and composition of the first (red), second (blue) and the third (green) partial least squares regression (PLS) component. Averaged explained variance is represented by the mean R2 for each category of environmental factors, obtained from different PLS models describing the biomass of mycorrhizal fungi, root-associated ascomycetes, yeasts, moulds, pathogenic fungi and unknown fungi. See Fig. 4 for the composition of the PLS components of the different fungal guilds
Biomass responses of fungal guilds against soil- and microclimate variables
Regression coefficients of the PLS models (Table 3) revealed that total fungal biomass decreased significantly, with thinning (standardised regression coefficient of − 2.20) and soil Mg content (− 0.77), but increased significantly with Na (1.15), K (1.09), N (0.90) and cation-exchange capacity (0.34; CEC). Similarly, mycorrhizal biomass correlated negatively with thinning (− 1.56) and positively with Na (1.35), K (1.09), cation-exchange capacity (0.77), N (0.70) and Ca (0.64). However, unlike the total biomass, mycorrhizal fungi correlated positively with altitude (0.52). Biomass of root-associated ascomycetes also decreased with thinning (− 1.56), but opposed to mycorrhizal fungi, biomass of ascomycetes decreased with altitude (− 0.90) and cation-exchange capacity (− 0.47) and soil Mg content (− 1.26) and displayed positive correlations with soil P- (0.60) and N content (0.43). Biomass of yeast fungi correlated negatively with thinning (− 1.45) and positively with soil contents of Ca (0.79), N (0.69) and K (0.66). Biomass of yeasts also correlated negatively with air- and soil temperature (− 0.75 and − 0.89) and positively with soil moisture content (0.78) and soil P content (1.16). These trends were also observed for moulds, which also decreased with thinning (− 1.20) and displayed positive correlations with soil contents of N (0.50) and K (0.40). Pathogenic fungi correlated positively with soil N content (0.91) and correlated negatively with soil moisture content (− 0.78), soil Mg content (− 0.60) and altitude (0.30). Biomass of moss-associated fungi correlated positively with soil moisture content (0.94) and negatively with altitude (− 0.97), soil contents of Mg (− 0.72) and Na (− 0.63), stand basal area (− 0.57) and cation-exchange capacity (− 0.29). Biomass of fungal taxa with unknown functions shared responses with root-associated ascomycetes and yeasts and correlated negatively with thinning (− 1.87) and soil Mg content (− 1.55), as well as positively with contents of P (0.96), K (0.57) and N (0.64). However, unlike the other fungal guilds, biomass of unknown fungi correlated negatively with pH (− 1.21). Biomass of saprotrophic fungi correlated with several environmental variables. However, given the low predictive capacity of the PLS models (low Q2), the PLS model was considered unreliable.
Discussion
In the present study, we quantified the extent to which environmental factors related to soil chemistry, microclimate, weather and forest stand density and management (basal area and effects of thinning) contribute to biomass dynamics of different fungal guilds in Mediterranean P. pinaster forests. Variables related to soil chemistry were the overall strongest drivers of fungal biomass, followed by the effects of season and direct influences of microclimate, weather and forest stand density and management. On average, soil chemistry variables explained 15%, while the combination of season, microclimate and weather together explained 10% of the variation in total fungal biomass. Forest density and management only explained 1.5% of the overall biomass dynamics, still thinning displayed having the single strongest influence among all the factors, contributing to a significant reduction in biomass of mycorrhizal fungi (e.g. Inocybe, Russula and Tricholoma), root-associated ascomycetes, yeasts (e.g. Cryptococcus and Rhodotorula) and moulds (e.g. Mortierella and Umbelopsis).
Variations in fungal biomass were primarily driven by variation in soil nutrients
In disagreement with our first hypothesis, biomass of non-mycorrhizal taxa was primarily driven by factors related to chemical- and nutrient characteristics of the soil rather than direct influences of season and microclimate. Additionally, variables related to soil conditions explained the majority (22%) of the biomass variation of mycorrhizal fungi. Thus, our results highlight that factors related to the availability of resources soil are the main drivers of fungal biomass dynamics in Mediterranean forests. This is in line with Sun et al. (2020) reporting that soil properties have an overall stronger influence on microbial biomass C than climate and ecosystem type over a global scale. Soil properties representing the strongest regulatory factor also corroborates the findings by Awad et al. (2019) reporting that variation in soil conditions is the main driver of fungal biomass dynamics in temperate European forests. The effect of season explained the second largest variation in fungal biomass, and all fungal guilds displayed seasonality in biomass. This agrees with the findings of Castaño et al. (2018), reporting that seasonal changes in climatic conditions underpin shifts in fungal community composition in Mediterranean forests. However, when disentangling the effects of season from the direct effects of precipitation, temperature and soil moisture content, we observed that those factors had a relatively small influence on the overall fungal biomass dynamics, likely because the climatic factors were largely embedded within the effect of season. However, biomass of yeasts and moulds was directly related to monthly variation in soil moisture content and temperature, which may indicate that free-living, opportunistic fungi, are more sensitive to drought and precipitation events compared to root-associated fungi (Castaño et al. 2018). Conversely, biomass of pathogenic fungi correlated negatively with soil moisture content. Unlike other fungal guilds, pathogenic fungi often exhibit a large plasticity and can thrive at water potentials well below the minimum for plant growth (Desprez-Loustau et al. 2006). Drought-induced diseases are usually caused by latent pathogenic fungi with endophytic abilities that are living inside dead parts of living trees. Thus, the negative correlation between the pathogenic biomass and soil moisture content corroborates previous findings that pathogenic fungi may interact with plant water stress to mediate drought-induced infection in trees (Desprez-Loustau et al. 2006; Allen et al. 2010). No fungal guild displayed any direct relationship with precipitation alone, likely because of a higher explanatory power of soil moisture content and season. The explanatory power was low (Q2 < 14) for root-associated ascomycetes and moss-associated fungi, suggesting that some more complex mechanisms are regulating their biomass. Additionally, we were unable to explain biomass dynamics of saprotrophic fungi, likely because the upper litter layer was discarded upon sampling and only sampled well-decomposed organic layers and mineral soil.
Overall fungal biomass dynamics were primarily driven by mycorrhizal fungi
In agreement with our second hypothesis, variation in soil fungal biomass was primarily driven by mycorrhizal biomass dynamics. Thus, the factors explaining variation in mycorrhizal biomass were the overall strongest predictors of the total fungal biomass. Mycorrhizal fungi being the main driver of total fungal biomass is in line with Cheeke et al. (2021), who reported that standing fungal biomass in temperate hardwood forests in US Indiana was primarily regulated by mycorrhizal mycelial production, across a gradient in mycorrhizal association. However, in disagreement with the third hypothesis we did not observe any interaction between the levels of extractable P and N in terms of correlating with the mycorrhizal biomass, nor did we observe any significant increase in biomass with increasing soil P content. Biomass of mycorrhizal fungi relates to the availability of macronutrients, and the mycorrhizal biomass of Pinus sylvestris (L.) appears to peak under conditions of low P and high N (Wallander and Nylund 1992; Ekblad et al. 1995). In the present study, the fraction of easily available P was estimated using Olsen’s method and soil P ranged from 4 to 21 mg kg−1, which is low considering that an Olsen-P value of 20 mg kg−1 has been regarded as a threshold for optimal plant growth (Li et al. 2011). Despite the apparent low P availability, it seems likely that the availability of P, relative to N, was not sufficiently low to stimulate an accumulation of mycorrhizal biomass. Furthermore, given the fact that the mycorrhizal biomass correlated positively with soil N concentration, it seems likely that the N conditions impose greater constraints on mycorrhizal growth than the apparent low P availability. However, it is difficult to establish the extent to which N and P are limited, as mycorrhizal fungi can acquire N by degrading soil organic matter (Lindahl and Tunlid 2015) and can accelerate the dissolution of mineral bound P by exuding organic acids (Schmalenberger et al. 2015).
Contrary to our findings, studies from boreal forests report decreased accumulation of mycorrhizal biomass with increased N supply (Nilsson and Wallander 2003; Kjøller et al. 2012; Bahr et al. 2013). This inconsistency between results could be related to mycorrhizal biomass accumulation not being linearly related to N availability. For example, Treseder and Allen (2002) proposed a parabolic relationship between mycorrhizal fungal growth and soil nutrient availability, with maximal mycorrhizal growth when nutrient availability constrains plant growth but not that of the mycorrhizal fungi and diminished fungal growth under low and high N availabilities. Low biomass production has also been proposed as a mechanism for limiting immobilisation in the N in fungal biomass to enable a proportionally larger host N allocation (Hagenbo et al. 2019). In addition to soil N, the mycorrhizal biomass correlated with several factors (Na, Ca, K, CEC, thinning, altitude and season) which implies that an assembly of drivers regulates the mycorrhizal biomass dynamics in Mediterranean forests. For instance, thinning had a strong negative influence on the mycorrhizal biomass and contributed to the largest variability in biomass among all the separate factors. Although thinning increases growth of the remaining trees, stand-level productivity usually declines over the short term after thinning (Amiro et al. 2010), which may contribute to reduced C allocation to roots and associated symbionts belowground, which may impair fungal growth (Noormets et al. 2015; Collado et al. 2020).
Soil salinity may mediate variation in total- and mycorrhizal fungal biomass
In agreement with our fourth hypothesis, we observed positive correlations between the total and mycorrhizal biomass and availability of soil K and Ca, which are both involved in mechanisms mitigating drought stress in plants (Sardans and Peñuelas, 2007, 2015). Whereas Ca is important for plasma membrane stability and cell extension in roots (Thor 2019), K plays a fundamental role in maintaining osmotic homeostasis and is important for stomatal control, water conductance and root hydraulic conductivity (Sardans and Peñuelas 2015). Because restricted water access constraints photosynthesis and thus plant growth, allocation of C to belowground may decrease under severe water limitation (Staddon et al. 2004; Swaty et al. 2004), leading to reduced growth of roots and associated mycorrhizal fungi. The biomass of most fungal guilds displayed positive correlations with the extractable K content and given the importance of K in plant water uptake, it is likely that the increases in biomass are related to improved water conditions. Opposed to mycorrhizal fungi, biomass of root-associated ascomycetes displayed no correlation with K and this could be related to them being less sensitive to drought and warm conditions compared to mycorrhizal fungi and other fungal guilds (Castaño et al. 2018).
Furthermore, the positive correlation between soil Na, K, Ca and total- and mycorrhizal fungal biomass suggests that variation in salinity stress may contribute to variation in mycorrhizal biomass. In particular, the excess of Na in soil (salinity) adversely affect plant growth, by decreasing water potential in soil which impairs the uptake of water (Bolaños and Longstreth 1984). Mycorrhizal symbiosis has been proposed as a key factor for better salt tolerance of woody species by reducing Na uptake (Guerrero-Galán et al. 2019). While high salinity imposes Na toxicity and reduces fungal growth, mild-moderate Na occurrence has been reported to stimulate the growth of some mycorrhizal species (Bois et al. 2006; Matsuda et al. 2006; Zhang et al. 2008), which may contribute to the positive correlations between soil Na and mycorrhizal biomass. However, the mechanisms driving mycorrhizal biomass responses to salinity variation are not known, and the effects of salinity on mycorrhizal-related processes need further investigation.
Conclusion
Our study shows that biomass of fungal guilds in Mediterranean P. pinaster forests are primarily driven by environmental factors related to soil conditions, in particular content of N and K in soil. Overall, mycorrhizal fungi and root-associated ascomycetes displayed similar biomass dynamics, yeasts and moulds were the only fungal guilds displaying strong linkages with microclimate and pathogenic and moss-associated fungi largely diverged in their responses to environmental factors. Variation in total fungal biomass was mainly driven by mycorrhizal biomass dynamics. Thus, the factors explaining variation in mycorrhizal biomass were the main contributors to total fungal biomass variation. Namely, variability in extractable soil K and Ca content had a significant influence on total and mycorrhizal biomass variation, potentially because both K and Ca being involved in mitigating drought stress in plants, thus sustaining plant growth and belowground allocation of C to roots and mycorrhizae. Additionally, soil salinity appeared to have an influence on the mycorrhizal biomass, although the mechanistic link between biomass and salinity is uncertain. All fungal guilds displayed a seasonality in biomass, whereas factors related to weather- and microclimate conditions had a limited direct influence on fungal biomass dynamics. Potentially, the lack of direct linear responses in microclimate conditions and fungal biomass dynamics is related to variation in climatic conditions being embedded within the effect of season, as well as fungal biomass dynamics operating over longer temporal scales compared to short-term meteorological conditions. While the results of our study highlight several correlations between fungal biomass dynamics and environmental factors, further research is needed to establish a mechanistic understanding of the processes driving biomass dynamics to improve our understanding of how ecosystem functions may shift with climate change.
Availability of data and material
Sequence data are archived at NCBI’s Sequence Read Archive under accession number PRJNA309233 (ncbi.nlm.nih.gov/sra).
Change history
17 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00374-022-01629-w
References
Alday JG, Martínez de Aragón J, de-Miguel S, Bonet JA (2017) Mushroom biomass and diversity are driven by different spatio-temporal scales along Mediterranean elevation gradients. Sci Rep 7:45824. https://doi.org/10.1038/srep45824
Algora Gallardo C, Baldrian P, López-Mondéjar R (2021) Litter-inhabiting fungi show high level of specialization towards biopolymers composing plant and fungal biomass. Biol Fertil Soils 57:77–88. https://doi.org/10.1007/s00374-020-01507-3
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH (Ted), Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim J-H, Allard G, Running SW, Semerci A, Cobb N (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684. https://doi.org/10.1016/j.foreco.2009.09.001
Amiro BD, Barr AG, Barr JG, Black TA, Bracho R, Brown M, Chen J, Clark KL, Davis KJ, Desai AR, Dore S, Engel V, Fuentes JD, Goldstein AH, Goulden ML, Kolb TE, Lavigne MB, Law BE, Margolis HA, Martin T, McCaughey JH, Misson L, Montes‐Helu M, Noormets A, Randerson JT, Starr G, Xiao J (2010) Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J Geophys Res Biogeosci. https://doi.org/10.1029/2010JG001390
Awad A, Majcherczyk A, Schall P, Schröter K, Schöning I, Schrumpf M, Ehbrecht M, Boch S, Kahl T, Bauhus J, Seidel D, Ammer C, Fischer M, Kües U, Pena R (2019) Ectomycorrhizal and saprotrophic soil fungal biomass are driven by different factors and vary among broadleaf and coniferous temperate forests. Soil Biol Biochem 131:9–18. https://doi.org/10.1016/j.soilbio.2018.12.014
Bahr A, Ellström M, Akselsson C, Ekblad A, Mikusinska A, Wallander H (2013) Growth of ectomycorrhizal fungal mycelium along a Norway spruce forest nitrogen deposition gradient and its effect on nitrogen leakage. Soil Biol Biochem 59:38–48. https://doi.org/10.1016/j.soilbio.2013.01.004
Baldrian P (2017) Forest microbiome: diversity, complexity and dynamics. FEMS Microbiol Rev 41:109–130. https://doi.org/10.1093/femsre/fuw040
Bois G, Bertrand A, Piché Y, Fung M, Khasa DP (2006) Growth, compatible solute and salt accumulation of five mycorrhizal fungal species grown over a range of NaCl concentrations. Mycorrhiza 16:99–109. https://doi.org/10.1007/s00572-005-0020-y
Bolaños JA, Longstreth DJ (1984) Salinity effects on water potential components and bulk elastic modulus of Alternanthera philoxeroides (Mart.) Griseb. Plant Physiol 75:281–284. https://doi.org/10.1104/pp.75.2.281
Bonet JA, de Miguel S, Martínez de Aragón J, Pukkala T, Palahí M (2012) Immediate effect of thinning on the yield of Lactarius group deliciosus in Pinus pinaster forests in Northeastern Spain. For Ecol Manag 265:211–217. https://doi.org/10.1016/j.foreco.2011.10.039
Botha A (2011) The importance and ecology of yeasts in soil. Soil Biol Biochem 43:1–8. https://doi.org/10.1016/j.soilbio.2010.10.001
Brum M, Teodoro GS, Abrahão A, Oliveira RS (2017) Coordination of rooting depth and leaf hydraulic traits defines drought-related strategies in the campos rupestres, a tropical montane biodiversity hotspot. Plant Soil 420:467–480. https://doi.org/10.1007/s11104-017-3330-x
Castaño C, Lindahl BD, Alday JG, Hagenbo A, Martínez de Aragón J, Parladé J, Pera J, Bonet JA (2018) Soil microclimate changes affect soil fungal communities in a Mediterranean pine forest. New Phytol 220:1211–1221. https://doi.org/10.1111/nph.15205
Castaño C, Berlin A, Brandström Durling M, Ihrmark K, Lindahl BD, Stenlid J, Clemmensen KE, Olson Å (2020) Optimized metabarcoding with Pacific biosciences enables semi-quantitative analysis of fungal communities. New Phytol 228:1149–1158. https://doi.org/10.1111/nph.16731
Chapin FS, Matson PA, Vitousek PM (2011) Plant carbon budgets. In: Chapin FS, Matson PA, Vitousek PM (eds) Principles of terrestrial ecosystem ecology. Springer, New York, pp 157–181
Chapman HD (1965) Cation exchange capacity. In: Black CA (ed) Methods of sol analysis. ASA, Madison, pp 891–901
Cheeke TE, Phillips RP, Kuhn A, Rosling A, Fransson P (2021) Variation in hyphal production rather than turnover regulates standing fungal biomass in temperate hardwood forests. Ecology 102:e03260. https://doi.org/10.1002/ecy.3260
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD (2013) Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339:1615–1618. https://doi.org/10.1126/science.1231923
Collado E, Castaño C, Bonet JA, Hagenbo A, Martínez de Aragón J, de-Miguel S (2020) Divergent above- and below-ground responses of fungal functional groups to forest thinning. Soil Biol Biochem 150:108010. https://doi.org/10.1016/j.soilbio.2020.108010
De Cáceres M, Martin-StPaul N, Turco M, Cabon A, Granda V (2018) Estimating daily meteorological data and downscaling climate models over landscapes. Environ Model Softw 108:186–196
Desprez-Loustau M-L, Marçais B, Nageleisen L-M, Piou D, Vannini A (2006) Interactive effects of drought and pathogens in forest trees. Ann For Sci 63:597–612. https://doi.org/10.1051/forest:2006040
Ekblad A, Wallander H, Carlsson R, Huss-Danell K (1995) Fungal biomass in roots and extramatrical mycelium in relation to macronutrients and plant biomass of ectomycorrhizal Pinus sylvestris and Alnus incana. New Phytol 131:443–451. https://doi.org/10.1111/j.1469-8137.1995.tb03081.x
Ekblad A, Wallander H, Godbold DL, Cruz C, Johnson D, Baldrian P, Björk RG, Epron D, Kieliszewska-Rokicka B, Kjøller R, Kraigher H, Matzner E, Neumann J, Plassard C (2013) The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: role in carbon cycling. Plant Soil 366:1–27. https://doi.org/10.1007/s11104-013-1630-3
Ericsson T (1995) Growth and shoot: root ratio of seedlings in relation to nutrient availability. Plant Soil 168:205–214. https://doi.org/10.1007/BF00029330
Fernández FG, Brouder SM, Volenec JJ, Beyrouty CA, Hoyum R (2011) Soybean shoot and root response to localized water and potassium in a split-pot study. Plant Soil 344:197–212. https://doi.org/10.1007/s11104-011-0740-z
Galmés J, Pou A, Alsina MM, Tomàs M, Medrano H, Flexas J (2007) Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): relationship with ecophysiological status. Planta 226:671–681. https://doi.org/10.1007/s00425-007-0515-1
Gill AL, Finzi AC (2016) Belowground carbon flux links biogeochemical cycles and resource-use efficiency at the global scale. Ecol Lett 19:1419–1428. https://doi.org/10.1111/ele.12690
Godbold DL, Hoosbeek MR, Lukac M, Cotrufo MF, Janssens IA, Ceulemans R, Polle A, Velthorst EJ, Scarascia-Mugnozza G, Angelis PD, Miglietta F, Peressotti A (2006) Mycorrhizal hyphal turnover as a dominant process for carbon input into soil organic matter. Plant Soil 281:15–24. https://doi.org/10.1007/s11104-005-3701-6
Graham EB, Knelman JE, Schindlbacher A, Siciliano S, Breulmann M, Yannarell A, Beman JM, Abell G, Philippot L, Prosser J, Foulquier A, Yuste JC, Glanville HC, Jones DL, Angel R, Salminen J, Newton RJ, Bürgmann H, Ingram LJ, Hamer U, Siljanen HMP, Peltoniemi K, Potthast K, Bañeras L, Hartmann M, Banerjee S, Yu R-Q, Nogaro G, Richter A, Koranda M, Castle SC, Goberna M, Song B, Chatterjee A, Nunes OC, Lopes AR, Cao Y, Kaisermann A, Hallin S, Strickland MS, Garcia-Pausas J, Barba J, Kang H, Isobe K, Papaspyrou S, Pastorelli R, Lagomarsino A, Lindström ES, Basiliko N, Nemergut DR (2016) Microbes as engines of ecosystem function: when does community structure enhance predictions of ecosystem processes? Front Microbiol 214.https://doi.org/10.3389/fmicb.2016.00214
Guerrero-Galán C, Calvo-Polanco M, Zimmermann SD (2019) Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza 29:291–301. https://doi.org/10.1007/s00572-019-00894-2
Hagenbo A, Clemmensen KE, Finlay RD, Kyaschenko J, Lindahl BD, Fransson P, Ekblad A (2017) Changes in turnover rather than production regulate biomass of ectomycorrhizal fungal mycelium across a Pinus sylvestris chronosequence. New Phytol 214:424–431. https://doi.org/10.1111/nph.14379
Hagenbo A, Hadden D, Clemmensen KE, Grelle A, Manzoni S, Mölder M, Ekblad A, Fransson P (2019) Carbon use efficiency of mycorrhizal fungal mycelium increases during the growing season but decreases with forest age across a Pinus sylvestris chronosequence. J Ecol 107:2808–2822. https://doi.org/10.1111/1365-2745.13209
Hagenbo A, Piñuela Y, Castaño C, de Aragón JM, de-Miguel S, Alday JG, Bonet JA, (2021) Production and turnover of mycorrhizal soil mycelium relate to variation in drought conditions in Mediterranean Pinus pinaster, Pinus sylvestris and Quercus ilex forests. New Phytol 230:1609–1622. https://doi.org/10.1111/nph.17012
Ihrmark K, Bödeker ITM, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, Strid Y, Stenlid J, Brandström-Durling M, Clemmensen KE, Lindahl BD (2012) New primers to amplify the fungal ITS2 region—evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol 82:666–677. https://doi.org/10.1111/j.1574-6941.2012.01437.x
IUSS Working Group WRB (2015) World reference base for soil resources 2014, update 2015 international soil classification system for naming soils and creating legends for soil maps. World soil resources reports no. 106. FAO, Rome
Kjeldahl J (1883) Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern (New method for the determination of nitrogen in organic substances). Fresenius Z Anal Chem 22:366–382. https://doi.org/10.1007/BF01338151
Kjøller R, Nilsson L-O, Hansen K, Schmidt IK, Vesterdal L, Gundersen P (2012) Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol 194:278–286. https://doi.org/10.1111/j.1469-8137.2011.04041.x
Koide RT, Fernandez C, Malcolm G (2014) Determining place and process: functional traits of ectomycorrhizal fungi that affect both community structure and ecosystem function. New Phytol 201:433–439. https://doi.org/10.1111/nph.12538
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lücking R, Martín MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüßler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson K-H (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277. https://doi.org/10.1111/mec.12481
Kucheryavskiy S (2019) mdatools: multivariate data analysis for chemometrics R package version 0.9.4
Li H, Huang G, Meng Q, Ma L, Yuan L, Wang F, Zhang W, Cui Z, Shen J, Chen X, Jiang R, Zhang F (2011) Integrated soil and plant phosphorus management for crop and environment in China. A review. Plant Soil 349:157–167. https://doi.org/10.1007/s11104-011-0909-5
Lindahl BD, Tunlid A (2015) Ectomycorrhizal fungi - potential organic matter decomposers, yet not saprotrophs. New Phytol 205:1443–1447. https://doi.org/10.1111/nph.13201
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Hogberg P, Stenlid J, Finlay RD (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620. https://doi.org/10.1111/j.1469-8137.2006.01936.x
Maron JL, Marler M, Klironomos JN, Cleveland CC (2011) Soil fungal pathogens and the relationship between plant diversity and productivity. Ecol Lett 14:36–41. https://doi.org/10.1111/j.1461-0248.2010.01547.x
Martin TL, Anderson DA (1943) Organic matter decomposition, mold flora, and soil aggregation relationships. Soil Sci Soc Am J 7:215–217. https://doi.org/10.2136/sssaj1943.036159950007000C0032x
Matsuda Y, Sugiyama F, Nakanishi K, Ito S (2006) Effects of sodium chloride on growth of ectomycorrhizal fungal isolates in culture. Mycoscience 47:212–217. https://doi.org/10.1007/S10267-006-0298-4
McLachlan GJ, Do K-A, Ambroise C (2004) Analyzing microarray gene expression data. Wiley, Hoboken
Meisner A, Jacquiod S, Snoek BL, ten Hooven FC, van der Putten WH (2018) Drought legacy effects on the composition of soil fungal and prokaryote communities. Front Microbiol 294.https://doi.org/10.3389/fmicb.2018.00294
Montgomery HJ, Monreal CM, Young JC, Seifert KA (2000) Determination of soil fungal biomass from soil ergosterol analyses. Soil Biol Biochem 32:1207–1217. https://doi.org/10.1016/S0038-0717(00)00037-7
Nilsson LO, Wallander H (2003) Production of external mycelium by ectomycorrhizal fungi in a Norway spruce forest was reduced in response to nitrogen fertilization. New Phytol 158:409–416. https://doi.org/10.1046/j.1469-8137.2003.00728.x
Nogués-Bravo D, Araújo M, Lasanta T, Moreno J (2008) Climate change in Mediterranean mountains during the 21st century. Ambio 37:280–285. https://doi.org/10.1579/0044-7447(2008)37[280:ccimmd]2.0.co;2
Noormets A, Epron D, Domec JC, McNulty SG, Fox T, Sun G, King JS (2015) Effects of forest management on productivity and carbon sequestration: A review and hypothesis. For Ecol Manag 355:124–140. https://doi.org/10.1016/j.foreco.2015.05.019
Nylund J, Wallander H (1992) Ergosterol analysis as a means of quantifying mycorrhizal biomass. Methods Mircrobiol 24:77–88. https://doi.org/10.1016/S0580-9517(08)70088-6
Olsen SR, Cole CV, Watanabe FS, Dean LA, United States, Department of Agriculture (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Dept. of Agriculture, Washington, D.C.
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993. https://doi.org/10.1126/science.1201609
Querejeta J, Egerton-Warburton LM, Allen MF (2003) Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia 134:55–64. https://doi.org/10.1007/s00442-002-1078-2
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reynolds HL, Packer A, Bever JD, Clay K (2003) Grassroots ecology: plant–microbe–soil interactions as drivers of plant community structure and dynamics. Ecology 84:2281–2291. https://doi.org/10.1890/02-0298
Salmanowicz B, Nylund JE (1988) High-performance liquid-chromatography determination of ergosterol as a measure of ectomycorrhiza infection in Scots pine. Eur J Plant Pathol 18:291–298
Sardans J, Peñuelas J (2007) Drought changes phosphorus and potassium accumulation patterns in an evergreen Mediterranean forest. Funct Ecol 21:191–201. https://doi.org/10.1111/j.1365-2435.2007.01247.x
Sardans J, Peñuelas J (2015) Potassium: a neglected nutrient in global change. Glob Ecol Biogeogr 24:261–275. https://doi.org/10.1111/geb.12259
Sayer EJ, Oliver AE, Fridley JD, Askew AP, Mills RTE, Grime JP (2017) Links between soil microbial communities and plant traits in a species-rich grassland under long-term climate change. Ecol Evol 7:855–862. https://doi.org/10.1002/ece3.2700
Schmalenberger A, Duran AL, Bray AW, Bridge J, Bonneville S, Benning LG, Romero-Gonzalez ME, Leake JR, Banwart SA (2015) Oxalate secretion by ectomycorrhizal Paxillus involutus is mineral-specific and controls calcium weathering from minerals. Sci Rep 5:12187. https://doi.org/10.1038/srep12187
Schulze E-D, Mooney HA, Sala OE, Jobbagy E, Buchmann N, Bauer G, Canadell J, Jackson RB, Loreti J, Oesterheld M, Ehleringer JR (1996) Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia 108:503–511. https://doi.org/10.1007/BF00333727
Shi L, Guttenberger M, Kottke I, Hampp R (2002) The effect of drought on mycorrhizas of beech (Fagus sylvatica L.): changes in community structure, and the content of carbohydrates and nitrogen storage bodies of the fungi. Mycorrhiza 12:303–311. https://doi.org/10.1007/s00572-002-0197-2
Smith ES, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London
Staddon PL, Gregersen R, Jakobsen I (2004) The response of two Glomus mycorrhizal fungi and a fine endophyte to elevated atmospheric CO2, soil warming and drought. Glob Change Biol 10:1909–1921. https://doi.org/10.1111/j.1365-2486.2004.00861.x
Sun T, Wang Y, Hui D, Jing X, Feng W (2020) Soil properties rather than climate and ecosystem type control the vertical variations of soil organic carbon, microbial carbon, and microbial quotient. Soil Biol Biochem 148:107905. https://doi.org/10.1016/j.soilbio.2020.107905
Swaty RL, Deckert RJ, Whitham TG, Gehring CA (2004) Ectomycorrhizal abundance and community composition shifts with drought: predictions from tree rings. Ecology 85:1072–1084. https://doi.org/10.1890/03-0224
Thor K (2019) Calcium—nutrient and messenger. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00440
Thornton PE, Hasenauer H, White MA (2000) Simultaneous estimation of daily solar radiation and humidity from observed temperature and precipitation: an application over complex terrain in Austria. Agric For Meteorol 104:255–271. https://doi.org/10.1016/S0168-1923(00)00170-2
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515. https://doi.org/10.1046/j.1469-8137.2002.00470.x
Treseder KK, Balser TC, Bradford MA, Brodie EL, Dubinsky EA, Eviner VT, Hofmockel KS, Lennon JT, Levine UY, MacGregor BJ, Pett-Ridge J, Waldrop MP (2012) Integrating microbial ecology into ecosystem models: challenges and priorities. Biogeochemistry 109:7–18. https://doi.org/10.1007/s10533-011-9636-5
van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA, Suding KN, de Voorde TFJV, Wardle DA (2013) Plant–soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276. https://doi.org/10.1111/1365-2745.12054
Vašutová M, Edwards-Jonášová M, Baldrian P, Čermák M, Cudlín P (2017) Distinct environmental variables drive the community composition of mycorrhizal and saprotrophic fungi at the alpine treeline ecotone. Fungal Ecol 27:116–124. https://doi.org/10.1016/j.funeco.2016.08.010
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wallander H (1995) A new hypothesis to explain allocation of dry matter between mycorrhizal fungi and pine seedlings in relation to nutrient supply. Plant Soil 168–169:243–248. https://doi.org/10.1007/BF00029334
Wallander H, Nylund J-E (1992) Effects of excess nitrogen and phosphorus starvation on the extramatrical mycelium of ectomycorrhizas of Pinus sylvestris L. New Phytol 120:495–503. https://doi.org/10.1111/j.1469-8137.1992.tb01798.x
Wang M, Ding L, Gao L, Li Y, Shen Q, Guo S (2016) The interactions of aquaporins and mineral nutrients in higher plants. Int J Mol Sci 17:1229. https://doi.org/10.3390/ijms17081229
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511. https://doi.org/10.1093/aob/mcg164
White TJ, Burns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wold S (1978) Cross-validatory estimation of the number of components in factor and principal components models. Technometrics 20:397–405. https://doi.org/10.1080/00401706.1978.10489693
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab Syst 58:109–130. https://doi.org/10.1016/S0169-7439(01)00155-1
Zhang H, Li J, Chen S, Lu C, Wang R, Dai S, Zhu H, Zhang Y, Shi Y, Wang M, Lu Y, Li L, Langenfeld-Heyser R, Eberhard F, Polle A (2008) Effect of NaCl on growth and ion relations in two salt-tolerant strains of Paxillus involutus. For Stud China 10:95–100. https://doi.org/10.1007/s11632-008-0025-7
Funding
Open access funding provided by Norwegian Institute of Bioeconomy Research. This work was supported by the Spanish Ministry of Science, Innovation and Universities, grant RTI2018-099315-A-I00. J.G.A. was supported by the Ramon y Cajal fellowship (RYC-2016–20528) and J.A.B. and S.dM benefitted from a Serra-Húnter Fellowship provided by the Generalitat of Catalunya. A.H. was supported by the Norwegian Institute for Bioeconomy Research (NIBIO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hagenbo, A., Alday, J.G., Martínez de Aragón, J. et al. Variations in biomass of fungal guilds are primarily driven by factors related to soil conditions in Mediterranean Pinus pinaster forests. Biol Fertil Soils 58, 487–501 (2022). https://doi.org/10.1007/s00374-022-01621-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-022-01621-4