Abstract
Identifying traits for adaptation to different management and environmental regimes is key to maintaining robust honey bee populations under global climate change. We compared mite-resistant (Pol-line and Russian) and Italian honey bee stocks in variable-temperature cage experiments (200 bees per cage) with respect to food consumption, thermoregulation, gene expression, and lifespan, in 3 experiments over 2 years. The Italian stock bees consumed more syrup and pollen on average than the mite-resistant stocks, but the mite-resistant stocks maintained higher cluster temperatures and had median lifespans 8 days longer, consistent with the increased expression of vitellogenin relative to Italian stock. Model results indicated that, to maintain the same colony size as the mite-resistant stocks, Italian stock colonies would need about 13% more sealed brood to offset reduced worker lifespans. These differences among bee stocks likely influence colony-level productivity and health, and showed the importance of experimental replication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Honey bee colonies adapt quickly to new environments due to their organization and behavioral plasticity. Colonies modulate their energy and nutrient investments in brood production, foraging activity, thermoregulation, and other colony-level behaviors in response to changes in their environment, including forage availability, ambient temperatures, daylength, exposure to other bee colonies, and other factors (Southwick and Mugaas 1971). Modern beekeeping operations often relocate hives to new environments that have variable forage and different levels of pathogen exposure (see for example Meikle et al. 2020) and which may induce stress in the colonies. Managed honey bee populations in the USA are experiencing high levels of colony loss, often exceeding 40% (Bee Informed Partnership 2023), suggesting that improved management strategies are needed to sustain beekeeping operations in a rapidly changing world.
Queen quality is paramount for beekeeping operations as it directly impacts colony genetic traits, productivity, and overall health. The queen’s genetic composition ultimately determines her physiological limitations and the heritable characteristics that are passed on to workers, including resistance to Varroa mites (Varroa destructor Anderson and Trueman) (Mondet et al. 2020). A variety of honey bee stocks (isolated populations propagated for specific traits) are produced by queen breeders and shipped throughout the USA. Some of these stocks, such as Pol-line and Russian bees, have been selected for specific traits (e.g., mite resistance) or are the result of research-based breeding programs (Saelao et al. 2020). However, intensive breeding programs can unintentionally reduce genetic diversity and increase vulnerability to disease or environmental changes (Panziera et al. 2022). A better understanding of how genetic variation contributes to honey bee performance at the colony and individual levels could help identify traits that are beneficial in diverse management contexts and a changing global climate.
In this study, hoarding cages of newly emerged adult honey bees from three different stocks, Pol-line (obtained from USDA-ARS, Baton Rouge, LA, USA), Russian, or a commercial Italian stock (hereafter “Italian”), were placed in a variable-temperature incubator, their food consumption and cluster temperature were monitored, and individuals were sampled for gene expression. This experimental design has been used to detect the effects of sublethal pesticide exposure on bee behavior (Meikle et al. 2018). Food consumption and survivorship are key traits that may impact resource utilization and colony growth patterns in the field, and by using groups of bees of the same age, we can easily measure how these variables change over the course of the life of an adult bee. Similarly, thermoregulation is an important behavior and a useful response variable in field studies (see Meikle et al. 2017, 2022, 2023). Since biomarker-guided assessments of bee physiology have been informative at both the colony and individual levels, we monitored a panel of genes associated with longevity and overwinter survival, specifically vitellogenin, heat shock proteins (HSPs) 70 and 90, and deformed wing virus B (DWV-B). Vitellogenin is an important nutritional storage protein associated with longevity (Amdam et al. 2005); HSPs are linked to cellular protein homeostasis and reducing damage from reactive oxygen species (McMenamin et al. 2020; Zhao et al. 2021); and DWVs are important bee pathogens (de Miranda and Genersch 2010). Overall, we aimed to identify differences among genetic stocks of bees that could inform future breeding efforts for vigorous honey bee colonies.
2 Materials and methods
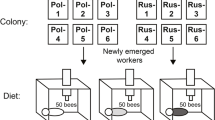
In general, published recommendations for cage studies of honey bees (Williams et al. 2013) were employed in these studies. In March 2022, at the Carl Hayden Bee Research Center (CHBRC) in Tucson, AZ (31° 46′ 38.08″ N, 110° 51′ 47.39″ W), several frames of mature brood were removed from an apiary consisting of six colonies each of three bee stocks: Pol-line, Russian, or Italian. The colonies were visually assessed for disease (e.g., dead or deformed adult bees, chalkbrood mummies, cleanliness of the bottom board, the presence and characteristics of any dead or chewed brood) and were monitored for Varroa infestation by inserting paperboards coated with petroleum jelly and covered with mesh onto hive bottom boards (Dietemann et al. 2013) and left 2–3 days. All hives had been treated against Varroa using thymol (Apiguard, Vita BeeHealth, UK) the previous October, followed up with amitraz (Apivar, Véto-pharma, France). The frames were placed in an incubator (Percival model I36VL) at 32 °C and 50% RH. Adult bees emerging over the following 48 h were distributed among 24 hoarding cages until each cage had about 200 worker bees (8 replicate cages per stock). Deformed bees were rejected. The cage’s internal dimensions were 115 mm × 100 mm × 65 mm; the narrow sides and top were made of Plexiglas® and the broadsides and bottom were made of 3-mm aperture galvanized steel mesh. A plastic 50-mL bottle for water and a plastic 30-mL bottle for syrup, each with a small hole in the lid, were inverted and placed on top of each cage. A 4 × 4 cm square of wire mesh was suspended, using 2 thin gauge wires, about 2–3 cm from the top of the cage, under the syrup and water bottles. Two Thermochron iButtons (Maxim Integrated, San Jose, CA, USA), set to record the temperature every 5 min, were attached to the center of the mesh, one on either side, and the entire mesh and sensors were covered in wax foundation. All cages were placed on three shelves in the incubator, dividing each stock group among all shelves; the position of each cage on the shelf was random with respect to stock. Temperature sensors were placed in Petri dishes on each shelf in the incubator to monitor temperature gradients within the incubator. Sucrose solution was prepared with 500-g sucrose and 500-mL distilled water in a mixing flask heated to no more than 60 °C. A mixture of 10 g each of pollen (Natural Foods Inc., Toledo, OH, USA), sucrose, and inverted sucrose was placed inside a rubber gasket accessed via a hole in the side of the cage at stocking.
Newly emerged bees were kept for 1 week at 30 °C. Thereafter, the incubator temperature varied: 12 h at 30 °C and 12 h at 15 °C. During the 15 °C phase, bees tended to cluster in the cage, usually on the wax square, and clusters would disperse during the 30 °C phase. Dead bees were removed and counted every 2–3 days. At the same time, syrup consumption was monitored by weighing the syrup feeder bottles at each observation day. At least once a week, the bottles were emptied, the syrup replaced, and the new full bottle weight recorded. Pollen paste consumption was monitored before and after the experiment. After 47 days, when all cages had lost at least 50% of their bees, all cages were placed in a freezer at − 20 °C, the remaining bees were counted, and temperature data was downloaded. The experiment was repeated in May 2022 with 10 cages per stock from the same apiary and again in April 2023 with 10 cages per stock from colonies established in April 2022, with different queens; a total of 16,783 bees were used (Supplementary data). For the experiments in May 2022 and April 2023, 10 bees were sampled per stock prior to placing bees in cages (newly emerged bees), and 10 bees were sampled from four cages per stock (= 12 cages total) on days 10, 14, and 21 after the start of the experiment. Sampled bees were immediately placed at − 80 °C for gene expression analysis. The same four cages were sampled each time. Twenty bees per stock per experiment were placed individually in Eppendorf tubes, weighed, dried for 168 h at 60 °C, and then re-weighed to determine the average dry weight per bee.
2.1 Gene expression analyses
Individual bees were sorted into 2-mL bead beater tubes (MP Biomedicals) on dry ice and stored at − 80 °C prior to processing. Whole bees were homogenized in 600 µL of Maxwell® simplyRNA homogenization solution (Promega). Four hundred microliters of the homogenized sample was added to 400 µL of Maxwell® simplyRNA lysis buffer and centrifuged, and 400 µL of the supernatant was transferred to Maxwell® RSC-48 cartridges (Promega). RNA extractions were carried out according to the Maxwell® RSC simplyRNA tissue kit protocol (Promega). cDNA synthesis was carried out using 1 µg of DNase-treated total RNA and QuantiTect Reverse Transcription Kits (Qiagen) according to the manufacturer’s protocols. Quantitative PCR (qPCR) was performed using a 1:10 dilution of cDNA in triplicate to quantify transcript levels of vitellogenin (vg), heat shock protein 70, heat shock protein 90, and DWV-B. All reactions were carried out as follows: initial denaturation at 95 °C for 5 min; 40 cycles with denaturation at 95 °C for 10 s; and a primer-pair-specific annealing and extension temperature (Supplementary data) for 30 s. A melt curve step was included in each reaction and data was captured at 65 °C with temperature increment of 5 °C up to 95 °C for 5 s. The reactions were carried out using Luna® Universal qPCR Master Mix (New England Biolabs Inc.) in triplicate on a CFX96 Real-Time PCR Detection System (Bio-rad, Hercules, CA, USA). Relative expression levels were determined based on the Δ Ct method using honey bee β-actin for normalization. Therefore, relative expression was calculated as 2^ -(target gene—β-actin). All qPCR Primer efficiencies were within the acceptable range of 90–110%.
2.2 Statistical analyses
Average syrup and pollen daily consumption rates per bee were calculated as the total observed consumption in milligrams for each cage for the duration of the experiment divided by the total number of “bee-days” for that cage. Bee-days were calculated by averaging the number of bees observed in a cage on each of two consecutive observation dates multiplied by the number of days between those dates; total bee-days per cage were calculated by summing these values across the duration of the experiment. Average daily syrup and pollen consumption rates were examined for normality using Kolmogorov–Smirnov statistic, log10-transformed to improve normality if needed (which was then tested), and analyzed across all three experiments using mixed-model ANOVA (α = 0.05) with stock, experiment, and their interaction as fixed factors; cage as a random factor; an autoregressive covariance matrix; and post hoc comparisons of stock and experiment using the Bonferroni error rate correction (Glimmix, SAS, Inc.). Gene expression data were examined for normality, log10-transformed if needed, and analyzed in the same manner with stock, experiment, sampling occasion, and their interactions as fixed effects. Data that were not acceptably normal after transformation were pooled across time, and pairwise comparisons were conducted within each experiment using the nonparametric Wilcoxon test (Npar1way, SAS, Inc.) Mite fall data onto paperboards in the field prior to the experiments were analyzed using an ANOVA with stock, year, and their interaction as fixed effects. Effect sizes and associated 95% confidence intervals for the post hoc contrasts of all ANOVA and MANOVA were estimated using Hedge’s g (Nakagawa and Cuthill 2007).
Cluster temperatures were calculated by averaging the data from two sensors in each cage and subtracting from that the ambient temperature recorded by the sensor placed on the same shelf. Data analysis was limited to the average of the first 6 h of the 15 °C cycle, when clusters were warmest and differences among groups were expected to be greatest. Daily average cluster temperatures decline markedly as the bees age (Meikle et al. 2018), so analyses were limited to the first 25 days. Temperature data were log10-transformed and analyzed using repeated-measures MANOVA (α = 0.05) with stock, experiment, day, and all two-way interactions as fixed factors; the number of bees in the cage as a covariate; and the same covariance matrix and post hoc comparisons as above. The number of bees in a given cage was estimated for non-observation days by linearly interpolating between values from consecutive observation days.
Bee survivorship in cages was analyzed using the mixed-model Cox regression model coxme in R (R Development Core Team 2020), with stock and experiment as fixed effects and cage number as a random effect. Data were censored for live bees at the end of the experiments and for bees sampled during the last two experiments. Adult bee survivorship for the bees that died during the course of the experiments was modeled in R using a distributed delay function. Distributed delay models use an iterative method to simulate, via a series of artificial substages, a population of objects moving through a process—in this case, the survivorship of bees moving through their adult stage. The distribution of the times of individuals leaving the process (in this case by dying) is assumed to have an Erlang distribution of parameter k equal to the number of substages per time step (1 day), with k = 1 (exponential distribution) to k = infinity (normal distribution). Data were pooled by stock across experiments and cages to estimate the k parameter. Once the model was implemented for the longest-lived stock, it was modified by a daily attrition rate, to provide a rough estimate of the daily additional loss in terms of adult workers for the shorter-lived stock, estimated using the least sums of squares.
3 Results
3.1 Food consumption
Kolmogorov–Smirnov statistics on the normality of raw and log-transformed syrup and pollen consumption are provided (Supplementary Information Table S1). Food consumption was significantly affected by both stock and experiment (Supplementary Information Table S2). Post hoc contrasts showed that, in terms of both syrup and pollen consumption, Italian bees > Russian bees > Pol-line bees, and all effect sizes were significant (the effect size interval did not include zero) (Table I). Italian bees consumed on average 29.2 ± 0.5 mg syrup and 5.1 ± 0.1 mg pollen patty per bee per day, while Russian bees consumed 27.1 ± 0.5 mg syrup and 4.3 ± 0.1 mg pollen patty, and Pol-line bees consumed 23.3 ± 0.6 mg syrup and 4.1 ± 0.1 mg pollen patty per bee per day (Figure 1). Experiments were also significantly different with respect to food consumption. In terms of average syrup consumption, the relationships were March 2022 > April 2023 > May 2022, while in terms of pollen consumption, the relationships were May 2022 > April 2023 > March 2022.
3.2 Cluster temperature
Both stock and experiment significantly affected cluster temperature (Supplementary Information Table S2). Post hoc contrasts showed that, among queen stocks, cluster temperatures were Pol-line > Russian > Italian, with all pairwise comparisons significant (Table I, Figure 2). Pol-line bee clusters were on average 4.5 ± 0.1 °C above ambient temperatures in the incubator, while Russian clusters were 3.8 ± 0.1 °C and Italian clusters were 2.8 ± 0.1 °C above ambient. Cluster temperatures declined significantly over time, and were also significantly correlated with the number of bees in the cage.
3.3 Gene expression
Kolmogorov–Smirnov statistics on the normality of raw and log-transformed data vitellogenin, HSP 70, and HSP 90 expression and on newly emerged bee dry weights are provided (Supplementary Information Table S1). Data on DWV-B expression could not be satisfactorily transformed and so were pooled across time and analyzed using nonparametric statistics. Bee stock was a significant factor in the expression of vitellogenin, HSP 70 (Supplementary Information Table S3, Figure 3), and DWV-B (Supplementary Information Table S4). Bee stock was not a significant factor in the expression of HSP90. “Experiment” (either May 2022 or April 2023) and “Time” (the four sampling occasions at 0, 10, 14, and 21 days after bee emergence) were significant for vitellogenin, HSP 70, and HSP 90, indicating that expression varied with respect to bee age and to colony differences between years (Table II). However, post hoc contrasts showed that the only pairwise comparison with a significant effect size was vitellogenin expression (with Pol-line stock producing more than the Italian stock). DWV-B levels, analyzed using nonparametric statistics, were significantly different among all bee stocks in the May 2022 experiment (all contrasts P < 0.005), with Italian > Pol-line > Russian (Figure 4). DWV-B is transmitted by Varroa mites; analyses of mite fall did not detect a significant difference among stocks (P = 0.21) but did detect a difference between years (P < 0.001), with (mean ± s.e.) 11.7 ± 3.3 mites per day in 2022 and 1.7 ± 0.4 mites per day in 2023. Dry weights of newly emerged bees were only significantly different between Russian bees (21.5 ± 0.5 mg) and Pol-line bees (20.1 ± 0.4 mg), but they were also significantly different between experiments (22.1 ± 0.3 mg in May 2002 and 19.6 ± 0.3 mg in April 2023).
Log10 relative expression of the vitellogenin gene in sampled bees over time. A May 2022 experiment; B April 2023 experiment. Boxes are defined as 1.58 × IQR/n0.5, where IQR is the inter-quartile range and n is the number of data. Points represent data considered outliers within the respective treatment group. Relative expression levels were determined based on the Δ Ct method using honey bee β-actin for normalization using the Eq. 2^ -(vitellogenin—β-actin).
Log10 relative expression of deformed wing virus B (DWV-B) transcripts in sampled bees over time. A May 2022 experiment; B April 2023 experiment. Boxes are defined as 1.58 × IQR/n0.5, where IQR is the inter-quartile range and n is the number of data. Points represent data considered outliers within the respective treatment group. Relative expression levels were determined based on the Δ Ct method using honey bee β-actin for normalization using the Eq. 2^ -(DWV-B—β-actin).
3.4 Bee survivorship
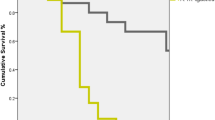
Analysis of all data, with the Italian stock as a reference group, showed that the Pol-line and Russian stocks had significant proportional hazards, indicating that the survivorship of those groups was longer than the Italian group (Table III, Figure 5), but they were not different from each other (P = 0.42). Experiments were also different; compared to the third experiment (April 2023) as the reference group, proportional hazards for the March and May 2022 experiments were significant, and those experiments also had a significant proportional hazard (hazard = 0.968, s.e. = 0.059, z = 16.52, P < 0.0001). The most rapid general decline in survivorship occurred in the May 2022 experiment. The median age at termination for Italian stock bees across all experiments was 25 days, while the median age for both Pol-line and Russian bees was 33 days.
3.5 Model results
The model generated using parameters from the Pol-line bee data, and with no attrition, fit the observed data well (Figure 6). Caution should be exercised in interpreting the initial model fit since the data included only bees that died during the experiment, which was 90.8% of the Italian bees but only 69.7% of the Pol-line bees. The mean survival time, 32.50 ± 0.14 day for Pol-line bees and 24.78 ± 0.11 day for Italian bees, would have been longer if the experiment had continued until all bees had died. Given that more Pol-line bees survived, the difference in the mean survival times between the groups would likely have also been larger as well, even if bees from both groups died at identical rates after the ends of the respective experiments.
Using the least sum of squares to fit the most optimal attrition rate, but retaining the Pol-line data parameters, showed that a daily attrition rate of 0.0104 per day fit the Italian stock data best. The modified model was overall a less optimal fit, because the population parameters from the Pol-line bees were used rather than those of the Italian bees. Also, the attrition rate itself is applied equally across all time steps (days) whereas the data showed no differences in attrition for at least a week, so the attrition rate should be treated as very approximate.
4 Discussion
Comparative physiological and behavioral assessments of honey bee populations could enable selective breeding to enhance their resilience to diverse management and environmental conditions. This includes identifying differences in climate adaptation, nutritional responses, and stress resistance. Syrup and pollen consumption were significantly different among these bee stocks and the relationships among the stocks were largely consistent among experiments, although the experiments themselves were significantly different from each other. Italian bees had the highest syrup and pollen consumption rates across all experiments, and Pol-line bees had the lowest, which was similar to previous reports (Penn et al. 2022). These data suggest that one or more other physiological parameters, such as energy storage (such as lipoproteins), activity levels, gland production (e.g., hypopharyngeal or wax), or tissue growth, would also be different among bee stocks. Here, we measured mRNA transcript levels of vitellogenin, an important nutritional storage protein associated with longevity (Amdam et al. 2005). Vitellogenin has been used as a biomarker to assess bee nutritional status since expression is linked to diet quality and quantity (Alaux et al. 2011; Ricigliano et al. 2021). The observed expression differences among bee stocks were, however, contrary to expectations: despite higher pollen consumption, Italian bees had significantly lower vitellogenin expression levels than Pol-line bees (and no other comparisons were significant).
Average cluster temperature per cage may be considered a proxy variable for activity levels, since both require energy expenditure. Cluster temperatures were significantly different among bee stocks, with significant effect sizes, but the direction of the differences was contrary to expectations; that is, Italian bees had the lowest average cluster temperatures although they consumed significantly more food per day than the other stocks. Bee cluster formation has a certain level of complexity (Stabentheiner et al. 2010), and it is not clear from this study whether the higher cluster temperatures resulted from higher thoracic temperatures among bees heating the cluster (a difference in individual bee behavior) or from a more effective cluster formation that better insulated the inner part of cluster, near the sensor, from ambient temperatures (a difference in collective behavior). This observation affords a prediction for field result: Italian colonies would be expected to have lower average hive temperatures than colonies of the other stocks, if other factors, such as colony size, are equal. Variability in cluster temperatures in bee hives, itself a function of the size and quality of the brood cluster, has been found inversely proportional to the quantity of sealed brood (the more constant the temperature, the greater the expected amount of sealed brood) (Meikle et al. 2016).
Differences in adult bee survivorship among the different stocks were stark: bees from Pol-line and Russian stocks lived significantly longer than bees from the Italian stock. That the same relationships were observed across three separate experiments over 2 years suggests the results are robust. While Italian bees consumed significantly more syrup and pollen than the other stocks on a daily basis, since they died significantly earlier, their lifetime consumption of syrup, 710 ± 26 mg per bee, was significantly lower than either Pol-line or Russian stocks (768 ± 37 and 857 ± 34 mg per bee, respectively). Pol-line bees had significantly higher vitellogenin expression levels, with an important effect size, than Italian bees, which agreed with survivorship results. Russian bees likewise lived significantly longer than Italian bees, but the effect size of the vitellogenin comparison, while significant as a post hoc contrast, was negligible. Expression of heat shock protein genes—which are associated with longevity in diverse species (Calderwood et al. 2009)—has been suggested to protect honey bee cells from damage under exposure to environmental stressors and pathogens (Abou-Shaara 2024), and heat shock protein expression has been found inversely proportional to the humeral immune response (McKinstry et al. 2017). HSP expression showed significant effects of bee stock with respect to one (HSP 70) but not the other (HSP 90). Russian bees had HSP 70 expression levels that were significantly less than that of other stocks in terms of post hoc analyses, but the magnitudes of the effect sizes were negligible and expression of HSP genes did not correspond to survivorship results.
Relative DWV-B expression showed that in the second experiment, clear differences existed among all bee stocks, while in the third experiment, no differences were found. Both Pol-line and Russian bees are considered “Varroa-resistant” lines (Ricigliano et al. 2021), while the Italian stock used here was not. The failure to repeat the results from the second experiment indicates that further work is needed to clarify the role of DWV-B with respect to other response variables. Why the DWV-B viral load was different between the two experiments is not known. Varroa mite fall was not significantly different among donor colonies prior to the experiments, and any bees exhibiting physical deformities were excluded from the cages, so such an explanation is possible but not likely. DWV-B, classified as having low virulence compared to other honey bee RNA viruses, is known to reduce the lifespan of adults (Martin and Brettell 2019). The relationship between DWV-B infection and the incidence of deformed wings is not always strong, and therefore, deformed wings may not be a reliable indicator of viral load (de Miranda and Genersch 2010). However, the lifespans of the Italian bees were significantly shorter than Pol-line and Russian bees, whether or not DWV-B levels were different.
Given the different worker lifespans among the different bee stocks, how would these differences be observed in field data? The distributed delay approach (Manetsch 1976; Vansickle 1977) used to model bee survivorship has been applied in insect ecology (see Gutierrez and Baumgaertner 1984, Meikle et al. 2003) and is comparatively simple yet can capture important parts of temporal dynamics. The modeling exercise suggested that cages with Italian stock lost on average about 1.04% more per day compared to the Pol-line bees. Confirming that result with bee colonies in the field, using devices such as dead bee traps (e.g., Human et al. 2013) would be difficult since the associated measurement error would be comparatively high compared to the average of any expected differences. However, honey bee colonies produce brood in order to generate the adult population, and since the brood is sealed for 12 days prior to the emergence of the adult bee (Winston 1992), the sealed brood itself may be considered a 2-week stock of bees that is easier to measure than daily worker loss. Thus, for an Italian colony to maintain the same size as a Pol-line colony, while losing 1.04% more per day, the sealed brood population would need to be larger by a factor of 1.010412 ~ 1.13, or about 13%, than that of a Pol-line colony. According to this analysis, all else being equal, one should expect about 13% more brood in the commercial Italian stock than in the Pol-line stock.
The role of “experiment,” that is, the importance of data resulting from a particular experiment, was significant as a main effect in the analysis of every dependent variable subjected to MANOVA (consumption rates, cluster temperature, NEB dry weights, and gene expression except for DWV-B) yet difficult to interpret. Because the source brood for each experiment was collected on the same day, the bees represent a “snapshot” of the colony at a given moment, reflecting colony differences over time and between years due to uncontrolled factors (e.g., rainfall, daily temperatures, pathogen exposure, or competition due to the presence of other colonies in the area). We believe this underscores the importance of experiment-wide replication. The age of the bees, included as “Time” in the analyses, was also clearly important as an explanatory variable. Changes in food consumption and cluster temperature over time are logical as the bees age and have been reported in cage studies (Meikle et al. 2018). The observed changes over time in the expression of vitellogenin and HSP (DWV-B data were pooled across time) may be due largely to the sampling protocol (destructive sampling of four bees per cage at each time point) combined with population variability.
Identifying traits for adaptation to different management and environmental factors is key to maintaining robust honey bee populations under climate change. Bee stock had a significant impact on food consumption (Italian > Russian > Pol-line), and mite-resistant stocks had higher cluster temperatures and survival relative to Italian stock. This was consistent with the observed higher expression of vitellogenin—a gene that influences worker lifespan. Differences in DWV-B were inconsistent between experiments, and future work may include a more in-depth exploration of viral loads among these bee stocks. These findings provide novel fine-scale insights regarding the effects of genetic variation on bee attributes that may influence colony-level productivity and health.
Availability of data and materials
The datasets generated during the current study may be found in the Supplementary data file.
Code availability
R code used for analysis is available upon request.
References
Abou-Shaara HF (2024) The response of heat shock proteins in honey bees to abiotic and biotic stressors. J Thermal Biol 119:103784. https://doi.org/10.1016/j.jtherbio.2024.103784
Alaux C, Dantec C, Parrinello H, Le Conte Y (2011) Nutrigenomics in honey bees: digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genomics 12:496. https://doi.org/10.1186/1471-2164-12-496
Amdam GV, Norberg K, Omholt SW, Kryger P, Lourenço AP, Bitondi MMG, Simões ZLP (2005) Higher vitellogenin concentrations in honey bee workers may be an adaptation to life in temperate climates. Insectes Soc 52:316–319
Bee Informed Partnership (2023) https://beeinformed.org/2023/06/22
Calderwood SK, Murshid A, Prince T (2009) The shock of aging: molecular chaperones and the heat shock response in longevity and aging–a mini-review. Gerontology 55(5):550–558
de Miranda JR, Genersch E (2010) Deformed Wing Virus J Invert Pathol 103:S48–S61. https://doi.org/10.1016/j.jip.2009.06.012
Dietemann V, Nazzi F, Martin SJ, Anderson D, Locke B, Delaplane KS, Wauquiez Q, Tannhill C, Frey E, Zigelmann B, Rosenkranz P, Ellis JD (2013) Standard methods for Varroa research. In: Dietemann, V, Ellis JD, Neumann P (Eds.) The COLOSS BeeBook, Vol. II: Standard methods for Apis mellifera pest and pathogen research. J Apicult Res 52(1). https://doi.org/10.3896/IBRA.1.52.1.09
Gutierrez AP, Baumgaertner JU (1984) Multi-trophic level models of predator-prey energetics: I. Age-specific energetics models – pea aphid Acyrthosiphon pisum (Harris) (Homoptera: Aphididae) as an example. Can Entomol 116:924–932
Human H, Brodschneider R, Dietemann V, Dively G, Ellis J, Forsgren E, Fries I, Hatjina F, Hu F-L, Jaffé R, Jensen AB, Köhler A, Magyar J, Özkýrým A, Pirk CWW, Rose R, Strauss U, Tanner G, Tarpy DR, van der Steen JJM, Vaudo A, Vejsnæs F, Wilde J, Williams GR, Zheng H-Q (2013) Miscellaneous standard methods for Apis mellifera research. In: Dietemann V, Ellis JD, Neumann P (Eds.) The COLOSS BeeBook, Volume I: Standard methods for Apis mellifera research. J Apicult Res 52(4). https://doi.org/10.3896/IBRA.1.52.4.10
Manetsch TJ (1976) Time varying distributed delays and their use in aggregative models of large systems. IEEE Trans Syst Man Cybern 6:547–553
Martin SJ, Brettell LE (2019) Deformed wing virus in honeybees and other insects. Annu Rev Virol 6:49–69. https://doi.org/10.1146/annurev-virology-092818-015700
McKinstry M, Chung C, Truong H, Johnston BA, Snow JW (2017) The heat shock response and humoral immune response are mutually antagonistic in honey bees. Sci Rep 7:8850. https://doi.org/10.1038/s41598-017-09159-4
McMenamin AJ, Daughenbaugh KF, Flenniken ML (2020) The heat shock response in the Western honey bee (Apis mellifera) is antiviral. Viruses 12(2):245. https://doi.org/10.3390/v12020245
Meikle WG, Adamczyk JJ, Weiss M, Gregorc A (2018) Effects of bee density and sublethal imidacloprid exposure on cluster temperatures of caged honey bees. Apidologie 49(5):581–593. https://doi.org/10.1007/s13592-018-0585-z
Meikle WG, Corby-Harris V, Ricigliano V, Snyder L, Weiss W (2023) Cold storage as part of a Varroa management strategy: effects on honey bee colony performance mite levels and stress biomarkers abstract. Sci Rep 13(1). https://doi.org/10.1038/s41598-023-39095-5
Meikle WG, Colin T, Adamczyk JJ, Weiss M, Barron AB (2022) Traces of a neonicotinoid pesticide stimulate different honey bee colony activities, but do not increase colony size or longevity. Ecotoxicol Environ Safety 231:113202. https://doi.org/10.1016/j.ecoenv.2022.113202
Meikle WG, Jaronski S, Mercadier G, Quimby PC (2003) A distributed delay routine-based simulation model of Beauveria bassiana conidial stability in response to environmental stressors. Biocontrol 48:561–578
Meikle WG, Weiss M, Maes PW, Fitz W, Snyder LA, Sheehan T, Mott BM, Anderson KE (2017) Internal hive temperature as a means of monitoring honey bee colony health in a migratory beekeeping operation before and during winter. Apidologie 48:666–680. https://doi.org/10.1007/s13592-017-0512-8
Meikle WG, Weiss M, Beren E (2020) Landscape factors influencing honey bee colony behavior in Southern California commercial apiaries. Sci Rep 10:5013. https://doi.org/10.1038/s41598-020-61716-6
Meikle WG, Weiss M, Stilwell AR (2016) Monitoring colony phenology using within-day variability in continuous weight and temperature of honey bee hives. Apidologie 47:1–14. https://doi.org/10.1007/s13592-015-0370-1
Mondet F, Beaurepaire A, McAfee A, Locke B, Alaux C, Blanchard S, Danka B, Le Conte Y (2020) Honey bee survival mechanisms against the parasite Varroa destructor: a systematic review of phenotypic and genomic research efforts. Internat J Parasitol 50:433–447. https://doi.org/10.1016/j.ijpara.2020.03.005
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605. https://doi.org/10.1111/j.1469-185X.2007.00027.x
Panziera D, Requier F, Chantawannakul P, Pirk CWW, Blacquière T (2022) The diversity decline in wild and managed honey bee populations urges for an integrated conservation approach. Front Ecol Evol 10:767950. https://doi.org/10.3389/fevo.2022.767950
Penn HJ, Simone-Finstrom MD, de Guzman LI, Tokarz PG, Dickens R (2022) Viral species differentially influence macronutrient preferences based on honey bee genotype. Biol Open 11:bio059039. https://doi.org/10.1242/bio.059039
R Development Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing
Ricigliano VA, Ihle KE, Williams ST (2021) Nutrigenetic comparison of two Varroa-resistant honey bee stocks fed pollen and spirulina microalgae. Apidologie 52:873–886. https://doi.org/10.1007/s13592-021-00877-3
Saelao P, Simone-Finstrom M, Avalos A, Bilodeau L, Danka RG, de Guzman L, Rinkevich F, Tokarz P (2020) Genome-wide patterns of differentiation within and among US commercial honey bee stocks. BMC Genomics 21:1–12. https://doi.org/10.1186/s12864-020-07111-x
Southwick EE, Mugaas JN (1971) A hypothetical homeotherm: the honey bee hive. Comp Biochem Physiol 40A:935–944
Stabentheiner A, Kovac H, Brodschneider R (2010) Honeybee colony thermoregulation—regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE 5(1):e8967. https://doi.org/10.1371/journal.pone.0008967
Vansickle J (1977) Attrition in distributed delay models. IEEE Trans Syst Man Cybern 7:635–638
Williams GR, Alaux C, Costa C, Csáki T, Doublet V, Eisenhardt D, Fries I, Kuhn R, McMahon DP, Medrycki P, Murray TE, Natsopoulu ME, Neumann P, Oliver R, Paxton RJ, Pernal SF, Shutler D, Tanner G, van der Steen JJM, Brodschneider R (2013) Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. In: Dietemann V, Ellis JD, Neumann P (Eds.) The COLOSS BeeBook Vol. I: Standard methods for Apis mellifera research. J Apicult Res 52(1). https://doi.org/10.3896/IBRA.1.52.1.04
Winston ML (1992) The honey bee colony: life history. In: Graham JM (ed) The hive and the honey bee, Dadant & Sons. Hamilton, IL, USA, pp 73–101
Zhao H, Li G, Guo D, Li H, Liu Q, Xu B, Guo X (2021) Response mechanisms to heat stress in bees. Apidologie 52:388–399. https://doi.org/10.1007/s13592-020-00830-w
Acknowledgements
The authors would like to thank E. Beren for valuable technical assistance and V. Corby-Harris, J.J. Adamczyk, and two anonymous reviewers for improving the manuscript.
Author information
Authors and Affiliations
Contributions
MW and DA performed material preparation and data collection. WGM and VAR conducted data analyses and led manuscript writing, and all authors edited and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor: Michelle L Flenniken
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meikle, W.G., Weiss, M., Adjaye, D. et al. Comparative assessment of food consumption, longevity, thermoregulation, and molecular health markers in mite-resistant and Italian honey bee stocks. Apidologie 55, 28 (2024). https://doi.org/10.1007/s13592-024-01071-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-024-01071-x