Abstract
Tropical soils often contain less soil organic C (SOC) and microbial biomass C (MBC) than temperate soils and, thus, exhibit lower soil fertility. The addition of plant residues and N fertilizers can improve soil fertility, which might be reflected by microbial C use efficiency (CUE) and functional diversity. A 42-day incubation study was carried out, adding leaf litter of the C4 plant finger millet (Eleusine coracana Gaertn.) and inorganic 15N fertilizer. The aim was to investigate amendment effects on CUE and functional diversity in a tropical Nitisol and a temperate Luvisol. At day 42, 28% of the millet litter-derived C (C4) added was mineralised to CO2C4 in the temperate Luvisol and only 18% in the tropical Nitisol, averaging all N treatments. In contrast, none of the different fractions used for calculating CUE values, i.e. CO2C4, MBC4, microbial residue C4, and particulate organic matter C4, differed between the soils in the N0 (no N addition) treatment. CUE values considering microbial residues varied around 0.63, regardless of soil type and sampling day, which needs further evaluation. Millet litter increased autochthonous SOC-derived CO2C3 production, but N addition did not. This priming effect was apparently not caused by N mining. The respiratory response to most substrates added by multi-substrate-induced respiration (MSIR) and, thus, functional diversity was higher in the Luvisol than in the Nitisol. Millet litter had positive and N addition negative effects on the functional diversity of Nitisol, indicating that MSIR is a useful tool for evaluating soil fertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil organic matter (SOM) and soil microorganisms are central components for soil fertility (Joergensen 2010), i.e. the ability of soils to maintain key ecological functions, such as decomposition of plant residues and provision of nutrients for plant growth (Craswell and Lefroy 2001; Joergensen and Castillo 2001; Cerri et al. 2006). Soil microorganisms maintain the majority of enzymatic processes in soil and preserve energy and nutrients in their biomass (Jenkinson and Ladd 1981). As these processes are controlled by temperature, the turnover rates of microbial biomass C (MBC) and soil organic C (SOC) are higher in tropical regions than in temperate climates (Jenkinson and Ayanaba 1977; Diels et al. 2004; Sierra and Desfontaines 2018). This shortens the response time to changes in management practices and increases the risk of permanent damage to soil fertility (Cerri et al. 2003; Bationo et al. 2007).
In contrast to humid temperate areas, the tropical monsoonal climate of India is characterised by extreme changes between dry and rainy seasons (Srivastava and Singh 1991; Manna et al. 2007; Vineela et al. 2008). Dystric Nitisols are the dominating soil type of the old landscapes around tropical Bangalore in South India (Murugan et al. 2019). These soils are characterised by high contents of Al and Fe oxides, which reduce P availability, as well as low contents of SOC, which intensify drought effects. Consequently, these Nitisols currently exhibit considerably lower soil fertility in comparison with Luvisols of the young landscapes in Central Germany. However, SOC contents, and thus fertility, of tropical Nitisol soils can be improved by adding plant residues (Agegnehu et al. 2016; Sierra and Desfontaines 2018), especially in combination with N fertilizers (Geisseler and Scow 2014).
SOC sequestration can be promoted by increasing the C input or by decreasing microbial turnover, which is the product of maintenance coefficient × C use efficiency (CUE). Often CUE values are calculated as MBC growth/MBC uptake (Manzoni et al. 2012; Spohn et al. 2016a, b; Geyer et al. 2019). There, MBC growth is usually substrate C incorporated into the microbial biomass, while MBC uptake is the sum of substrate-derived MBC and CO2 (Manzoni et al. 2012). Accurate CUE estimates using this approach depend upon a relatively narrow window of incubation time that ensures complete metabolisation of an added substrate (Joergensen and Wichern 2018).
The view of Manzoni et al. (2012) and Spohn et al. 2016a, b was challenged by Joergensen and Wichern (2018), who asked for all microbial metabolites to be included in the CUE calculation. Research has been focused on CUE of 13C- and 14C-labelled sugars, mainly glucose (Bardgett and Saggar 1994; Bremer and Kuikman 1994), and other simple organic components (Jones et al. 2018) but rarely SOM (Spohn et al. 2016a, b). Less information is available on the CUE of complex plant residues (Muhammad et al. 2006; Rottmann et al. 2010; Sauvadet et al. 2018), which can be determined by the recovery of added substrate as particulate organic matter (Magid and Kjærgaard 2001). This approach is often combined with the difference in δ13C of C4 plants and SOC mainly originated from C3 plants (Ryan and Aravena 1994; Balesdent and Mariotti 1996; Faust et al. 2019). However, nothing is known about the effects of soil type and incubation time on this type of CUE value. As the metabolisation of added complex substrates is usually incomplete at a specific sampling date (Faust et al. 2019), CUE values of complex plant litter might vary over incubation time, like that of simple glucose (Joergensen and Wichern 2018).
Lower MBC and SOC levels of Nitisols might be caused by lower CUE values of an N limited microbial community (Kaiser et al. 2014; Poeplau et al. 2019). Microbial C and N limitation can be reflected by a variable, but often lower respiratory response to simple low-molecular-weight organic substrates added to soil, depending on the metabolic demand (Struecker and Joergensen 2015). This creates differences in functional diversity of the soil microbial community, using multi-substrate-induced respiration (Campbell et al. 2003). This approach has been shown to respond sensitively to soil type (Moscatelli et al. 2018) and to the addition of organic N fertilizers (Sradnick et al. 2013; Murugan et al. 2014; Podmirseg et al. 2019). However, nothing is known about the immediate effect of the combined addition of plant litter and inorganic N fertilizers on functional diversity. Microbial C limitation is certainly reduced by the addition of plant litter (Struecker and Joergensen 2015), but the response to inorganic N fertilisation remains largely unknown.
The central objective of the current incubation study was to investigate the following hypotheses: (1) CUE of millet litter does not depend on the incubation time if all microbial metabolites are included. (2) The Nitisol with less MBC has a lower CUE of millet litter than the Luvisol with more MBC. (3) Microbial functional diversity is increased by millet litter and reduced by inorganic N addition. (4) Amendment effects on CUE and functional diversity are stronger in the Nitisol than in the Luvisol.
Materials and methods
Sites, soils, and litter
The temperate soil is a Haplic Luvisol (IUSS Working Group WRB 2015). Samples were taken at 0–15 cm from the site Teilanger I in Neu-Eichenberg, Germany (51° 22′ 50.8″ N 9° 54′ 34.3″ E) in March 2018. The site was cultivated with a mixture of alfalfa (Medicago sativa L.) and Italian rye grass (Festuca perennis Lam.) under a non-ploughing system. The soil had a pH-H2O of 6.83 with a texture of 3% sand, 83% silt, and 13% clay. The soil contained 11.4 ± 0.3 mg SOC and 1.48 ± 0.03 mg total N g−1 soil with a δ13C of −27.4 ± 0.1‰ and a δ15N of 8.2 ± 0.3‰. Mean annual rainfall is 579 ± 165 mm (2013–2017). Mean annual air temperature is 10.5 ± 0.8 °C.
The tropical soil is a Dystric Nitisol (IUSS Working Group WRB 2015). Soil samples were taken from the irrigated field experiment (FOR2432) at the University of Agricultural Sciences (UAS) in Bangalore, India (13° 05′ 23.9″ N 77° 33′ 58.8″ E) (Hoffmann et al. 2017). Soils were sampled at 0–20 cm in four replicated plots after harvest of lablab (Lablab purpureus (L.) Sweet) under a non-ploughing system in November 2017. The soil had a pH-H2O of 6.69 with a texture of 58% sand, 10% silt, and 32% clay. The soil contained 8.9 ± 0.6 mg SOC and 0.90 ± 0.04 mg total N g−1 soil with a δ13C of − 22.6 ± 0.2‰ and a δ15N of 5.4 ± 0.3‰. Long-term mean annual rainfall is 935 mm and mean annual air temperature is 24.0 °C.
All soil samples were sieved (< 2 mm), homogenised, and, after the removal of roots and stones, stored in plastic bags at 4 °C in the dark until the start of the experiment. Soil pH was measured electrochemically at a soil to water ratio of 1 to 2.5. Water holding capacity was determined by weighing 10 g moist soil into a glass tube (2 × 10 cm) with a porous membrane at the bottom that allows water flow. The tubes were transferred into a water bath with a filling level of 1 cm height, followed by water addition to the tubes until the soil was completely covered, and left for 3 h. Then, the tubes were removed from the water bath, placed onto absorbent tissue paper for 10 min, and weighed. Finally, the tubes were dried at 105 °C for 24 h and weighed again (Wilke 2005).
Finger millet (Eleusine coracana Gaertn.) is a C4 plant and common in India. The millet leaf litter used in this experiment originated from the rainfed field experiment of the DGF project FOR2432 at the UAS, Bangalore (Hoffmann et al. 2017; Murugan et al. 2019). The millet litter contained 384.7 ± 1.4 mg total C and 6.8 ± 0.1 mg total N g−1 dry weight (DW) with a δ13C of − 13.4 ± 0.4‰ and a δ15N of 15.0 ± 1.0‰.
Experimental treatments
The experimental set-up was designed as a three-factorial experiment with the following factors in quadruplicate: (1) soil (temperate Luvisol and tropical Nitisol), (2) millet litter addition (with and without), and (3) N level: N0 (no N addition), N20 (= 20 μg N g−1 soil) and N50 (= 50 μg N g−1 soil) applied as 10 atom% NH4-15NO3 (Chemotrade, Leipzig, Germany). The amount of millet leaf litter (5 mm cuttings) corresponding to 1 mg C g−1 soil was added to the soil of ML + treatments. Litter and soil were mixed in the plastic bag and transferred into 50-ml Falcon tubes. The water content was adjusted to 50% water holding capacity (WHC) by adding the NH4NO3 solution to the N20 and N50 treatments and water to the N0 treatment, respectively. Two Falcon tubes per treatment, vial A (15 g moist soil for sampling day 7) and vial B (35 g moist soil for sampling day 42), were placed into a Mason jar, equipped with sealing rings. Soils were incubated at 25 °C. For measuring particulate organic matter (POM), moist soil of 100 g per replicate was mixed with 1 mg C g−1 soil of millet litter and transferred into 250-ml plastic vessels, placed in another set of Mason jars, placed alongside the Mason jars with the Falcon tubes. The POM experiment was restricted solely to the N0 treatment, due to limited quantity of the Nitisol.
CO2 evolution
The CO2 evolved was trapped during the incubation in 0.25 M NaOH solution, which was changed after 2, 4, 7, 14, 21, 28, 35, and 42 days. The trapped CO2 was precipitated with 5 ml of a saturated BaCl2 solution (Zibilske 1994) and stored under CO2-free atmosphere. Then, the NaOH not consumed was back titrated with 0.25 M HCl, using a TITRONIC 500 (Xylem Analytics, Weilheim, Germany) system to the transition point of phenolphthalein at a pH of 8.3. For the determination of δ13C values, BaCO3 samples from the titration events day 2, 7, and 42 were centrifuged (3000 g for 10 min at 20 °C), washed three times with H2O to remove excess ions, and freeze-dried before analysis. The δ13C values of the titration events day 4, 14, 21, 28, and 35 were estimated by linear interpolation between the sampling days.
Microbial biomass
Soil microbial biomass C (MBC) and N (MBN) were determined by fumigation extraction (Brookes et al. 1985; Vance et al. 1987) at day 7 (vial A) and day 42 (vial B) of incubation. To reduce the amount of inorganic N in the sample, 15 g moist soil were pre-extracted for 30 min by oscillating shaking at 200 rev min−1 with 40 ml 0.05 M K2SO4 (Widmer et al. 1989). Non-fumigated and fumigated 5-g portions were extracted for 30 min by oscillation shaking at 200 rev min−1 with 20 ml 0.05 M K2SO4 (Potthoff et al. 2003), centrifuged (3000 g for 10 min at 10 °C), filtered (hw3, Sartorius Stedim Biotech, Göttingen, Germany), and stored at −18 °C before analysis. Organic C and total N in the extracts were determined with a Multi N/C 2100S analyser (Analytik Jena, Germany). MBC was calculated as EC/kEC, where EC = (organic C extracted from fumigated soils) − (organic C extracted from non-fumigated soils) and kEC = 0.45 (Wu et al. 1990). MBN was calculated as EN/kEN, where EN = (total N extracted from fumigated soils) − (total N extracted from non-fumigated soils) and kEN = 0.54 (Brookes et al. 1985). About 14 ml of the soil extracts were freeze-dried for isotope analysis (Alpha 1-4 LD plus, Christ, Osterode, Germany).
Particulate organic matter
POM was recovered as described by Magid and Kjærgaard (2001) at day 0, 7, and 42 of incubation. Briefly, 100 g moist soil was dispersed in 400 ml 5% NaCl solution, stirred by hand, and allowed to stand overnight (Muhammad et al. 2006). Samples were poured gradually onto a 400-μm sieve and washed with tap water. Soil aggregates were destroyed by pushing them through the sieve during the washing process. A 5% NaCl solution was added to the washed soil and the procedure was repeated until organic particles were no longer visible in the mineral fraction and the washing water was clear. POM was transferred into crucibles, dried at 60 °C, and weighed.
C and N analyses and calculations
For analyses of total C, δ13C, total N, and δ15N, samples were dried for 24 h at 105 °C (soil) and 60 °C (POM and litter), respectively, and ball-milled. The δ13C and δ15N in K2SO4 extracts as well as δ13C of BaCO3 were analysed in freeze-dried samples. Isotope values were measured by elemental analyser–isotope ratio mass spectrometry and are expressed in δ-notation relative to the Vienna Pee Dee Belemnite (VPDB) for 13C and in atom% relative to air for 15N. The fraction of millet-derived C in the K2SO4 extracts of fumigated and non-fumigated samples in CO2C as well as in POMC was calculated for each individual replicate of all treatments from the δ13C data according to a two pool-mixing model (Balesdent and Mariotti 1996) using the following equation:
where δ13Csample represents the millet treatments, δ13Ccontrol the no millet treatments at the respective sampling days 7 and 42. The fraction of addition-derived 15N in the K2SO4 extracts of fumigated and non-fumigated samples was calculated using the following equation (Dijkstra et al. 2006; Zareitalabad et al. 2010):
where 15Nsample represents the N20 and N50 treatments, δ15Ncontrol the N0 treatment at the respective sampling days in atom%, and 15Naddition = 10 atom%.
CUE calculations
CUE values of millet litter were calculated at sampling days 7 and 42 in the N0 treatment according to Joergensen and Wichern (2018), considering all microbial metabolites, i.e. millet-derived microbial residue C (MRC4):
Millet-derived C4 in MBC, POM, and CO2C are abbreviated as MBC4, POMC4, and CO2C4. MRC (Joergensen and Wichern 2018) or microbial necromass (Liang et al. 2019) embraces all freshly synthesised microbial products that leave the cells as non-biomass metabolites, such as exo-enzymes (Burns et al. 2013), extracellular polymeric substances (Redmile-Gordon et al. 2014, 2015), e.g. the glomalin-related protein (Wright and Upadhyaya 1996; Spohn and Giani 2011), other secondary metabolites, e.g. antibiotics (Boruta 2018; Keller 2019), and dead tissue remains (Joergensen and Wichern 2018). In addition, CUE of millet litter at sampling days 7 and 42 in the N0 treatment was calculated in the classical way, as proposed e.g. by Manzoni et al. (2012) and Spohn et al. (2016a, b). This calculation approach solely considers the incorporation of millet-derived C4 into the MBC4 and is thus abbreviated as CUEMB:
All millet litter-derived fractions were considered in the calculations as % of the added substrate.
Multi-substrate-induced respiration
Multi-substrate-induced respiration (MSIR) was determined according to Campbell et al. (2003). MSIR was conducted on the soil from vial B, which was stored after the incubation experiment at 4 °C in the dark until analysis. The soil samples were dried from 50 to 40%WHC by monitoring the gravimetric reduction, before 300 mg soil was weighed in triplicate per substrate, divided on different deep-well plates (Nunc, Langenselbold, Germany). Then, the soil was pre-incubated for 7 days in the dark at 25 °C prior to MSIR analysis.
The physiological profiles were determined by applying H2O, 6 amino acids [γ-aminobutyric acid (Aba), l-alanine (Ala), l-arginine (Arg), dl-aspartic acid (Asp), l-glutamine (GluN), l-leucine (Leu)], 2 amino sugars [N-acetyl glucosamine (NAG), d-glucosamine (GlcN)], 5 neutral sugars [l-arabinose (Ara), d-galactose, d-glucose (Glc), d-fructose (Fru), d-trehalose (Tre)], 3 carboxylic acids [citric acid (Cit), l-malic acid (Mal), oxalic acid (Oxa)], and phenolic protocatechuic acid (ProCa). These substrates present a cross section of root exudates (Campbell et al. 2003) and microbial components and products (Amelung et al. 2001) and have been used in similar studies before (Campbell et al. 2008; Sradnick et al. 2013; Murugan et al. 2014; Struecker and Joergensen 2015). To achieve a final concentration of 8 mg substrate g−1 soil, 20 μl substrate solution was added to 300 mg moist soil. Exceptions were aspartic acid (0.04 mg g−1 soil), l-leucine (1.33 mg g−1 soil), and protocatechuic acid (0.8 mg g−1 soil), due to their lower solubility in water. The colorimetric (12.5 ppm cresol red, 150 mM KCl, and 2.5 NaHCO3) CO2 trap was produced according to Campbell et al. (2003) and stored under CO2 free atmosphere at 25 °C in the dark for at least 72 h prior to measurement (Murugan et al. 2014). Addition of aqueous substrates was performed 40 min prior to the initial (t0) measurement, to avoid the first wetting peak. Detection plates were measured immediately before sealing (t0) and after 6 h of incubation (t6) at 25 °C in the dark with a microtiter plate reader (FLUO Star Omega, BMG, Ortenberg, Germany) at 572 nm (Sradnick et al. 2013). Absorbance was converted to CO2 concentration, using the following equation:
where ABS is the difference in absorption (572 nm) between t6 and t0 (Murugan et al. 2014, r = 0.98). Finally, μg CO2 was calculated by multiplying μl CO2 by 0.49. The Shannon diversity index (H) was calculated according to Zak et al. (1994):
where pi is the ratio of the respiration rate on a particular substrate minus the blank of the plate to the sum of respiration rates on all substrates.
Statistical analysis
Data are presented as arithmetic means on an oven-dry weight basis. All data used for ANOVA analysis exhibited normality of residuals and homogeneity of variances according to the Shapiro-Wilk test and Levene test, respectively. The significance of soil, millet litter, and N addition effects on autochthonous SOC-derived ΣCO2C3, MBC3, total MBN, and MB15N were tested using a 3-way ANOVA. The significance of soil and N effects on ΣCO2C4 and MBC4 were tested using a 2-way ANOVA. The significance of soil effects on POMC4, the POM-C/N ratio, and all fractions required for CUE calculations were tested using an unpaired t test. The significance of changes between days 7 and 42 was tested using a paired t test. ANOVA and t test analyses were carried out using SigmaPlot 13.0 (Systat, San José, USA). For MSIR, discriminant function analysis was used to identify classification success according to respiratory response of 17 substrates and H2O, using SPSS 21.0 (IBM, Armonk, USA).
Results
Millet litter decomposition
At the end of the incubation, 28% of the added millet litter C was mineralised to CO2 (ΣCO2C4) in the temperate Luvisol, but only 18% in the tropical Nitisol, averaging all N treatments (Table 1). In contrast, similar percentages of millet litter C were incorporated as MBC4 in the two soils, approximately 3% at day 7, significantly (P < 0.04, paired t test) increasing to 4% at day 42. N addition did not generally affect ΣCO2C4 and MBC4. However, in the N50 treatment, the lower MBC4 of the Luvisol (25 μg g−1 soil) in comparison with the Nitisol (53 μg g−1 soil) caused a significant soil × N interaction. In contrast to ΣCO2C4, the recovery of particulate organic matter C did not differ between the soils (Table 2). The C/N ratio of the recovered millet litter was always higher in the Luvisol than in the Nitisol, but this difference declined during the incubation.
MBC3 and total MBN in the Luvisol were roughly twice those in the Nitisol at day 7 (Table 3). MBC3 and total MBN generally (P < 0.01, paired t test) increased in both soils from day 7 to 42, so that the relative differences between the two soils declined. Millet litter addition did not affect MBC3, but moderately increased total MBN. In contrast, millet litter addition strongly increased cumulative ΣCO2C3 production. The absolute increase in the evolution of autochthonous CO2 was roughly the same in both soils at days 7 (+ 73 μg C g−1 soil) and 42 (+ 234 μg g−1 soil), although the basal respiration rate of the Luvisol was twice that of the Nitisol. However, the significant soil × millet interactions on ΣCO2C3 production at day 42 and on MBN at day 7 were in both cases caused by the significantly larger differences in the Nitisol than in the Luvisol.
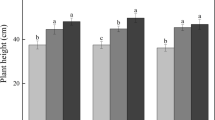
N addition did not generally affect ΣCO2C3 production, MBC3 and MBN. However, the significant soil × N interaction at day 7 was caused by 47 and 22% higher ΣCO2C3 production in the N0 treatment of the Luvisol compared with the N20 and N50 treatments, respectively. Microbial uptake of 15N was proportionate to the addition rate (Table 4), i.e. approximately 3.5% of the added N was incorporated as MB15N without significant (P = 0.14, paired t-test) changes from day 7 to 42. However, significantly less 15N was incorporated into the MBN of the Nitisol at day 7 in the N50 treatment, whereas all differences between the two soils diminished at day 42. At day 7, the MB-C/N ratio of the Nitisol generally exceeded that of the Luvisol (Fig. 1). At this day, the MB-C/N ratio was significantly increased by millet litter, but decreased by N addition. At day 42, all differences diminished and the MB-C/N ratio varied around 7.6, 23% larger than the overall average at day 7 (P < 0.01, paired t test).
Main effects on the microbial biomass C/N ratio at day 7 in the two soils and different treatments of the incubation experiment at 25 °C; probability values of a three-way ANOVA, using soil, millet litter, and N addition as factors: soil: P = 0.04, millet: P = 0.04; N addition: P = 0.01; interactions were all not significant
Carbon use efficiency
None of the different fractions used for calculating CUE differed between the soils in the N0 treatment (Table 5). This was also true for the ΣCO2C4 evolution. For this reason, the microbial CUE values of millet litter varied around 0.63 (Table 5), regardless of soil type. The CUE values did not differ between sampling days 7 and 42, whereas those obtained by the classical CUEMB approach declined with incubation time. They were, thus, 20 and 40% smaller than the CUE values, considering MRC4. However, the CUEMB also did not differ between the two soils.
Multi-substrate-induced respiration
All substrates and water additions resulted in a significantly (P < 0.01) stronger respiratory response in the Luvisol than in the Nitisol (Fig. 2). Arginine (Arg) addition created the lowest respiratory response, with 1.43 μl CO2C g−1 soil h−1 in the Luvisol (Fig. 2a) and 0.44 μl CO2C g−1 soil h−1 in the Nitisol (Fig. 2b). Citric acid (Cit) addition led to the highest respiratory response, with 13.9 μl CO2C g−1 soil h−1 in the Luvisol (Fig. 2a) and 11.4 μl CO2C g−1 soil h−1 in the Nitisol (Fig. 2b). Millet addition significantly (P < 0.03) increased the respiratory response of most substrates on average by 14% in both soils, except that of arginine and oxalic acid (Fig. 2). In contrast, N addition had no effect on the respiratory response of most substrates, except a mean significant (P < 0.04) reduction of glutamine (GluN), leucine (Leu), asparagine (Asp), and protocatechuic acid (ProCA) by approximately 7%. In contrast to soil × millet, numerous significant (P < 0.05) millet × N addition interactions were observed, because the respiratory response of the N20 treatment was significantly reduced for most substrates in the Luvisol.
The Shannon indices of all Luvisol treatments exceeded those of the respective Nitisol treatments (Fig. 3). In the Nitisol, millet addition increased the Shannon index, whereas N addition had negative effects. Discriminant function 1a strongly separated the functional diversity of the Luvisol from that of the Nitisol (Fig. 4a), whereas discriminant function 2a weakly separated the treatments with and without millet addition solely in the Nitisol. Discriminant function 1b separated the functional diversity between the N20 and N50 treatments (Fig. 4b), whereas discriminant function 2b separated the N0 from the N20 and N50 treatments.
Boxplots of the Shannon diversity index for the two soils with different fertilizer levels and millet litter addition; probability values of a three-way ANOVA, using soil, millet litter, and N addition as factors: soil: P < 0.01, millet: P < 0.01; N addition: not significant; soil × millet: P = 0.01, soil × N: not significant; millet × N: not significant
Discussion
N effects on litter decomposition
N addition had no effect on millet litter decomposition, indicating that neither of the soils were N limited. The lower millet decomposition rate of the Nitisol is most likely caused by the shortage of nutrients other than N, P, and K, which have all been applied as fertilizers in India. Consequently, it is more likely that SOC and MBC levels are generally low (de Castro Lopes et al. 2013; Fang et al. 2018; Luo et al. 2018). There were also no indications that N addition has general increasing effects on litter decomposition, MBC and MBN in both soils. However, the MB-C/N ratio declined with increasing N addition rate. This suggests an additional N uptake without physiological demand of soil microorganisms in both soils (Rasul et al. 2009), e.g. in fungal vacuoles (Klionsky et al. 1990; Khan and Joergensen 2019). However, the uptake of mineral N was low and did not change from day 7 to 42; an exception was the N50 treatment of the Luvisol, indicating low microbial immobilisation rates of inorganic N. Microbial uptake rates for manure N were higher, at 10% and 5% after 2 and 6 weeks, respectively (Bohlen et al. 1999). N addition did not cause a shift in functional diversity according to the MSIR approach.
Litter-induced priming of SOM mineralisation
The current increase in ΣCO2C3 evolution derived from autochthonous SOM mineralisation after millet litter addition is a strong true positive priming effect (Kuzyakov et al. 2000). This was apparently not caused by N mining, as N addition did not reduce the priming response. However, the possibility cannot be excluded that in other cases N mining might be an important reason for priming effects (Dijkstra et al. 2013; Mason-Jones et al. 2018; Tian et al. 2019). The significant soil × N interaction for ΣCO2C3 at day 7 in the Luvisol points to the possibility of N mining even in the current experiment. However, the absolute priming effect was similar in both soils, i.e. it was not related to different MBC and SOC levels but to the identical millet litter addition rate.
A large part of litter mineralisation is usually carried out by surface colonizing microorganisms (Flessa et al. 2002; Potthoff et al. 2005). In the current study, the extracellular enzymes produced by millet litter decomposing saprotrophic fungi were most likely also able to break down SOC as a co-substrate (Scheller and Joergensen 2008; Maynard et al. 2017; Finley et al. 2018). This means that priming mechanisms strongly differ between simple amendments, such as glucose or complex plant residues (Wu et al. 1993; Finley et al. 2018; Hicks et al. 2019). This might explain why true priming effects have rarely been observed under field conditions.
CUE calculations and constraints
A mean CUE of 0.63, considering MRC, indicates the strong ability of soil microbial communities to use the organic substrates that enter soil nearly as efficiently as glucose (Joergensen and Wichern 2018). This mean CUE value is in line with those reviewed by Joergensen and Wichern (2018), which were obtained by much longer incubation times of between 56 (Muhammad et al. 2006; Zareitalabad et al. 2010) and 57 days (Rottmann et al. 2010). The absence of a significant difference in CUE values of millet litter between days 7 and 42 is remarkable. Between these two sampling dates, MBC slightly increased, whereas MRC exponentially increased with time. As the classical approach does not consider this formation of MRC, the CUEMB values decline with time, indicating serious limitations (Joergensen and Wichern 2018). This exclusion of MRC from CUE calculations may cause the effects of nutrient supply (Fang et al. 2018), substrate quality (Öquist et al. 2017), temperature (Li et al. 2019; Qiao et al. 2019; Ye et al. 2019), and clay content or soil pH (Fang et al. 2018; Jones et al. 2019; Zheng et al. 2019) on CUE values.
However, other incubation periods than the two used in the current study might be even more appropriate for determining CUE values of plant residues, due to minor methodological constraints of the current approach. An incubation period of 7 days might be too short for soils with low turnover rates or for substrates that need more time to be colonised by decomposers (Eck et al. 2015). In this case, it is possible that not enough POMC was lost within 7 days to obtain a significant decline in comparison with initial values. Another problem is that only small MRC values might be formed within 7 days, as in the current Nitisol. The assumption that all POM recovered is non-decomposed substrate is not fully true, as all plant litter is colonised by microorganisms, which account for up to 2% of organic matter (Potthoff et al. 2008; Scheller and Joergensen 2008; Rottmann et al. 2011). This percentage is roughly counterbalanced by the negligible substrate losses during sieving, as indicated by the high day-0 recovery of 95% and more.
The current results contradict the view that the CUE declines with increasing ratio of substrate to MBC (Bardgett and Saggar 1994; Witter and Kanal 1998; Chander and Joergensen 2001). This view is probably less important for plant litter than low-molecular-weight substances such as glucose (Bremer and Kuikman 1994).
Differences in functional diversity between the soils
In the N0 treatment, the ΣCO2C4 evolution did not differ between the two soils (Table 5), contrasting the results of Delgado-Baquerizo et al. (2015), who observed strong soil type effects on leaf litter decomposition. However, less millet litter added was ultimately mineralised to CO2 in the Nitisol than in the Luvisol, averaging all N treatments (Table 1). This observation might be explained by the negative effects of N addition on the functional diversity expressed as the Shannon index (Fig. 3).
The Luvisol exhibited a higher functional diversity in combination with a higher respiratory response to most of the substrates added by the MSIR approach (Fig. 2). The temperate Luvisol with its higher MBC and SOC levels seems to buffer millet litter and N addition effects without further changes in functional diversity, which is in line with the study of van der Boma et al. (2018). However, the lower buffering capacity of the Nitisol led to a more variable response to the experimental treatments. This means that the functional diversity of the tropical Nitisol responded positively to the millet litter addition but negatively to N addition. Consequently, the Nitisol might also react rapidly to a continuous higher supply of organic matter.
Conclusions
Nitrogen addition did not generally affect millet litter decomposition but reduced its mineralisation to CO2 in the tropical Nitisol. This reduction is in line with the negative N effects on the functional diversity of the Nitisol. The strong priming effect on autochthonous SOC mineralisation was apparently not caused by N mining but using SOC as a co-substrate after adding easily available millet litter. In contrast to N addition, millet litter increased the functional diversity of the Nitisol but not that of the Luvisol. The functional diversity of the temperate Luvisol was generally higher than that of the Nitisol and was not affected by millet litter or by N addition. This suggests that improved soil fertility is not only expressed by SOC and MBC but also by a higher functional diversity, all increasing the buffering capacity of a soil to environmental changes. Without N addition, similar percentages of millet leaf litter were mineralised to CO2, recovered as POM, and incorporated into MBC. Consequently, also the microbial CUE values of millet litter, including microbial residue C, did not differ between the two soils. They varied around 0.63, regardless of whether the incubation period was 7 or 42 days. However, the optimum incubation time within this period still needs further evaluation. Future research should also test the effects of plant residues, differing in N and lignin concentrations, on CUE values in a larger range of soils, especially also under field conditions.
References
Agegnehu G, Nelson PN, Bird MI (2016) Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Till Res 160:1–13
Amelung W, Miltner A, Zhang X, Zech W (2001) Fate of microbial residues during litter decomposition as affected by minerals. Soil Sci 166:598–606
Balesdent J, Mariotti A (1996) Measurement of soil organic matter turnover using 13C natural abundance. In: Boutton TW, Yamasaki SI (eds) Mass spectrometry of soils. Marcel Dekker, New York, pp 83–111
Bardgett RD, Saggar S (1994) Effects of heavy metal contamination onthe short-term decomposition of labelled 14C glucose in a pasture soil. Soil Biol Biochem 26:727–733
Bationo A, Kihara J, Vanlauwe B, Waswa B, Kimetu J (2007) Soil organic carbon dynamics, functions and management in West African agro-ecosystems. Agric Syst 94:13–25
Bohlen PJ, Parmelee RW, Allen MF, Ketterings QM (1999) Differential effects of earthworms on nitrogen cycling from various nitrogen 15N labeled substrates. Soil Sci Soc Am J 63:882–890
Boruta T (2018) Uncovering the repertoire of fungal secondary metabolites: from Fleming’s laboratory to the International Space Station. Bioengineered 9:12–16
Bremer E, Kuikman P (1994) Microbial utilization of 14C[U]glucose in soil is affected by the amount and timing of glucose additions. Soil Biol Biochem 26:511–517
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method for measuring microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from C substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69:3593–3599
Campbell CD, Cameron CM, Bastias BA, Chen C, Cairney JWG (2008) Long term repeated burning in a wet sclerophyll forest reduces fungal and bacterial biomass and responses to carbon substrates. Soil Biol Biochem 40:2246–2252
Cerri CEP, Coleman K, Jenkinson DS, Bernoux M, Victoria R, Cerri CC (2003) Modeling soil carbon from forest and pasture ecosystems of Amazon, Brazil. Soil Sci Soc Am J 67:1879–1887
Cerri CEP, Piccolo MC, Feigl BJ, Paustian K, Cerri CC, Victoria RL, Melillo JM (2006) Interrelationships among soil total C and N, microbial biomass, trace gas fluxes and internal N-cycling in soils under pasture of the Amazon region. J Sustain Agric 27:45–69
Chander K, Joergensen RG (2001) Decomposition of 14C glucose in two soils with different levels of heavy metal contamination. Soil Biol Biochem 33:1811–1816
Craswell ET, Lefroy RDB (2001) The role and function of organic matter in tropical soils. Nutr Cycl Agroecosyst 61:7–18
de Castro Lopes AA, de Sousa DMG, Chaer GM, dos Reis Junior FB, Goedert WJ, de Carvalho Mendes I (2013) Interpretation of microbial soil indicators as a function of crop yield and organic carbon. Soil Sci Soc Am J 77:461–472
Delgado-Baquerizo M, García-Palacios P, Milla R, Gallardo A, Maestre FT (2015) Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biol Biochem 81:134–142
Diels J, Vanlauwe B, van der Meersch MK, Sanginga N, Merckx R (2004) Long-term soil organic carbon dynamics in a subhumid tropical climate: 13C data in mixed C3/C4 cropping and modeling with ROTHC. Soil Biol Biochem 36:1739–1750
Dijkstra P, Ishizu A, Doucett R, Hart SC, Schwartz E, Menyailo OV, Hungate BA (2006) 13C and 15N natural abundance of the soil microbial biomass. Soil Biol Biochem 38:3257–3266
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4:216
Eck T, Potthoff M, Dyckmans J, Wichern F, Joergensen RG (2015) Priming effects of Aporrectodea caliginosa on young rhizodeposits and old soil organic matter following wheat straw addition. Eur J Soil Biol 70:38–45
Fang Y, Singh BP, Collins D, Li B, Zhu J, Tavakkoli E (2018) Nutrient supply enhanced wheat residue-carbon mineralization, microbial growth, and microbial carbon-use efficiency when residues were supplied at high rate in contrasting soils. Soil Biol Biochem 126:168–178
Faust S, Koch HJ, Dyckmans J, Joergensen RG (2019) Response of maize leaf decomposition in litterbags and soil bags to different tillage intensities in a long-term field trial. Appl Soil Ecol 141:38–44
Finley BK, Dijkstra P, Rasmussen C, Schwartz E, Mau RL, Liu XJA, van Gestel N, Hungate BA (2018) Soil mineral assemblage and substrate quality effects on microbial priming. Geoderma 322:38–47
Flessa H, Potthoff M, Loftfield N (2002) Laboratory estimates of CO2 and N2O emissions following surface application of grass mulch: importance of indigenous microflora of mulch. Soil Biol Biochem 34:875–879
Geisseler D, Scow KM (2014) Long-term effects of mineral fertilizers on soil microorganisms – a review. Soil Biol Biochem 75:54–63
Geyer KM, Dijkstra P, Sinsabaugh R Frey, S.D (2019) Clarifying the interpretation of carbon use efficiency in soil through methods comparison. Soil Biol Biochem 128:J79–J88
Hicks LC, Meir P, Nottingham AT, Reay DS, Stott AW, Salinas N, Whitaker J (2019) Carbon and nitrogen inputs differentially affect priming of soil organic matter in tropical lowland and montane soils. Soil Biol Biochem 129:212–222
Hoffmann E, Jose M, Nölke N, Möckel T (2017) Construction and use of a simple index of urbanisation in the rural–urban interface of Bangalore, India. Sustainability 9:2146
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. FAO, Rome
Jenkinson DS, Ayanaba A (1977) Decomposition of carbon-14 labeled plant material under tropical conditions. Soil Sci Soc Am J 41:912–915
Jenkinson DS, Ladd JN (1981) Microbial biomass in soil: measurement and turnover. In: Paul EA, Ladd JN (eds) Soil biochemistry. Volume 5. Marcel Dekker, New York, pp 415–471
Joergensen RG (2010) Organic matter and micro-organisms in tropical soils. In: Dion P (ed) Soil biology and agriculture in the tropics. Springer, Berlin, pp 17–44
Joergensen RG, Castillo X (2001) Interrelationships between microbial and soil properties in young volcanic ash soils of Nicaragua. Soil Biol Biochem 33:1581–1589
Joergensen RG, Wichern F (2018) Alive and kicking: why dormant soil microorganisms matter. Soil Biol Biochem 116:419–430
Jones DL, Hill PW, Smith AR, Farrell M, Ge T, Banning NC, Murphy DV (2018) Role of substrate supply on microbial carbon use efficiency and its role in interpreting soil microbial community-level physiological profiles (CLPP). Soil Biol Biochem 123:1–6
Jones DL, Cooledge EC, Hoyle FC, Griffiths RI, Murphy DV (2019) pH and exchangeable aluminium are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities. Soil Biol Biochem 138:107584
Kaiser C, Franklin O, Dieckmann U, Richter A (2014) Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol Lett 17:680–690
Keller NP (2019) Fungal secondary metabolism: regulation, function and drug discovery. Nature Rev Microbiol 17:167–180
Khan KS, Joergensen RG (2019) Stoichiometry of the soil microbial biomass in response to amendments with varying C/N/P/S ratios. Biol Fertil Soils 55:265–274
Klionsky DJ, Herman PK, Emr SD (1990) The fungal vacuole composition, function, and biogenesis. Microbiol Rev 54:266–292
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Li J, Wang G, Mayes MA, Allison SD, Frey SD, Shi Z, Hu XM, Luo Y, Melillo JM (2019) Reduced carbon use efficiency and increased microbial turnover with soil warming. Glob Change Biol 25:900–910
Liang C, Amelung W, Lehmann J, Kästner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Chang Biol 25:3578–3590
Luo G, Lia L, Friman VP, Guo J, Guo S, Shen Q, Ling N (2018) Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: a meta-analysis. Soil Biol Biochem 124:105–115
Magid J, Kjærgaard C (2001) Recovering decomposing plant residues from the particulate soil organic matter fraction: size versus density separation. Biol Fertil Soils 33:252–257
Manna MC, Swarup A, Wanjari RH, Ravankar HN (2007) Long-term effects of NPK fertiliser and manure on soil fertility and a sorghum-wheat farming system. Austr J Experim Agric 47:700–711
Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91
Mason-Jones K, Schmücker N, Kuzyakov Y (2018) Contrasting effects of organic and mineral nitrogen challenge the N-mining hypothesis for soil organic matter priming. Soil Biol Biochem 124:38–46
Maynard DS, Crowther TW, Bradford MA (2017) Fungal interactions reduce carbon use efficiency. Ecol Lett 20:1034–1042
Moscatelli MC, Secondi L, Marabottini R, Papp R, Stazi SR, Mania E, Marinari S (2018) Assessment of soil microbial functional diversity: land use and soil properties affect CLPP-MicroResp and enzymes responses. Pedobiol 66:36–42
Muhammad S, Müller T, Joergensen RG (2006) Decomposition of pea and maize straw in Pakistani soils along a gradient in salinity. Biol Fertil Soils 43:93–101
Murugan R, Loges R, Taube F, Sradnick A, Joergensen RG (2014) Changes in soil microbial biomass, residues and functional diversity after conversion of permanent to modified grassland or maize crop. Microb Ecol 67:907–918
Murugan R, Parama VRR, Madan B, Muthuraju R, Ludwig B (2019) Short-term effect of nitrogen intensification on aggregate size distribution, microbial biomass and enzyme activities in a semi-arid soil under different crop types. Pedosphere 29:483–491
Öquist MG, Erhagen B, Haei M, Sparrman T, Ilstedt U, Schleucher J, Nilsson MB (2017) The effect of temperature and substrate quality on the carbon use efficiency of saprotrophic decomposition. Plant Soil 414:113–125
Podmirseg SM, Waldhuber S, Knapp BA, Insam H, Goberna M (2019) Robustness of the autochthonous microbial soil community after amendment of cattle manure or its digestate. Biol Fertil Soils 55:565–576
Poeplau C, Helfrich M, Dechow R, Szoboszlay M, Tebbe CC, Don A, Greiner B, Zopf D, Thumm U, Korevaar H, Geerts R (2019) Increased microbial anabolism contributes to soil carbon sequestration by mineral fertilization in temperate grasslands. Soil Biol Biochem 130:167–176
Potthoff M, Loftfield N, Wick B, John B, Buegger F, Joergensen RG, Flessa H (2003) The determination of δ13C in soil microbial biomass using fumigation-extraction. Soil Biol Biochem 35:947–954
Potthoff M, Dyckmans J, Flessa H, Muhs A, Beese F, Joergensen RG (2005) Dynamics of maize (Zea mays L.) leaf straw mineralization as affected by the presence of soil and the availability of nitrogen. Soil Biol Biochem 37:1259–1266
Potthoff M, Dyckmans J, Flessa H, Beese F, Joergensen RG (2008) Decomposition of maize residues after manipulation of colonization and its contribution to the soil microbial biomass. Biol Fertil Soils 44:891–895
Qiao Y, Wang J, Liang G, Du Z, Zhou J, Zhu C, Huang KX, Luo Y, Yan L, Xia J (2019) Global variation of soil microbial carbon-use efficiency in relation to growth temperature and substrate supply. Sci Rep 9:5621
Rasul G, Khan AA, Khan KS, Joergensen RG (2009) Immobilization and mineralization of nitrogen in a saline and alkaline soil during microbial use of sugarcane filter cake amended with glucose. Biol Fertil Soils 45:289–296
Redmile-Gordon MA, Brookes PC, Evershed RP, Goulding KWT, Hirsch PR (2014) Measuring the soil-microbial interface: extraction of extracellular polymeric substances (EPS) from soil biofilms. Soil Biol Biochem 72:163–171
Redmile-Gordon MA, Evershed RP, Hirsch PR, White RP, Goulding KWT (2015) Soil organic matter and the extracellular microbial matrix show contrasting responses to C and N availability. Soil Biol Biochem 88:257–267
Rottmann N, Dyckmans J, Joergensen RG (2010) Microbial use and decomposition of maize leaf straw incubated in packed soil columns at different depths. Eur J Soil Biol 46:27–33
Rottmann N, Siegfried K, Buerkert A, Joergensen RG (2011) Litter decomposition in fertilizer treatments of vegetable crops under irrigated subtropical conditions. Biol Fertil Soils 47:71–80
Ryan MC, Aravena R (1994) Combining 13C natural abundance and fumigation-extraction methods to investigate soil microbial biomass turnover. Soil Biol Biochem 26:1583–1585
Sauvadet M, Lashermes G, Alavoine G, Recous S, Chauvat M, Maron PA, Bertrand I (2018) High carbon use efficiency and low priming effect promote soil C stabilization under reduced tillage. Soil Biol Biochem 123:64–73
Scheller E, Joergensen RG (2008) Decomposition of wheat straw differing in N content in soils under conventional and organic farming management. J Plant Nutr Soil Sci 171:886–892
Sierra J, Desfontaines L (2018) Predicting the in situ rate constant of soil carbon mineralisation from laboratory-based measurements in tropical soils under contrasting tillage management systems. Soil Till Res 180:175–181
Spohn M, Giani L (2011) Impacts of land use change on soil aggregation and aggregate stabilizing compounds as dependent on time. Soil Biol Biochem 43:1081–1088
Spohn M, Klaus K, Wanek W, Richter A (2016a) Microbial carbon use efficiency and biomass turnover times depending on soil depth - implications for carbon cycling. Soil Biol Biochem 96:74–81
Spohn M, Pötsch EM, Eichorst SA, Woebken D, Wanek W, Richter A (2016b) Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol Biochem 97:168–175
Sradnick A, Murugan R, Oltmanns M, Raupp J, Joergensen RG (2013) Changed in functional diversity of the soil microbial community in a heterogeneous sandy soil after long-term fertilisation with cattle manure and mineral fertilizer. Appl Soil Ecol 63:23–28
Srivastava SC, Singh JS (1991) Microbial C, N and P in dry tropical forest soils: effects of alternate land-uses and nutrient flux. Soil Biol Biochem 23:117–124
Struecker J, Joergensen RG (2015) Microorganisms and their substrate utilization patterns in topsoil and subsoil layers of two silt loams, differing in soil organic C accumulation due to colluvial processes. Soil Biol Biochem 91:310–317
Tian P, Mason-Jones K, Liu S, Wang Q, Sun T (2019) Form of nitrogen deposition affects soil organic matter priming by glucose and cellulose. Biol Fertil Soils 55:383–391
van der Boma F, Nunes I, Raymond NS, Hansen V, Bonnichsen L, Magid J, Nybroe O, Jensen LS (2018) Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field. Soil Biol Biochem 122:91–103
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vineela C, Wani SP, Srinivasarao C, Padmaja B, Vittal KPR (2008) Microbial properties of soils as affected by cropping and nutrient management practices in several long-term manurial experiments in the semi-arid tropics of India. Appl Soil Ecol 40:165–173
Widmer P, Brookes PC, Parry LC (1989) Microbial biomass nitrogen measurements in soils containing large amounts of inorganic nitrogen. Soil Biol Biochem 21:865–867
Wilke BM (2005) Determination of chemical and physical soil properties. In: Margesin R, Schinner F (eds) Manual of soil analysis – monitoring and assessing soil bioremediation. Soil biology, Volume 5. Springer, Berlin, pp 47–95
Witter E, Kanal A (1998) Characteristics of the soil microbial biomass in soils from a long-term field experiment with different levels of C input. Appl Soil Ecol 10:37–49
Wright SF, Upadhyaya A (1996) Extraction of an abundant and unusual protein from soils and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci 161:575–586
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C - an automated procedure. Soil Biol Biochem 22:1167–1169
Wu J, Brookes PC, Jenkinson DS (1993) Formation and destruction of microbial biomass during the decomposition of glucose and ryegrass in soil. Soil Biol Biochem 25:1435–1441
Ye JS, Bradford MA, Dacal M, Maestre FT, García-Palacios P (2019) Increasing microbial carbon use efficiency with warming predicts soil heterotrophic respiration globally. Glob Change Biol 25:3354–3364
Zak J, Willig M, Moorhead D, Wildman H (1994) Functional diversity of microbial communities. A quantitative approach. Soil Biol Biochem 26:1101–1108
Zareitalabad P, Heinze S, Rottmann N, Potthoff M, Dyckmans J, Joergensen RG (2010) Decomposition of 15N labelled maize leaf residues in soil affected by the feeding activity of endogeic geophagous Aporrectodea caliginosa. Soil Biol Biochem 42:276–282
Zheng Q, Hu Y, Zhang S, Noll L, Bockle T, Richter A, Wanek W (2019) Growth explains microbial carbon use efficiency across soils differing in land use and geology. Soil Biol Biochem 128:45–55
Zibilske LM (1994) Carbon mineralization. In: Weaver RW, Angle S, Bottomley P, Wollum DBSSATA (eds) Methods of soil analysis, part 2 – microbiological and biochemical processes, SSSA Book. Soil Science Society of America, Madison, pp 835–863
Acknowledgements
The Indian soil was sampled within the Research Unit 2432 and financially supported by the German Research Foundation (DFG) and the Indian Department of Biotechnology (DBT). We would like to thank Prof. Dr. Andreas Buerkert for organizing this Research Unit. We would also like to thank Dr. Ellen Hoffmann, Dr. Rüdiger Graß, and Dr. Thomas Fricke for providing soil and climate data. The technical assistance of Gabriele Dormann, Martin Lüttge, Larissa Krause, and Anna Kenn is highly appreciated.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schroeder, J., Jannoura, R., Beuschel, R. et al. Carbon use efficiency and microbial functional diversity in a temperate Luvisol and a tropical Nitisol after millet litter and N addition. Biol Fertil Soils 56, 1139–1150 (2020). https://doi.org/10.1007/s00374-020-01487-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-020-01487-4