Abstract
Bird migration is one of most salient annual events in nature. It involves predictable seasonal movements between breeding and non-breeding habitats. Both circadian and circannual clocks are entrained by photoperiodic cues and time daily and seasonal changes in migratory physiology and behavior. This mini-review provides an update on daily and seasonal rhythms of migratory behavior, and examines the neuroendocrine and molecular pathways involved in the timing of migration in songbirds. Recent findings have identified key neural substrates, and suggest the involvement of multiple neuroendocrine regulatory systems in controlling seasonal states in migrants. We propose that four distinct neural substrates are involved in the timing of migration and include (1) pineal gland and suprachiasmatic nucleus (mSCN); (2) a cluster of hypothalamic nuclei, the mediobasal hypothalamus (MBH); (3) dorsomedial hypothalamic nucleus (DMH); and (4) tanycytes along ependymal layer of the 3rd ventricle (3V). Cluster N, a nucleus in the telencephalon involved in the integration of geomagnetic cues, likely maintains functional connectivity with brain regions involved in timing songbird migration. These nuclei form an interconnected network that coordinates daily timing (pineal gland/mSCN), annual photoperiodic response (MBH, 3V), energetic state (MBH, DMH, 3V), and magnetic compass information (i.e., cluster N) for migration in songbirds.



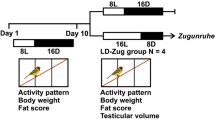
The data have been derived from Jain and Kumar (1995) and Trivedi and Kumar (unpublished)

Similar content being viewed by others
References
Bairlein F (2003a) The study of bird migrations—some future perspectives. Bird Study 50:243–253
Bairlein F (2003b) Nutritional strategies in migratory birds. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian migration. Springer, Berlin, pp 321–334
Bartell PA, Gwinner E (2005) A separate circadian oscillator controls nocturnal migratory restlessness in the songbird Sylvia borin. J Biol Rhythms 20:538–549
Berthold P (1974) Endogene jahresperiodik. Innere jahreskalender als grundlage der jahreszeitlichen orientierung bei tieren und pflanzen. Universitatsverlag, Konstanz, pp 46
Berthold P (1993) Bird migration: a general survey. Oxford University Press, Oxford
Berthold P, Querner U (1988) Was zugunruhe wirklich ist: eine quantitative bestimmmung mit hilfe von video-aufnahmen bei infrarotbeleuchtung. J Ornithol 129:372–375
Berthold P, Gwinner E, Klein H (1972) Circannuale periodik bei grasmücken. J Ornithol 113:170–190
Berthold P, Gwinner E, Sonnenschein E (2003) Avian migration. Springer-Verlag GmbH, Heidelberg
Bolborea M, Dale N (2013) Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci 36:91–100
Budki P, Rani S, Kumar V (2012) Persistence of circannual rhythms under constant periodic and aperiodic light conditions: sex differences and relationship with the external environment. J Exp Biol 215:3774–3785
Budki P, Malik S, Rani S, Kumar V (2014) Circadian rhythms are not involved in the regulation of circannual reproductive cycles in a sub-tropical bird, the spotted munia. J Exp Biol 217:2569–2579
Cantwell EL, Cassone VM (2006) The chicken suprachiasmatic nuclei: I. Efferent and afferent connections. J Comp Neurol 496:97–120
Cassone VM (2014) Avian circadian organization: a chorus of clocks. Front Neuroendocrinol 35:76–88
Cornelius JM, Boswell T, Jenni-Eiermann S, Breuner CW, Ramenofsky M (2013) Contribution of endocrinology of the migration life history of birds. Gen Comp Endocrinol 190:47–60
Dawson A, Goldsmith AR (1997) Changes in gonadotropin-releasing hormone in the preoptic area and median eminence of starlings during the recovery of photosensitivity and during photostimulation. J Reprod Fert 111:1–6
Dawson A, Sharp PJ (2007) Photorefractoriness in birds—photoperiodic and non-photoperiodic control. Gen Comp Endocrinol 153:378–384
Dingle H (2006) Animal migration: is there a common migratory syndrome? J Ornithol 147:212–220
Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, Hara E, Wada K, Mouritsen H, Jarvis ED (2008) Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS One 3:e1768
Follett BK, Sharp PJ (1969) Circadian rhythmicity in photoperiodically induced gonadotrophin release and gonadal growth in the quail. Nature 223:968–971
Fuchs T, Haney A, Jechura TJ, Moore FR, Bingman VP (2006) Daytime naps in night-migrating birds: behavioral adaptation to seasonal sleep deprivation in the Swainson’s thrush (Catharus ustulatus). Anim Behav 72:951–958
Fudickar AM, Peterson MP, Greives TJ, Atwell JW, Bridge ES, Ketterson ED (2016) Differential gene expression in seasonal sympatry: mechanisms involved in diverging life histories. Biol Lett 12:20160069
Fusani L, Coccon F, Rojas More A, Goymann W (2013) Melatonin reduces migratory restlessness in Sylvia warblers during autumnal migration. Front Zool 10:79
Garcia-Fernandez JM, Cernuda-Cernuda R, Davies WIL, Rodgers J, Turton M, Peirson SN, Follett BK, Halford S, Hughes S, Hankins MW, Foster RG (2015) The hypothalamic photoreceptors regulating seasonal reproduction in birds: a prime role for VA opsin. Front Neuroendocrinol 37:13–28
Gwinner E (1967) Circannuale periodic der mauser und der zugunrugebeieinem vogel. Naturwissenschaften 54:447
Gwinner E (1968) Circannuale periodik als grundlage des jahres zeitlichen funktionswandels bei zugvogeln. untersuchungen am fitis (Phylloscopus trochilus) und am Waldlaubsanger (P. sibilatrix). J Omithol 109:70–95
Gwinner E (1975) Circadian and circannual rhythms in birds. Avian Biol 5:221–285
Gwinner E (1986) Circannual rhythms in the control of avian migrations. Adv Stud Behav 16:191–228
Gwinner E (1996) Circadian and circannual programmes in avian migration. J Exp Biol 199:39–48
Gwinner E, Brandstätter R (2001) Complex bird clocks. Phil Trans R Soc B Biol Sci 356:1801–1810
Gwinner E, Helm B (2003) Circannual and circadian contributions to the timing of avian migration. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian migration. Springer, Berlin, pp 81–95
Gwinner E, Wiltschko W (1980) Circannual changes in migratory orientation of the garden warbler, Sylvia borin. Behav Ecol Sociobiol 7:73–78
Helm B, Schwabl I, Gwinner E (2009) Circannual basis of geographically distinct bird schedules. J Exp Biol 212:1259–1269
Heyers D, Manns M, Luksch H, Güntürkün O, Mouritsen H (2007) A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS One 2:e937
Holberton RL, Dufty AM Jr (2005) Hormones and variation in life history strategies of migratory and non-migratory birds. In: Marra P, Greenburg E (eds) Birds of two worlds: the ecology and evolution of migration. Johns Hopkins University Press, Baltimore, pp 290–302
Jain N, Kumar V (1995) Changes in food intake, body weight, gonads and plasma concentrations of thyroxine, luteinizing hormone and testosterone in captive buntings exposed to natural day lengths at 29°N. J Biosci 20:417–426
Johnston RA, Paxton KL, Moore FR, Wayne RY, Smit TB (2016) Seasonal gene expression in a migratory bird. Mol Ecol. doi:10.1111/mec.13879
Karagicheva J, Rakhimberdiev E, Dekinga A, Brugge M, Koolhaas A, Job Ten Horn JT, Piersma T (2016) Seasonal time keeping in a long-distance migrating shorebird. J Biol Rhythms 31:509–521
King JR, Farner DS (1963) The relationship of fat deposition to zugunruhe and migration. Condor 65:200–223
Kumar V (2001) Melatonin and circadian rhythmicity in birds. In: Dawson A, Chaturvedi CM (eds) Avian endocrinology. Narosa Publishing House, New Delhi, pp 93–112
Kumar V, Singh BP (2005) The timekeeping system in birds. Proc Indian Natl Sci Acad B 71:267–284
Kumar V, Singh BP, Rani S (2004) The bird clock: a complex, multi-oscillatory and highly diversified system. Biol Rhythm Res 35:121–144
Kumar V, Rani S, Singh BP (2006) Biological clocks help reduce the physiological conflicts in avian migrants. J Ornithol 147:281–286
Kumar V, Wingfield JC, Dawson A, Ramenofsky M, Rani S, Bartell P (2010) Biological clocks and regulation of seasonal reproduction and migration in birds. Physiol Biochem Zool 83:827–835
Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashia H, Aja S, Ford E, Fishell G, Blackshaw S (2012) Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci 15:700–702
Lewis JE, Ebling FJ (2017) Tanycytes as regulators of seasonal cycles in neuroendocrine function. Front Neurol 8:79
Lindström Å (2003) Fuel deposition rates in migrating birds: causes, constraints and consequences. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian migration. Springer, Berlin, pp 307–320
MacDougall-Shackleton SA, Stevenson TJ, Watts HE, Pereyra ME, Hahn TP (2009) The evolution of photoperiod response systems and seasonal GnRH1 plasticity in birds. Integr Comp Biol 49:580–589
Majumdar G, Rani S, Kumar V (2015) Hypothalamic gene switches control transitions between seasonal life history states in a night-migratory photoperiodic songbird. Mol Cell Endocrinol 399:110–121
Malik S, Singh J, Trivedi AK, Singh S, Rani S, Kumar V (2015) Nocturnal melatonin levels decode daily light environment and reflect seasonal states in night-migratory blackheaded bunting (Emberiza melanocephala). Photochem Photobiol Sci 14:963–971
McMillan JP (1972) Pinealectomy abolishes the circadian rhythm of migratory restlessness. J Comp Physiol 79:105–112
McWilliams SR, Karasov WH (2005) Migration takes guts. Birds Two Worlds 28:67–78
Mishra I, Kumar V (2017) Seasonal alterations in the daily rhythms in hypothalamic expression of genes involved in the photoperiodic transduction and neurosteroid-dependent processes in migratory blackheaded buntings. J Neuroendocrinol. doi:10.1111/jne.12469
Mishra I, Bhardwaj SK, Malik S, Kumar V (2017) Concurrent hypothalamic gene expression under acute and chronic long days: implications for initiation and maintenance of photoperiodic response in migratory songbirds. Mol Cell Endocrinol 439:81–94
Mouritsen H, Feenders G, Liedvogel M, Wada K, Jarvis ED (2005) Night-vision brain area in migratory songbirds. Proc Natl Acad Sci USA 102:8339–8344
Mouritsen H, Heyers D, Gunturkun O (2016) The neural basis of long-distance navigation in birds. Ann Rev Physiol 78:133–154
Nakane Y, Ikegami K, Ono H, Yamamoto N, Yoshida S, Hirunagi K, Ebihara S, Kubo Y, Yoshimura T (2010) A mammalian neural tissue opsin is a deep brain photoreceptor in birds. Proc Natl Acad Sci 107:15264–15268
Nicholls TJ, Goldsmith AR, Dawson A (1988) Photorefractoriness in birds and comparison with mammals. Physiol Rev 68:133–176
Orellana JA, Saez PJ, Cortes-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Figueroa V, Velarde V, Jian JX, Nualart F, Saez JC, Garcia MA (2012) Glucose increases intracellular free Ca(2+) in tanycytes via ATP release through connexin 43 hemichannels. Glia 60:53–68
Perez JH, Furlow JD, Wingfield JC, Ramenofsky M (2016) Regulation of vernal migration in Gambel’s white-crowned sparrows: role of thyroxine and triiodothyronine. Horm Behav 84:50–56
Piersma T, Pérez-Tris J, Mouritsen H, Bauchinger U, Bairlein F (2005) Is there a “migratory syndrome” common to all migrant birds? Ann New York Acad Sci 1046:282–293
Ramakrishnan S, Strader AD, Wimpee B, Chen P, Smith MS, Buntin JD (2007) Evidence for increased neuropeptide Y synthesis in mediobasal hypothalamus in relation to parental hyperphagia and gonadal activation in breeding ring doves. J Neuroendocrinol 19:163–171
Ramenofsky M, Wingfield JC (2007) Regulation of migration. Bioscience 57:135–143
Rani S, Kumar V (2013) Avian circannual systems: persistence and sex differences. Gen Comp Endocrinol 190:61–67
Rani S, Kumar V (2014) Photoperiodic regulation of seasonal reproduction in higher vertebrates. Indian J Exp Biol 52:413–419
Rani S, Malik S, Trivedi AK, Singh S, Kumar V (2006) A circadian clock regulates migratory restlessness in the blackheaded bunting, Emberiza melanocephala. Curr Sci 91:1093–1096
Rastogi A, Kumari Y, Rani S, Kumar V (2011) Phase inversion of neural activity in the olfactory and visual systems of a night-migratory bird during migration. Eur J Neurosci 34:99–109
Rastogi A, Kumari Y, Rani S, Kumar V (2013) Neural correlates of migration: activation of hypothalamic clock(s) in and out of migratory state in the blackheaded bunting (Emberiza melanocephala). PLoS One 8:e70065
Rattenborg NC, Mandt BH, Obermeyer WH, Winsauer PJ, Huber R, Wikelski M, Benca RM (2004) Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii). PLoS Biol 2:e212
Singh D, Rani S, Kumar V (2013) Daily expression of six clock genes in central and peripheral tissues of a night migratory songbird: evidence for tissue-specific circadian timing. Chronobiol Int 30:1208–1217
Singh D, Trivedi AK, Rani S, Kumar V (2015) Circadian timing in central and peripheral tissues in a migratory songbird: dependence on annual life-history states. FASEB J 29:4248–4255
Stevenson TJ, Ball GF (2012) Disruption of neuropsin mRNA expression via RNA interference facilitates the photoinduced increase in thyrotropin-stimulating subunit-β in birds. Eur J Neurosci 36:2859–2865
Stevenson TJ, Lincoln GA (2017) Epigenetic mechanisms regulating circannual rhythms. In: Kumar V (ed) Biological timekeeping: clocks, rhythms and behavior. Springer, New Delhi, pp 607–624
Stevenson TJ, Bernard DJ, Ball GF (2009) Photoperiodic condition is associated with region-specific expression of GNRH1 mRNA in the preoptic area of the male starling (Sturnus vulgaris). Biol Reprod 81:674–680
Stevenson TJ, MacDougall-Shackleton SA, Hahn TP, Ball GF (2012a) GnRH plasticity: a comparative perspective. Front Neuroendocrinol 33:287–300
Stevenson TJ, Hahn TP, Ball GF (2012b) Variation in gonadotropin releasing-hormone-1 gene expression in the preoptic area predicts transitions in seasonal reproductive state. J Neuroendocrinol 24:267–274
Stevenson TJ, Bernard DJ, McCarthy MM, Ball GF (2013) Photoperiod-dependent regulation of gonadotropin-releasing hormone 1 messenger ribonucleic acid levels in the songbird brain. Gen Comp Endocrinol 190:81–87
Surbhi A, Kumar V (2014) Avian photoreceptors and their role in the regulation of daily and seasonal physiology. Gen Comp Endocrinol 220:13–22
Surbhi Rastogi A, Rani S, Kumar V (2015) Seasonal plasticity in the peptide neuronal systems: potential roles of GnRH, GnIH, NPY and VIP in regulation of reproductive axis in subtropical Indian weaver birds. J Neuroendocrinol 27:357–369
Surbhi A, Rastogi S, Malik Rani S, Kumar V (2016) Changes in brain peptides associated with reproduction and energy homeostasis in photosensitive and photorefractory migratory redheaded buntings. Gen Comp Endocrinol 231:67–75
Trivedi AK, Kumar J, Rani S, Kumar V (2014) Annual life history–dependent gene expression in the hypothalamus and liver of a migratory songbird: insights into the molecular regulation of seasonal metabolism. J Biol Rhythms 29:332–345
Trivedi AK, Malik S, Rani S, Kumar V (2015) Adaptation of oxidative phosphorylation to photoperiod-induced seasonal metabolic states in migratory songbirds. Comp Biochem Physiol A Mole Integr Physiol 184:34–40
Trivedi AK, Malik S, Rani S, Kumar V (2016) Pinealectomy abolishes circadian behavior and interferes with circadian clock gene oscillations in brain and liver but not retina in a migratory songbird. Physiol Behav 156:156–163
Wikelski M, Martin LB, Scheuerlein A, Robinson M, Robinson N, Helm B, Hau M, Gwinner E (2008) Avian circannual clocks: adaptive significance and possible involvement of energy turnover in their proximate control. Philos Trans R Soc Lond B Biol Sci 363:411–423
Wingfield JC (2004) Allostatic load and life cycles: implications for neuroendocrine mechanisms. In: Schulkin J (ed) Allostasis, homeostasis, and the costs of physiological adaptation. Cambridge University Press, New York, pp 302–342
Yamamura T, Hirunagi K, Ebihara S, Yoshimura T (2004) Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial end feet in Japanese quail. Endocrinol 145:4264–4267
Yasuo S, Watanabe M, Okabayashi N, Ebihara S, Yoshimura T (2003) Circadian clock genes and photoperiodism: comprehensive analysis of clock gene expression in the mediobasal hypothalamus, the suprachiasmatic nucleus, and the pineal gland of Japanese quail under various light schedules. Endocrinol 144:3742–3748
Yoshimura T, Suzuki Y, Makino E, Suzuki T, Kuroiwa A, Matsuda Y, Namikawa T, Ebihara S (2000) Molecular analysis of avian circadian clock genes. Mole Brain Res 78:207–215
Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S (2003) Light induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature 426:178–181
Acknowledgements
The Science and Engineering Research Board (SERB) and Department of Biotechnology, Govt. of India have generously provided to VK the funding to carryout research on migration in buntings included in this review paper. TJS was funded by an International Exchange Programme from the Royal Society of Edinburgh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Stevenson, T.J., Kumar, V. Neural control of daily and seasonal timing of songbird migration. J Comp Physiol A 203, 399–409 (2017). https://doi.org/10.1007/s00359-017-1193-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-017-1193-5