Abstract
Honey bees (Apis mellifera) are one of the most crucial pollinators, providing vital ecosystem services. Their development and functioning depend on essential nutrients and substances found in the environment. While collecting nectar as a vital carbohydrate source, bees routinely encounter low doses of ethanol from yeast fermentation. Yet, the effects of repeated ethanol exposure on bees’ survival and physiology remain poorly understood. Here, we investigate the impacts of constant and occasional consumption of food spiked with 1% ethanol on honey bee mortality and alcohol dehydrogenase (ADH) activity. This ethanol concentration might be tentatively judged close to that in natural conditions. We conducted an experiment in which bees were exposed to three types of long-term diets: constant sugar solution (control group that simulated conditions of no access to ethanol), sugar solution spiked with ethanol every third day (that simulated occasional, infrequent exposure to ethanol) and daily ethanol consumption (simulating constant, routine exposure to ethanol). The results revealed that both constant and occasional ethanol consumption increased the mortality of bees, but only after several days. These mortality rates rose with the frequency of ethanol intake. The ADH activity remained similar in bees from all groups. Our findings indicate that exposure of bees to ethanol carries harmful effects that accumulate over time. Further research is needed to pinpoint the exact ethanol doses ingested with food and exposure frequency in bees in natural conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect pollinators play a crucial role in the functioning of both natural and agricultural ecosystems (Klein et al. 2007; Gallai et al. 2009). During pollination services, they collect pollen, essential for growth, and nectar, a primary energy source. The quality and composition of their diet are critical for development, health, and survival, making the abundance and diversity of resources in the surrounding environment crucial. While collecting pollen and nectar, pollinators are exposed to substances that directly impact them. For instance, insecticides widespread in agricultural environments significantly increase mortality and adversely affect the development and foraging abilities of pollinators (e.g. in solitary bees, Boff et al. 2021; Mokkapati et al. 2021). Conversely, certain phytochemicals found in pollen and nectar, such as caffeine, can significantly reduce parasitisation, acting both preventatively and therapeutically on pollinators’ health (e.g. in bumble and honey bees, Folly et al. 2021; Motta et al. 2023). Pollinators can be passively affected by environmental substances due to ingestion of contaminated resources, but they can also actively seek dietary components. For example, honey bees actively select food types advantageous to them in specific situations, such as opting for nectar with higher antibiotic potential during infection (Gherman et al. 2014) or gathering propolis to combat rising parasite levels (Pusceddu et al. 2021).
Honey bees (Apis mellifera L.), which are one of the key insect pollinators, likely encounter and consume ethanol in their natural environment. The main carbohydrate source for honey bees, floral nectar, is frequently colonised by yeasts capable of producing ethanol through fermentation (Lievens et al. 2015). Observations from tropical regions indicate that the concentration of ethanol in palm flower nectar can reach 6.9% (Hockings et al. 2015; Gochman et al. 2016). A concentration of about 1% ethanol, especially in more temperate climates, seems more realistic as a component of nectar in natural conditions (Ehlers and Olesen 1997; Jakubska et al. 2005; Goodrich et al. 2006; Wiens et al. 2008; Golonka et al. 2014; Rering et al. 2018). In any case, ethanol consumption must be significant enough to allow honey bees to biosynthesise ethyl oleate—a crucial pheromone that regulates colony demography by inhibiting the transition of workers into foragers (Leoncini et al. 2004; Castillo et al. 2012a, 2012b). Only foragers, the type of individuals that leave the hive and explore the surroundings for food, possess dehydrogenase enzymes likely responsible for breaking down ethanol (Miler et al. 2021). Furthermore, despite the aversive taste of ethanol in water for honey bees, when dissolved in sugar, it is willingly consumed both in the laboratory and field, even in extreme concentrations as high as 20% (Abramson et al. 2000, 2004a; Maze et al. 2006; Sokolowski et al. 2012, Mustard et al. 2019). Moreover, foragers prefer feeding solutions containing about 1% or even 2.5% ethanol over pure sugar solutions (Mustard et al. 2019; Varnon et al. 2018). All this suggests not only that ethanol frequently contaminates carbohydrate resources utilised by honey bees but also that they need this dietary component and are adapted for its encounter.
Nevertheless, ethanol has been observed to induce multiple negative effects on honey bees, both in social and non-social contexts. It disrupts social communication among individuals by directly influencing behaviours such as antennation or trophallaxis (Mixson et al. 2010; Wright et al. 2012) and through increasing aggression (Abramson et al. 2004b; Ammons and Hunt 2008). Moreover, ethanol consumption in foragers modifies proper dance communication (Bozic et al. 2006). Ethanol also impairs honey bees’ locomotion, foraging, and learning (Abramson et al. 2005; Giannoni-Guzmán et al. 2014; Maze et al. 2006; Mustard et al. 2008; Black et al. 2021; Ahmed et al. 2022). The extent of these behavioural changes occurs in a dose-dependent manner, with higher doses of ethanol producing larger effects on behaviour (Maze et al. 2006; Bozic et al. 2007; Wright et al. 2012). However, the majority of the studies that document effects resulting from ethanol consumption focus on single-exposure impact, often with concentrations that are relatively high and unlikely of ecological relevance. Not only the effects of low ethanol levels are understudied but so are those related to repeated ethanol exposure. Thus far, it has been demonstrated that bees show tolerance, expressed as lower motor impairment in response to ethanol in individuals previously exposed to it than those exposed for the first time (Miler et al. 2018 but see Stephenson et al. 2021). Moreover, individuals fed on food spiked with ethanol for a prolonged time exhibit withdrawal symptoms upon discontinuation of access to such food (Ostap-Chec et al. 2021). These characteristics can be considered primary hallmarks of alcohol dependence development in conditions of repeated encounters with ethanol. Still very little is known about the survivorship and physiological effects associated with repeated exposure to relatively low concentrations of ethanol in the diet.
Most research focuses on the behavioural and social effects of ethanol consumption in bees. However, these reactions are typically closely linked to physiological or biochemical responses. For instance, studies on Drosophila have shown that sensitivity and tolerance to ethanol are directly related to the activity of enzymes, such as alcohol dehydrogenase (ADH) (Ogueta et al. 2010). Research on vertebrates (Buris et al. 1985; Kishimoto et al. 1995; Tran et al. 2015) indicates that this enzyme tends to increase in activity with continuous, prolonged exposure to ethanol. In honey bee foragers, the presence of the most common type of ADH—type 1—has been identified (Bouga et al. 2005; Miler et al. 2021, 2022). However, it is unclear whether the consumption of ethanol by foragers leads to increases in the activity of this enzyme.
Here, we investigate the effects of occasional and constant low-level ethanol consumption on the survival and ADH activity of forager-age honey bees. We hypothesise that there would be no significant increase in mortality compared to abstinent individuals. Moreover, we hypothesise that the ADH activity would be highest in individuals exposed to ethanol most frequently, lower in those with occasional exposure, and lowest in workers with no access to ethanol. Our expectations are based on current knowledge regarding the likely routine presence of ethanol in food resources utilised by bees, their preferences for this dietary component, and adaptations towards its encounter.
Methods
General procedure
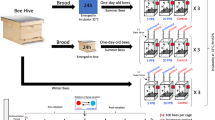
We performed the experiment in 4 bouts, with 2 days of delay between each bout. For each bout, we obtained newly emerged individuals that originated from 2 different colonies. For that purpose, we took a single bee-free frame with a capped brood from each colony participating in a bout and placed it in an incubator (KB53, Binder, Germany) at 32 °C overnight. The next morning we marked emerged workers with a coloured dot on the thorax using a non-toxic paint marker and then released them into an unrelated hive. Leaving bees in the hive for a period after emergence allows for their proper development and immune system strengthening, resulting in high subsequent survival rates in laboratory cages. This enables conducting extended experiments, as demonstrated in a previous study by Ostap-Chec et al. (2021). When bees were 7 days old, we recollected marked workers by opening the hive and picking them frame-by-frame using forceps, placing them in wooden cages and transporting them to the laboratory. The workers were kept 100 individuals per cage, 9 cages per bout, and with access to ad libitum water and food (2 M sucrose solution) in an incubator (KB400, Binder, Germany) at 32 °C and 60–70% RH. We applied a period of acclimation that lasted for 2 weeks, to be sure that all workers reached forager age (Dukas 2008) and remained naïve in terms of ethanol presence in their previous diet. We noted exactly how many individuals were in each cage as there was some mortality during the acclimation period (the number of workers ranged from 94–100 by the point of reaching 21 days of age). Then, we created 3 replicates in each bout, with each replicate comprising 3 groups, and started the diet. For the control group (CONTROL), we provided the workers with a 2 M sucrose water solution. For the group with occasional exposure (OCCASIONAL), we provided the workers with the same solution, but every 3rd day, we switched it to a sucrose water solution with the addition of 1% ethanol. For the group with constant exposure (CONSTANT), we provided the workers with a sucrose water solution with the addition of 1% ethanol (Fig. 1). Hence, we had 12 cages per group (4 bouts and 3 group replicates in each) totalling about 1200 individuals per group. The food with the addition of ethanol was prepared iso-caloric relative to the 2 M sucrose solution to avoid the confounding effect of ethanol caloric value when digested. Hence, assuming 5.5 kcal per 1 mL of ethanol, we added 1.38 g less sucrose to the food with ethanol to balance out the calories. Throughout the entire study, we refrained from providing protein sources to the workers and switched water and food to fresh every morning. During water and food renewal each day, we noted the number of dead individuals in each cage and removed the dead bodies. We exposed workers in each group to their respective diets for 21 days, and then the experiment was terminated. On the last day of the experiment, we froze 6 individuals from a randomly selected group replicate in each bout to analyse the alcohol dehydrogenase (ADH) activity in their bodies. Hence, we analysed 24 individuals per group in total. For that purpose, each body was thoroughly homogenised in 400 μl of PBS in a Bead Ruptor Elite homogenizer with ceramic beads (Omni International, USA) and spun in a centrifuge at 13,000 RPM for 7 min. Then, 10 µl of each supernatant was analysed using the ADH assay kit (ab102533, abcam, the Netherlands) as per the manufacturer's instructions. The kit enables the detection of type I ADH, which is present in bees (Miler et al. 2021). The optical density of the reaction product was read at 450 nm using the Multiskan FC microplate reader (Thermo Scientific, USA) after the initial incubation period (3 min) and in three measurement points between 7 and 35 min after the first measurement. The timeframe was determined to be within a range of linear reaction phase. The ADH activity (defined as nM NADH produced per min in 1 mL = mU/mL) was calculated based on the slope of the absorbance curve and translated into concentration values based on the prepared calibration curve slope. One sample was excluded from the analyses as the calculated activity had a slightly negative value (− 0.087, 95% CI: − 0.101–0.099), which was not biologically relevant.
Statistics
To analyse the data, we used R (R Core Team 2024). For the analysis of survivorship, we used the Cox mixed-effect regression (‘coxme’ and ‘survival’ packages, Therneau 2022, 2023) with a fixed factor of the group and random factors of the bout and replicate (coxme function). Individuals that remained alive when the experiment was terminated (day 21 of the diet) were censored. To analyse the ADH activity, we used the generalised linear mixed-effects model (GLMM, ‘lme4’ package, Bates et al. 2015) with a Gamma distribution and a fixed factor of the group and a random factor of the bout (lme function).
Results
Throughout the diet (21 days), the survival in the control groups (CONTROL) dropped to 79% (95% CI: 81–77%). Compared to that, the groups with occasional ethanol exposure (OCCASIONAL; Cox regression, z test = 7.24, p < 0.001) and with constant ethanol exposure (CONSTANT; Cox regression, z test = 18.02, p < 0.001) both demonstrated decreased survival. In the case of occasional exposure, the survival dropped to 65% (95% CI: 68–62%) whereas for constant exposure—to 41% (95% CI: 44–38%). Groups exposed to ethanol started to diverge from the control groups after 10 and 6 days of the diet for the OCCASIONAL and CONSTANT groups, respectively (Fig. 2). The ADH activity did not differ between groups (GLMM: χ2 = 1.029, p = 0.598, Fig. 3) and averaged 5.41 (SD: 3.16) mU/mL across groups.
Survival plot for honey bees exposed to different types of diets for 21 days (CONTROL = the control groups exposed to pure 2 M sucrose solution, OCCASIONAL = the groups with occasional exposure to ethanol (addition of 1% ethanol in food every third day), CONSTANT = the groups with constant exposure to ethanol (food with the addition of 1% ethanol)). There were 12 cages for each group, each with an initial number of individuals ranging from 94 to 100. Shadings show 95% CI
Violin plot for the ADH activity (mU/mL) in honey bees exposed to different types of diets for 21 days (CONTROL = the control groups exposed to pure 2 M sucrose solution (n = 24), OCCASIONAL = the groups with occasional exposure to ethanol (addition of 1% ethanol in food every third day) (n = 24), CONSTANT = the groups with constant exposure to ethanol (food with the addition of 1% ethanol) (n = 23)). Boxes indicate the median and interquartile range. Whiskers reach the smallest and largest values within 1.5 of the interquartile range. Coloured dots show individual data points
Discussion
Honey bees surely encounter and consume ethanol in their natural environment. They rely on ethanol for synthesising one of their crucial pheromones (Leoncini et al. 2004; Castillo et al. 2012a, 2012b), possess enzymes to break down ethanol (Miler et al. 2021), and willingly consume it when mixed with sugars (Abramson et al. 2000, 2004a; Maze et al. 2006; Sokolowski et al. 2012, Mustard et al. 2019). While previous research has focused on the effects of single and often high-concentration ethanol exposure on honey bees, this study investigates the consequences of repeated exposure to low levels of ethanol. Our findings reveal a significant decrease in lifespan due to ethanol exposure. Mortality rates in our study show that a significant drop in survival starts to appear after about 6 and 10 days of constant and occasional ethanol consumption, respectively. It is worth noting, however, that in natural conditions the average lifespan of forager-age bees is about a week (Dukas 2008). Therefore, while our results highlight the potentially harmful effects of even low ethanol concentrations and suggest cumulative impacts with each subsequent exposure, they need to be put in an ecological context. It seems likely that bees die for other reasons before the negative impact of routine, low-level ethanol exposure in their food appears. For comparison, in the study of Mustard et al. (2019), no discernible differences in mortality were observed in bees exposed daily to 1.25% ethanol and those abstinent. In their study, however, the authors used foragers of unknown age captured at hive entrances and a vast majority of all these bees died within a week. It is conceivable that in that study, the effects of diet had no chance to manifest (Mustard et al. 2019). Rasmussen et al. (2021) utilised overwintering bees with a longer-lived phenotype and demonstrated that 1% ethanol in their diet significantly decreased survival, but with a drop in mortality occurring only after a few days of ethanol exposure. In our study, even if we consider mortality over the entire 3 weeks of measurement, well over a third of all individuals survived daily ethanol consumption (Fig. 2). Together, these results highlight the extraordinary resilience to the potentially toxic effect of ethanol in honey bees. Previous research has demonstrated that ethanol induces an increase in heat shock protein concentration in honey bee brain tissue (Hranitz et al. 2010). Rasmussen et al. (2021) also showed that ethanol intake disrupts their DNA methylation patterns. These effects likely constitute the basis of the eventual harmfulness of ethanol consumption and its accumulative toxicity. Yet, it takes time for such effects to reflect on the survivorship of bees.
Bees likely ingest ethanol with their food as they collect floral nectar. Another potential source of ethanol is presented in spoiled or overripe fruits (e.g. Dudley 2002; Dominy 2004; Sánchez et al. 2004) because bees often collect juices from such fruits, especially during periods without major flowering events. Our understanding of the sources of honey bee exposure to ethanol, as well as the frequency and concentration at which honey bees come into contact with this compound, remains a gap that requires extensive research. The same is true for other pollinating nectarivores as well as frugivores, all of which probably meet with ethanol routinely (Dudley and Maro 2021).
Our results revealed no differences in the ADH activity between the groups of individuals (Fig. 3), suggesting that foragers consistently produce the enzyme, regardless of their ethanol exposure. This result is surprising, as it contradicts the expectation that prolonged alcohol consumption would induce ADH expression, as observed in vertebrates (Buris et al. 1985; Kishimoto et al. 1995; Tran et al. 2015). Notably, however, there are different types of ADH (Hernández-Tobías et al. 2011). In the case of fruit flies, in which ADH activity is closely related to alcohol tolerance and preferences (Ogueta et al. 2010), ADH type II was the focus of the study. Short-chain type II ADH is more typical for insects and might show higher activity with ethanol in bees, similar to Drosophila (Hernández-Tobías et al. 2011). In the case of honey bees, types I and II are present (Miler et al. 2021, 2022), but only the former is analysed here. Hence, our results regarding ADH activity cannot be treated as entirely conclusive. Similar results to ours were obtained in fruit-feeding butterflies, in which a diet containing ethanol did not alter ADH levels (Beaulieu et al. 2017). Miler et al. (2021) demonstrated ADH presence in honey bee foragers but not in intranidal workers that stay inside the hive and care for brood. Additionally, another study showed that even repeated ethanol exposure failed to induce ADH production in intranidal workers (Miler et al. 2022). Our current findings complement this prior research (Beaulieu et al. 2017; Miler et al. 2021, 2022) indicating that ADH type I activity remains constant, regardless of ethanol intake. The analysed type I ADH might not be restricted in its function to ethanol metabolism and this may confound the results. Additionally, considering our small sample size, we might have failed to detect an existing effect. We recommend future research to focus on this issue, including the activity of Drosophila-type ADH in bees.
Our findings stress the necessity for further research to find ecologically relevant levels of ethanol and the frequency of exposure to this substance in bees as well as their responses to this dietary component. The way for the comparative biology of ethanol exposure and response is open (Dudley and Maro 2021) and honey bees show promise in this context. Considering that ethanol is one of many neuroactive substances present in floral nectar that potentially mediate plant-pollinator interactions (Mustard 2020), this research field needs much more attention. It's important to acknowledge that much of the existing research, including our own, has been conducted under laboratory conditions. Although such studies have limitations, laboratory cage trials in the study of the honey bee remain a commonly utilised research method, offering high comparability between groups and minimising the influence of external factors. However, this controlled environment may not fully capture the complexities of real-world scenarios. Therefore, to gain a more comprehensive understanding of the impact of alcohol on bees, it's essential to complement laboratory studies with field research.
Data Availability
The datasets generated and analysed during the current study are available in the Zenodo repository (https://doi.org/10.5281/zenodo.11530862, Ostap-Chec et al. 2024).
References
Abramson CI, Stone SM, Ortez RA, Luccardi A, Vann KL, Hanig KD, Rice J (2000) The development of an ethanol model using social insects I: behavior studies of the honey bee (Apis mellifera L). Alcohol Clin Exp Res 24:1153–1166. https://doi.org/10.1111/j.1530-0277.2000.tb02078.x
Abramson CI, Sheridan A, Donohue D, Kandolf A, Božič J, Meyers JE, Benbassat D (2004a) Development of an ethanol model using social insects: III Preferences for Ethanol Solutions. Psychol Rep 94:227–239. https://doi.org/10.2466/pr0.94.1.227-239
Abramson CI, Place AJ, Aquino IS, Fernandez A (2004b) Development of an ethanol model using social insects: IV. Influence of ethanol on the aggression of Africanized honey bees (Apis mellifera L). Psychol Rep 94:1107–1115. https://doi.org/10.2466/pr0.94.3c.1107-1115
Abramson CI, Sanderson C, Painter J, Barnett S, Wells H (2005) Development of an ethanol model using social insects: V. Honeybee foraging decisions under the influence of alcohol. Alcohol 36:187–193. https://doi.org/10.1016/j.alcohol.2005.09.001
Ahmed I, Abramson CI, Faruque IA (2022) Honey bee flights near hover under ethanol-exposure show changes in body and wing kinematics. PLoS ONE 17:e0278916. https://doi.org/10.1371/journal.pone.0278916
Ammons AD, Hunt GJ (2008) Characterization of honey bee sensitivity to ethanol vapor and its correlation with aggression. Alcohol 42:129–136. https://doi.org/10.1016/j.alcohol.2007.12.005
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beaulieu M, Franke K, Fischer K (2017) Feeding on ripening and over-ripening fruit: interactions between sugar, ethanol and polyphenol contents in a tropical butterfly. J Exp Biol 220:3127–3134. https://doi.org/10.1242/jeb.162008
Black TE, Stauch KLN, Wells H, Abramson CI (2021) Effects of ethanol ingestion on aversive conditioning in honey bees (Apis mellifera L). J Comp Psychol 135:559–567. https://doi.org/10.1037/com0000296
Boff S, Scheiner R, Raizer J, Lupi D (2021) Survival rate and changes in foraging performances of solitary bees exposed to a novel insecticide. Ecotoxicol Environ Saf 211:111869. https://doi.org/10.1016/j.ecoenv.2020.111869
Bouga M, Kilias G, Harizanis PC, Papasotiropoulos V, Alahiotis S (2005) Allozyme variability and phylogenetic relationships in honey bee (Hymenoptera: Apidae: Apis mellifera) populations from Greece and Cyprus. Biochem Genet 43:471–483. https://doi.org/10.1007/s10528-005-8163-2
Bozic J, Abramson CI, Bedencic M (2006) Reduced ability of ethanol drinkers for social communication in honeybees (Apis mellifera carnica Poll). Alcohol 38:179–183. https://doi.org/10.1016/j.alcohol.2006.01.005
Bozic J, DiCesare J, Wells H, Abramson CI (2007) Ethanol levels in honeybee hemolymph resulting from alcohol ingestion. Alcohol 41:281–284. https://doi.org/10.1016/j.alcohol.2007.04.003
Buris L, Csabai G, Fodor M, Varga M (1985) Increase of alcohol dehydrogenase and protein content of liver following chronic ethanol administration. FEBS Lett 183:143–144. https://doi.org/10.1016/0014-5793(85)80972-8
Castillo C, Chen H, Graves C, Maisonnasse A, Le Conte Y, Plettner E (2012a) Biosynthesis of ethyl oleate, a primer pheromone, in the honey bee (Apis mellifera L). Insect Biochem Mol Biol 42:404–416. https://doi.org/10.1016/j.ibmb.2012.02.002
Castillo C, Maisonnasse A, Le Conte Y, Plettner E (2012b) Seasonal variation in the titers and biosynthesis of the primer pheromone ethyl oleate in honey bees. J Insect Physiol 58:1112–1121. https://doi.org/10.1016/j.jinsphys.2012.05.010
Dominy NJ (2004) Fruits, fingers, and fermentation: the sensory cues available to foraging primates. Integr Comp Biol 44:295–303. https://doi.org/10.1093/icb/44.4.295
Dudley R (2002) Fermenting fruit and the historical ecology of ethanol ingestion: is alcoholism in modern humans an evolutionary hangover? Addiction 97:381–388. https://doi.org/10.1046/j.1360-0443.2002.00002.x
Dudley R, Maro A (2021) Human evolution and dietary ethanol. Nutrients 13:2419. https://doi.org/10.3390/nu13072419
Dukas R (2008) Mortality rates of honey bees in the wild. Insect Soc 55:252–255. https://doi.org/10.1007/s00040-008-0995-4
Ehlers BK, Olesen JM (1997) The fruit-wasp route to toxic nectar in Epipactis orchids? Flora 192:223–229. https://doi.org/10.1016/S0367-2530(17)30787-9
Folly AJ, Koch H, Farrell IW, Stevenson PC, Brown MJ (2021) Agri-environment scheme nectar chemistry can suppress the social epidemiology of parasites in an important pollinator. Proc R Soc B 288:20210363. https://doi.org/10.1098/rspb.2021.0363
Gallai N, Salles JM, Settele J, Vaissière BE (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68:810–821. https://doi.org/10.1016/j.ecolecon.2008.06.014
Gherman BI, Denner A, Bobiş O, Dezmirean DS, Mărghitaş LA, Schlüns H, Moritz RFA, Erler S (2014) Pathogen-associated self-medication behavior in the honeybee Apis mellifera. Behav Ecol Sociobiol 68:1777–1784. https://doi.org/10.1007/s00265-014-1786-8
Giannoni-Guzmán MA, Giray T, Agosto-Rivera JL, Stevison BK, Freeman B, Ricci P, Brown EA, Abramson CI (2014) Ethanol-induced effects on sting extension response and punishment learning in the western honey bee (Apis mellifera). PLoS ONE 9:e100894. https://doi.org/10.1371/journal.pone.0100894
Gochman SR, Brown MB, Dominy NJ (2016) Alcohol discrimination and preferences in two species of nectar-feeding primate. R Soc Open Sci 3:160217. https://doi.org/10.1098/rsos.160217
Golonka AM, Johnson BO, Freeman J, Hinson DW (2014) Impact of nectarivorous yeasts on Silene caroliniana’s scent. East Biol 3:1–26
Goodrich KR, Zjhra ML, Ley CA, Raguso RA (2006) When flowers smell fermented: the chemistry and ontogeny of yeasty floral scent in pawpaw (Asimina triloba: Annonaceae). Int J Plant Sci 167:33–46. https://doi.org/10.1086/498351
Hernández-Tobías A, Julián-Sánchez A, Piña E, Riveros-Rosas H (2011) Natural alcohol exposure: is ethanol the main substrate for alcohol dehydrogenases in animals? Chem Biol Interact 191:14–25. https://doi.org/10.1016/j.cbi.2011.02.008
Hockings KJ, Bryson-Morrison N, Carvalho S, Fujisawa M, Humle T, McGrew WC, Nakamura M, Ohashi G, Yamanashi Y, Yamakoshi G, Matsuzawa T (2015) Tools to tipple: ethanol ingestion by wild chimpanzees using leaf-sponges. R Soc Open Sci 2:150150. https://doi.org/10.1098/rsos.150150
Hranitz JM, Abramson CI, Carter RP (2010) Ethanol increases HSP70 concentrations in honeybee (Apis mellifera L.) brain tissue. Alcohol 44:275–282. https://doi.org/10.1016/j.alcohol.2010.02.003
Jakubska A, Anioł-Kwiatkowska J, Kadej M, Prządo D (2005) Why do pollinators become „sluggish”? Nectar chemical constituents from Epipactis helleborine (L.) Crantz (Orchidaceae). App Ecol Env Res 3:29–38. https://doi.org/10.15666/aeer/0302_029038
Kishimoto R, Fujiwara I, Kitayama S, Goda K, Nakata Y (1995) Changes in hepatic enzyme activities related to ethanol metabolism in mice following chronic ethanol administration. J Nutr Sci Vitaminol 41:527–543. https://doi.org/10.3177/jnsv.41.527
Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B 274:303–313. https://doi.org/10.1098/rspb.2006.3721
Leoncini I, Le Conte Y, Costagliola G, Plettner E, Toth AL, Wang M, Huang Z, Bécard JM, Crauser D, Slessor KN, Robinson GE (2004) Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proc Natl Acad Sci 101:17559–17564. https://doi.org/10.1073/pnas.0407652101
Lievens B, Hallsworth JE, Pozo MI, Belgacem ZB, Stevenson A, Willems KA, Jacquemyn H (2015) Microbiology of sugar-rich environments: diversity, ecology and system constraints. Environ Microbiol 17:278–298. https://doi.org/10.1111/1462-2920.12570
Maze IS, Wright GA, Mustard JA (2006) Acute ethanol ingestion produces dose-dependent effects on motor behavior in the honey bee (Apis mellifera). J Insect Physiol 52:1243–1253. https://doi.org/10.1016/j.jinsphys.2006.09.006
Miler K, Kuszewska K, Privalova V, Woyciechowski M (2018) Honeybees show adaptive reactions to ethanol exposure. Sci Rep 8:8707. https://doi.org/10.1038/s41598-018-27117-6
Miler K, Stec D, Kamińska A, Pardyak L, Kuszewska K (2021) Alcohol intoxication resistance and alcohol dehydrogenase levels differ between the honeybee castes. Apidologie 52:230–241. https://doi.org/10.1007/s13592-020-00812-y
Miler K, Stec D, Pardyak L, Kamińska A, Kuszewska K (2022) No increase in alcohol dehydrogenase levels following repeated ethanol exposure in young honeybee workers. Physiol Entomol 47:110–116. https://doi.org/10.1111/phen.12380
Mixson TA, Abramson CI, Božič J (2010) The behavior and social communication of honey bees (Apis mellifera carnica Poll.) under the influence of alcohol. Psychol Rep 106:701–717. https://doi.org/10.2466/pr0.106.3.701-717
Mokkapati JS, Bednarska AJ, Laskowski R (2021) The development of the solitary bee Osmia bicornis is affected by some insecticide agrochemicals at environmentally relevant concentrations. Sci Total Environ 775:145588. https://doi.org/10.1016/j.scitotenv.2021.145588
Motta EV, Arnott RL, Moran NA (2023) Caffeine consumption helps honey bees fight a bacterial pathogen. Microbiol Spectrum 11:e00520-e523. https://doi.org/10.1128/spectrum.00520-23
Mustard JA (2020) Neuroactive nectar: compounds in nectar that interact with neurons. Arthropod-Plant Interact 14:151–159. https://doi.org/10.1007/s11829-020-09743-y
Mustard JA, Edgar EA, Mazade RE, Wu C, Lillvis JL, Wright GA (2008) Acute ethanol ingestion impairs appetitive olfactory learning and odor discrimination in the honey bee. Neurobiol Learn Mem 90:633–643. https://doi.org/10.1016/j.nlm.2008.07.017
Mustard JA, Oquita R, Garza P, Stoker A (2019) Honey bees (Apis mellifera) show a preference for the consumption of ethanol. Alcohol Clin Exp Res 43:26–35. https://doi.org/10.1111/acer.13908
Ogueta M, Cibik O, Eltrop R, Schneider A, Scholz H (2010) The influence of Adh function on ethanol preference and tolerance in adult Drosophila melanogaster. Chem Senses 35:813–822. https://doi.org/10.1093/chemse/bjq084
Ostap-Chec M, Opalek M, Stec D, Miler K (2021) Discontinued alcohol consumption elicits withdrawal symptoms in honeybees. Biol Lett 17:20210182. https://doi.org/10.1098/rsbl.2021.0182
Ostap-Chec M, Bajorek D, Antoł W, Stec D, Miler K (2024) Occasional and constant exposure to dietary ethanol shortens the lifespan of worker honey bees [Data set]. Zenodo. https://doi.org/10.5281/zenodo.11530863
Pusceddu M, Annoscia D, Floris I, Frizzera D, Zanni V, Angioni A, Satta A, Nazii F (2021) Honeybees use propolis as a natural pesticide against their major ectoparasite. Proc R Soc B 288:20212101. https://doi.org/10.1098/rspb.2021.2101
R Core Team (2024) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 16 Feb 2024
Rasmussen EM, Seier KL, Pedersen IK, Kreibich C, Amdam GV, Münch D, Dahl JA (2021) Screening bioactive food compounds in honey bees suggests curcumin blocks alcohol-induced damage to longevity and DNA methylation. Sci Rep 11:19156. https://doi.org/10.1038/s41598-021-98614-4
Rering CC, Beck JJ, Hall GW, McCartney MM, Vannette RL (2018) Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol 220:750–759. https://doi.org/10.1111/nph.14809
Sánchez F, Korine C, Pinshow B, Dudley R (2004) The possible roles of ethanol in the relationship between plants and frugivores: first experiments with Egyptian fruit bats. Integr Comp Biol 44:290–294. https://doi.org/10.1093/icb/44.4.290
Sokolowski MB, Abramson CI, Craig DPA (2012) Ethanol self-administration in free-flying honeybees (Apis mellifera L.) in an operant conditioning protocol. Alcohol Clin Exp Res 36:1568–1577. https://doi.org/10.1111/j.1530-0277.2012.01770.x
Stephenson L, Chicas-Mosier A, Black T, Wells H, Abramson C (2021) Inducing ethanol tolerance in free-flying honey bees (Apis mellifera L.). Int J Comp Psychol 34:1–13. https://doi.org/10.46867/ijcp.2021.34.00.03
Therneau T (2022) coxme: mixed effects Cox models. R package version 2.2–18.1. https://CRAN.R-project.org/package=coxme. Accessed 16 Feb 2024
Therneau T (2023) survival: Survival Analysis. R package version 3.5–7, https://CRAN.R-project.org/package=survival. Accessed 16 Feb 2024
Tran S, Nowicki M, Chatterjee D, Gerlai R (2015) Acute and chronic ethanol exposure differentially alters alcohol dehydrogenase and aldehyde dehydrogenase activity in the zebrafish liver. Prog Neuro-Psychopharmacol Biol Psychiatry 56:221–226. https://doi.org/10.1016/j.pnpbp.2014.09.011
Varnon CA, Dinges CW, Black TE, Wells H, Abramson CI (2018) Failure to find ethanol-induced conditioned taste aversion in honey bees (Apis mellifera L.). Alcohol Clin Exp Res 42(7):1260–1270. https://doi.org/10.1111/acer.13761
Wiens F, Zitzmann A, Lachance M-A, Yegles M, Pragst F, Wurst FM, von Holst D, Leng Guan S, Spanagel R (2008) Chronic intake of fermented floral nectar by wild treeshrews. Proc Natl Acad Sci 105:10426–10431. https://doi.org/10.1073/pnas.0801628105
Wright GA, Lillvis JL, Bray HJ, Mustard JA (2012) Physiological state influences the social interactions of two honeybee nest mates. PLoS ONE 7:e32677. https://doi.org/10.1371/journal.pone.0032677
Funding
This work was supported by the National Science Centre, Poland [grant number Sonata UMO-2021/43/D/NZ8/01044].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflicts of interest or competing interests are declared.
Additional information
Communicated by Gerhard Heldmaier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ostap-Chec, M., Bajorek, D., Antoł, W. et al. Occasional and constant exposure to dietary ethanol shortens the lifespan of worker honey bees. J Comp Physiol B 194, 403–410 (2024). https://doi.org/10.1007/s00360-024-01571-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-024-01571-3